INTRODUCTION
Q fever is a zoonosis caused by Coxiella burnetii, an intracellular bacterium that appears almost everywhere in the world [Reference Raoult, Marrie and Mege1]. The most common reservoirs for C. burnetii that cause human infections are ruminants, primarily cattle, sheep and goats, although some documented outbreaks have been associated with parturient cats or birds [Reference Pinsky2–Reference Stein and Raoult4]. Humans typically acquire an infection from inhaling infected aerosols or dust generated by infected animals or animal products [Reference Lyttikäinen5].
Q fever usually occurs sporadically, but common occupational exposures have been reported to cause outbreaks, mostly in abattoirs and among veterinarians or research staff using sheep as experimental animals [Reference Pedogy6–Reference Grilc8]. An old study in The Netherlands showed significantly higher prevalence of IgG antibodies against C. burnetii among veterinarians, residents of dairy farms and taxidermists compared to controls from the general population [Reference Richardus9]. Outbreaks not involving occupational exposure to C. burnetii have also been described, particularly in Europe. These are usually temporally linked to the lambing season in ruminants [10–Reference Orr12].
In The Netherlands Q fever is a mandatory notifiable disease in humans, but only since June 2008 in small ruminants. Five to 20 human cases are notified annually, but no community-acquired outbreak had been reported before 2007 [Reference van Lier, Rahamat-Langendoen and van Vliet13].
On 29 May 2007, a general practitioner (GP) from a rural village in the south of The Netherlands alerted the municipal health service about an unusual increase in pneumonia cases in adults [Reference Karagiannis14]. By the end of 2007, 178 Q fever cases with symptom onset in 2007 had been notified and appeared in the national surveillance database (Fig. 1). The peak of the outbreak was in week 21 with most cases occurring between 30 April and 30 June 2007 [Reference van Steenbergen15]. A substantial number of the cases (n=55) were notified in a well-defined cluster area in the east of the municipality of Oss (Fig. 2).
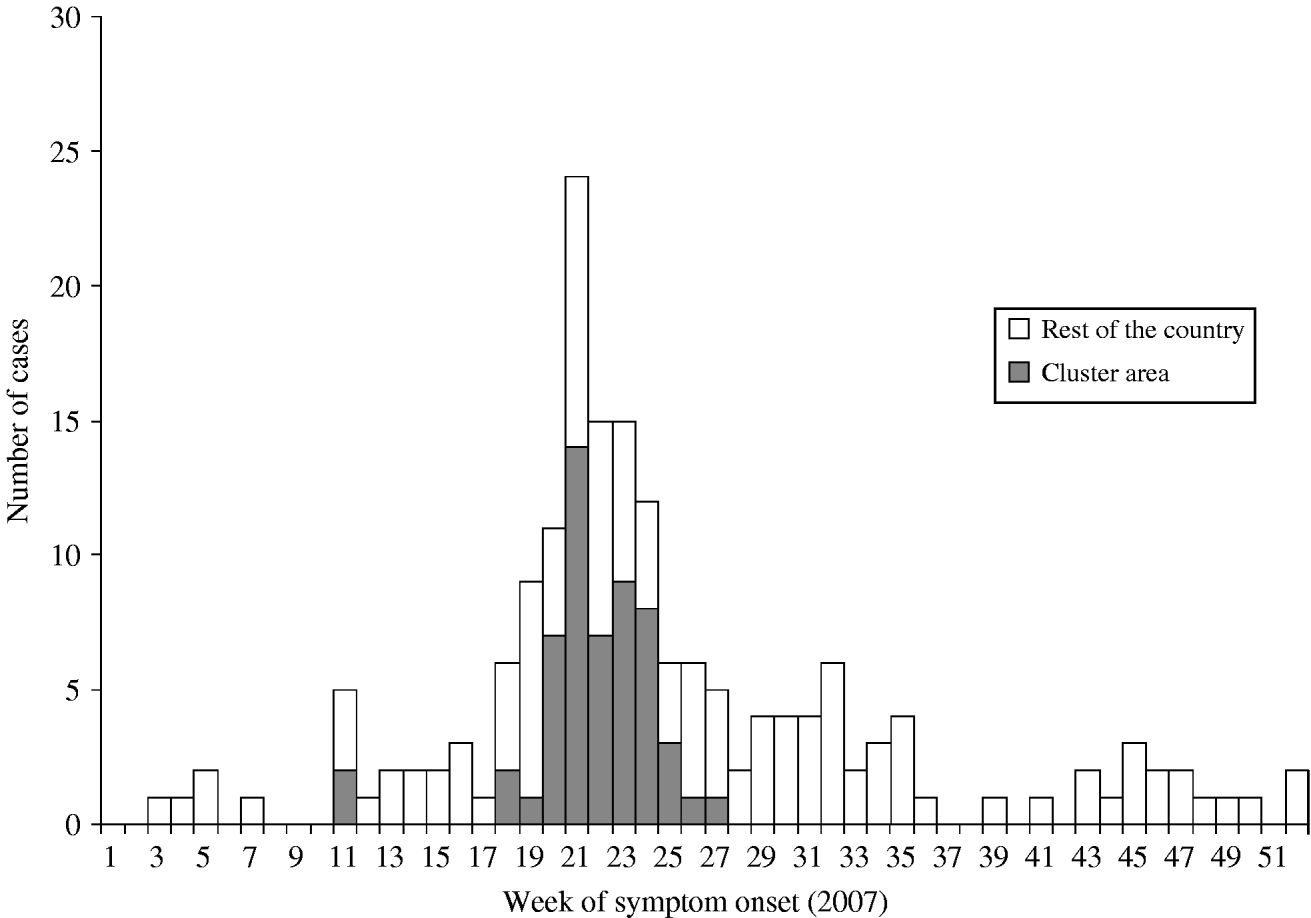
Fig. 1. Distribution of week of symptom onset for notified cases of Q fever in The Netherlands in 2007 (n=178). Cluster area (n=55).

Fig. 2. Participants with laboratory findings compatible with a recent or past C. burnetii infection, negative findings and cases previously identified in the centre of the cluster area.
As notification of further cases was received, an outbreak investigation was launched in this cluster area to describe the outbreak, find the source and route of transmission and investigate possible links to animal reservoirs in the region in order to decide on appropriate control measures. The present study describes this outbreak investigation.
MATERIALS AND METHODS
A frequency-matched case-control study with seronegative controls was used. Both cases and controls were restricted to the adult population of the cluster area.
Thirty-five confirmed cases had been identified in the cluster area by September 2007, when the case-control study was initiated. Our statistical assumptions and criteria were: (a) an achievement of a statistical power of 80%, (b) assumed overall attack rate of 15%, (c) participation rate of 33% in males and 40% in females (d) α error equal to 5%, (e) 1:2 case:control (seronegative for C. burnetii) ratio. Hence, we calculated that 696 inhabitants aged 18–84 years should be asked to complete a questionnaire and provide a blood sample. Previously identified cases were not excluded from the sampling procedure so as to achieve a truly random sample of the population. The overall estimated attack rate of 15% was based on a small survey in pregnant women in this area. The invited participants were frequency-matched with the known cases by village of residence, sex and age category (18–19, 20–29, 30–39, …, 70–79, 80–84 years). All eligible participants were invited by mail. Moreover, all of the 35 cases diagnosed before the study began were invited to complete the same questionnaire. The questionnaire included age, sex, postcode, working situation (including working in open air), residence information, distance of house to farms and meadows with livestock, animal possession, contact with animals, contact with unhandled animal products, consumption of raw milk products, visits to specific places or events, outdoor activities, house ventilation and health conditions – predispositions. Besides self-reported distances, distances from participants’ residence to farms with goats, sheep and cows in the cluster area and meadows where dung was spread were calculated using GIS software. We also calculated distance from participants' residence to all postcodes where ruminant farms were situated in the area, in order to identify neighbourhoods associated with recent infections. Participants were also asked about specific symptoms between 7 May and 8 July 2007. The recall period for the exposure variables was the end of April and the whole month of May 2007, i.e. up to 4 weeks before the peak of onset of cases (week 21 of 2007, i.e. 21–27 May 2007).
Cases included all laboratory-confirmed cases previously identified in the area at the time of the start of the study and all seropositive participants indicating a recent infection from the serological survey. The term ‘cases’ in the univariate and multivariable analyses of the case-control study refers to this whole group of seropositive participants. ‘Controls’ included participants from the serological survey with negative results for a recent C. burnetii infection. All participants with laboratory evidence for a past infection were excluded from the analysis.
The case-control study was approved by the Medical Ethical Committee of the University Medical Centre Utrecht (reference number: 07-241).
Statistical analysis
Odds ratios (OR) and corresponding 95% confidence intervals (CI) were calculated through logistic regression analysis to identify potential risk factors for the acquisition of a recent C. burnetii infection. Variables that were statistically significant at the 20% level and could explain at least 20% of the cases in the univariate analysis were included in the multivariable analyses. In the latter analysis, sex and age were always adjusted for. All other variables were tested with the use of manual backwards-elimination techniques. We also tested for interaction terms between variables in the final multivariable analysis model. All analyses were run in Stata version 10.0 (Stata Corp, College Station, TX, USA).
Laboratory screening
Study participants were screened for Q fever infection with an immunofluorescence assay (IFA) (Focus Diagnostics, Cypress, CA, USA) for IgG and IgM antibodies with a single 1:64 serum dilution. In order to harmonize the screening method, all 35 previously confirmed cases were reconfirmed by IFA and all met the case definition of a recent infection. Screening was performed to distinguish between uninfected, recently infected and individuals who had been infected in the past. Samples with unequivocal results were further analysed using twofold dilutions. A recent infection was defined as IgM phases 1 and 2⩾1:64 or an individual with an isolated titre of IgM phases 1 or 2⩾1:512. Individuals with a past infection did not match the IgM criteria in the definition of a recent infection, but had IgG phases 1 and 2⩾1:64 or an isolated titre of IgG phases 1 or 2⩾512. The remaining samples were scored negative and were used as seronegative controls in the case-control study.
Environmental investigation
Within the cluster area, environmental and animal samples were taken from a commercial (goat population: 3794) and a hobby goat farm, both probably related to incident cases, to track possible sources of C. burnetii. DNA was extracted using several modified NucliSens DNA extraction protocols (bioMérieux, France), depending on the environmental matrix examined. Detection of C. burnetii was performed by using a newly developed multiplex Q-PCR assay. Genomic targets that are most frequently used for detection of C. burnetii (icd, com1 [Reference Klee16] and IS1111 [Reference Zhang17]) were incorporated into one multiplex Q-PCR assay. For these three targets, primers and Taqman probes (Biolegio, The Netherlands) were designed using Visual OMP 6 for simultaneous detection. The specificity of the multiplex quantitative real-time PCR (Q-PCR) was tested on a large panel of non-target organisms to verify any cross-reaction with other closely related species. These non-target organisms included Bacillus cereus, B. mycoides, B. thuringiensis, Yersinia pseudotuberculosis, Y. agglomerans, Y. enterocolitica, Y. frederiksenii, Klebsiella pneumoniae, Pseudomonas aeruginosa, Legionella pneumophila, L. bozemonii, L. longbeachae, L. micdadei, L. dumoggii and L. anisa. No cross-reactions were observed for these organisms. The sensitivity of the assay was tested in a probit analysis (De Bruin et al., personal communication) and differed between the three targets. The minimal number of genome equivalents per reaction that could be detected with a 95% probability was found to be below five copies for the single copy targets icd and com1, and below three copies for the multicopy target IS1111.
Weather data for April 2007 and the same month over the last 30 years were acquired from the nearby weather station of the National Meteorological Institute (KNMI) at Eindhoven and weather conditions (temperature and wind direction) for this month were compared to mean long-term climatic values for April in the region.
RESULTS
Descriptive results
Of all 696 invited participants, 515 (74·0%) completed a questionnaire and 443 (63·6%) provided a blood sample. All participants who provided a blood sample also completed a questionnaire. Of the 35 previously identified cases, 30 (85·7%) completed the questionnaire. All the cases were eventually confirmed by IFA. Full respondents, i.e. participants who provided both a questionnaire and a blood sample, did not differ in age or sex distribution from the rest of the invited population.
Of all 443 people who provided a blood sample, 332 (74·9%) were seronegative for C. burnetii, 38 (8·6%) had a past infection and the remaining 73 (16·5%) had a recent infection. Of these recently infected participants, 67 (91·8%) lived in Herpen, the most affected village. The highest percentage of recent infections was observed in participants aged <30 years (median age of cases 49 years, for seronegative controls 54 years), while these percentages did not differ between the two sexes (Table 1). The median age in participants with a past infection was 54·2 years (36–80 years) and the male:female ratio was 2·2.
Table 1. Laboratory results for recent C. burnetii infection in participants in the most affected village of the cluster area (Herpen)

* Numbers include cases identified at the time of the study set-up.
† P(T+) refers to the percentage of participants with a laboratory-confirmed recent infection, including cases identified at the time of the study set-up. The crude P(T+) in Herpen, not standardized for age and sex distribution of the population, was 19·2% (18·6% for males and 20·3% for females). Excluding past infections from the denominator, the overall attack rate in Herpen was estimated to be 23·9% (24·8% for males and 23·0% for females).
‡ P(T+) adjusted percentages for the overall age and sex distribution of the village in inhabitants aged 18–84 years.
The most frequent symptoms in the 30 previously confirmed cases that provided a questionnaire were fever (100·0%), night sweating (92·6%) and general malaise (92·3%). Of these cases, 11 (36·7%) were hospitalized (Table 2). In the 73 seropositive participants in the serological study, severe fatigue (42·7%), headache (40·6%) and general malaise (38·8%) were the most common symptoms. Fever was only reported by a minority (25·4%). In this group, 25 (34·3%) individuals reported none of the symptoms they were asked about. Consultation with a physician and hospitalization were reported by 17 (23·3%) and one (1·4%) of these seropositive individuals, respectively (Table 2).
Table 2. Self-reported symptoms in patients with a serologically confirmed C. burnetii infection in the cluster area in spring/summer 2007
* Only 30 of the 35 cases identified at the time of the study set-up were supplied with a questionnaire.
Case-control study
The final group of cases for the analyses consisted of the 35 previously diagnosed cases at the time of the study's initiation and the 73 randomly selected participants with a recent infection, the latter including five of the confirmed cases. Therefore, the total number of cases in the case-control study was 103 (98 for risk-factor analyses; no questionnaire was available for five of the confirmed cases) and the number of seronegative controls was 332. The 38 participants with a past C. burnetii infection were excluded from the analysis (Fig. 3). The latter participants did not differ from the group of 103 cases in sex distribution, but were on average 5·2 years older.
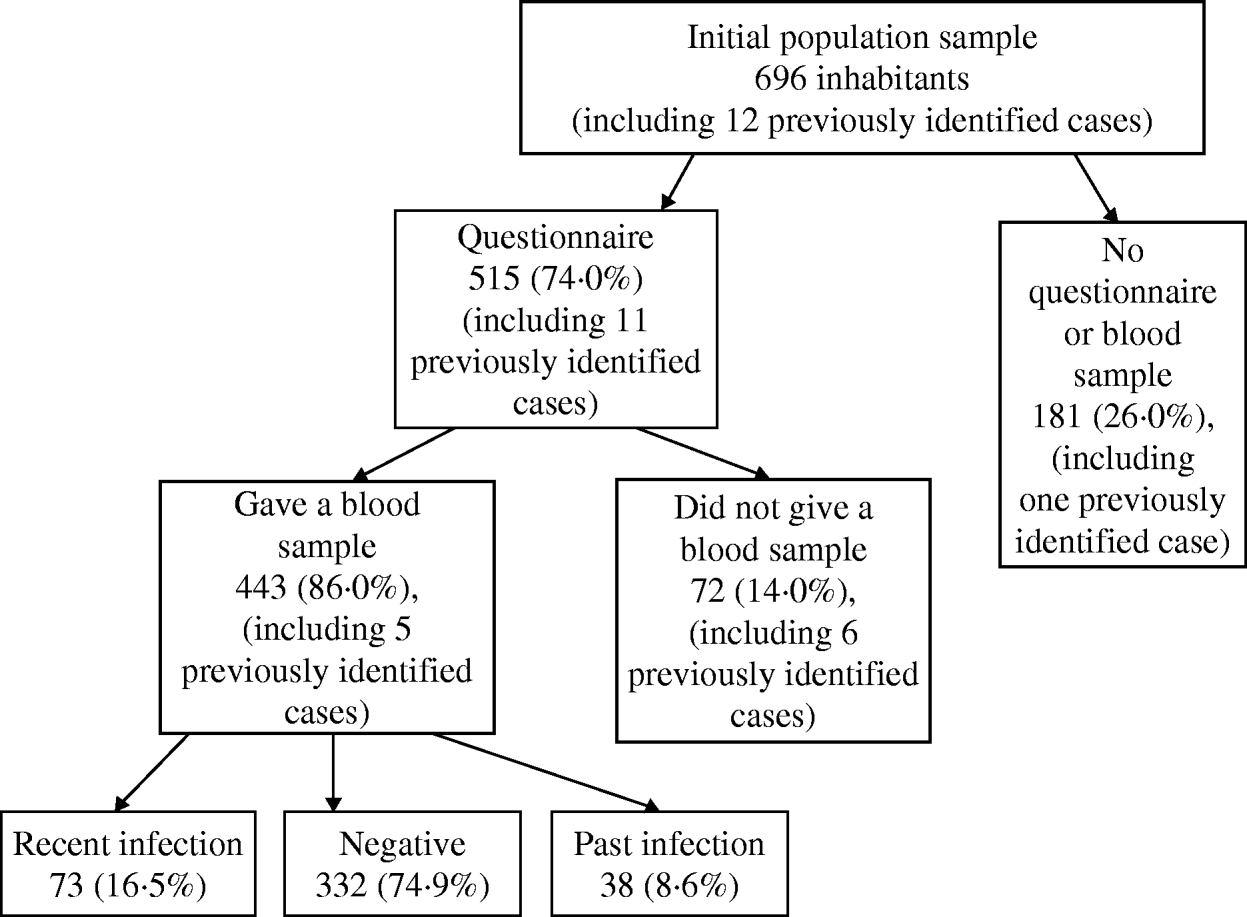
Fig. 3. Participation of the invited inhabitants and results of the serological study for C. burnetti, 2007.
Several variables were shown to be potential risk factors for the acquisition of a recent C. burnetii infection in the univariate analysis. An increase in age by 1 year resulted in a decrease in risk for infection by 3·1% (OR 0·97, 95% CI 0·95–0·99). People aged ⩽44 years had the highest risk of infection. Several risk factors that were associated with exposure to the open air and animals or animal/agricultural products, as well as distance to farms and meadows with goats, sheep and cows or where dung was spread were also significant (Table 3). Household size, type of residence and work in meat treatment, the agricultural sector and wool or leather treatment, as well as having spent nights in a different area during the incubation period, were not found to be related to the acquisition of a recent C. burnetii infection. The same applied to doing household-related activities outside the house as proxies for exposure to the open air.
Table 3. Results of the univariate and multivariable analyses of the case-control study for acquisition of C. burnetii infection in the cluster area, 2007
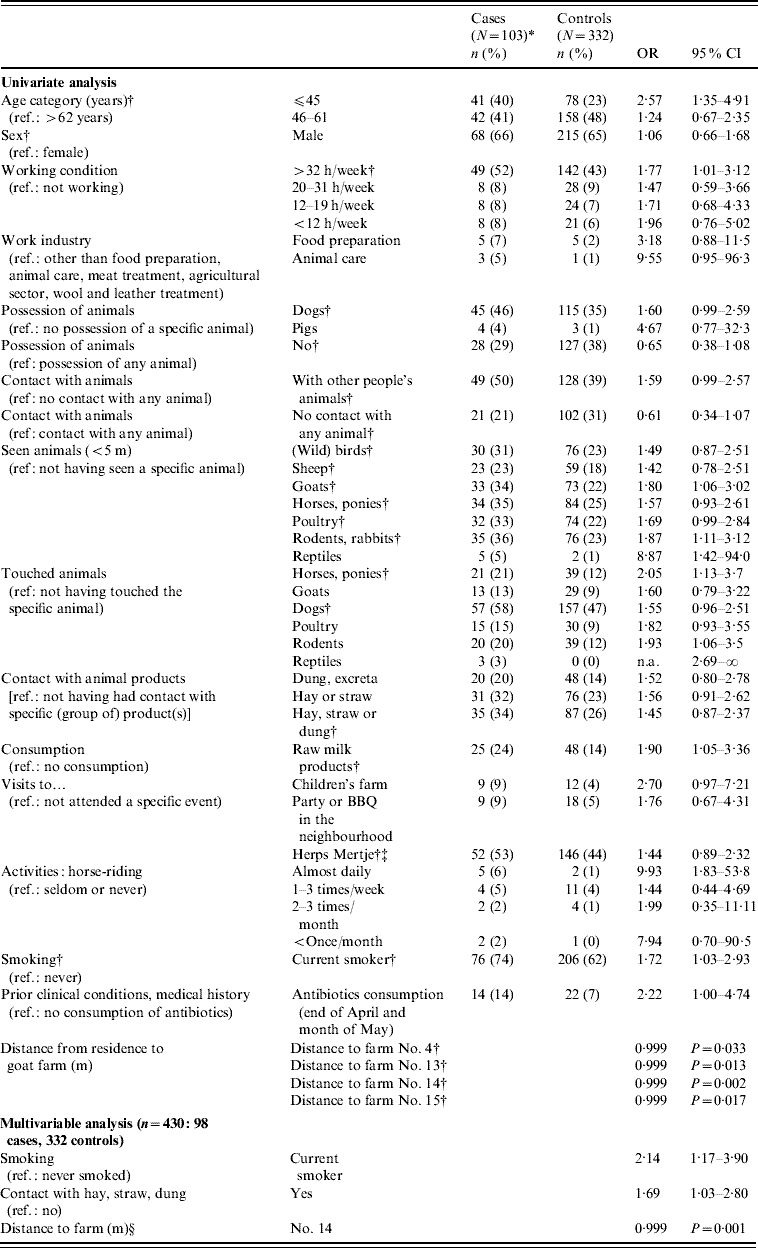
OR, Odds ratio; CI, confidence interval; n.a., not available.
* Ninety-eight for analysis of risk factors obtained from the questionnaire. Because distance to farm No. 14 was calculated with GIS software, this information was available for all 103 cases as residence address was also known for the five non-respondents.
† Variables included in the multivariable model before backward elimination.
‡ Large annual open-air market with among others small ruminants as part of a mobile pet farm.
§ Distance to farm No. 14 could be replaced by either one of four adjacent postal codes, with almost equal results for the model. Therefore, it was assumed that farm 14 was not uniquely associated with recent infections. It should be noted that odds ratios should be interpreted as living further away from the farm is protective.
In the multivariable analysis, smoking, contact with agricultural products such as manure, hay and straw and distance to a farm or one of four neighbourhood postal codes to the east of Herpen (‘area A’) remained statistically significant, also adjusted for sex and age (Table 3). Adding interaction terms between distance to area A and contact with agricultural products was not statistically significant. Smoking was not clearly associated with symptom acquisition, given an infection; in 60 seropositive participants for whom smoking habits were known, 15/24 (62·5%) non-current smokers developed symptoms compared to 27/36 current smokers (75%).
Environmental investigation
Seventy-nine environmental and animal samples were collected from two farms (10 and 69 samples from the hobby and commercial farm respectively), of which 75 were screened for C. burnetii. Twenty-five (33%) were positive for all three targets and six (8%) were positive for the IS1111 target only. Animal samples screened included: urine, milk and vaginal swabs from individual animals, a swab of a dead animal in a cadaver bin and manure from stable floor. Only urine and milk samples showed no positive results for the presence of C. burnetii. Environmental samples screened included straw (from stable floor), surface swabs (floors and walls), insects (collected from a UV lamp), and water (drinking buckets). C. burnetii was found to be present in all types of samples, apart from water. All positive samples originated from the same large commercial goat farm, where an abortion wave had occurred in April 2007.
April 2007 was a record warm and dry month in The Netherlands, with an average temperature of 13·4°C (4·4°C higher than the 1977–2006 average). Easterlies (wind direction from 45° to 135°) were the most prominent wind direction (11 out of the 30 days in April) and had never been so prominent during April for at least 30 years. This may have contributed to the spread of C. burnetii from area A to Herpen.
Control measures and other actions taken
In early 2008, while the lambing season was ongoing, an information leaflet was distributed by regular mail to all goat farms in Noord-Brabant, the province with the highest goat density in the country. This included background information about the disease in both animals and humans and recommended preventive and control measures to reduce spread of Coxiella, especially during and after the lambing season. This information was also put on several websites to reach ruminant farmers, including sheep farmers nationwide. Furthermore, it was agreed between the National Public Health Institute and the Animal Health Service (GD), which diagnose the majority of Coxiella problems in ruminants, to communicate all postal codes in a radius of 5 km from a newly diagnosed farm. This was done to help to alert physicians in high-risk areas to consider C. burnetii for patients with compatible symptoms. On 12 June, reporting of Q fever symptoms in small ruminants held in deep litter houses became notifiable for farmers and veterinarians, and a ban was introduced on the spreading of manure in the 90 days following Q fever-positive status at the farm. Finally, the outbreak triggered several studies to assess the prevalence of C. burnetii infections in small ruminants, their milk, the farmers, their families and the general human population, as well as studies on risk factors for infections in ruminants and (sporadic) human cases to generate more evidence-based control measures.
DISCUSSION
In 2007, the first community-acquired Q fever outbreak was identified in The Netherlands. The results of the outbreak investigation suggested that the source of the infections in the cluster area was situated in a rural zone with eight hobby and commercial ruminant farms. These include three dairy cattle farms (about 60–100 cows), one large dairy goat farm (at least 3700 goats), one small sheep breeding farm and three hobby farms with small numbers (<10) of goats or sheep. Although a specific farm or meadow could not be pinpointed, at least one large commercial goat farm in this area was known to suffer from abortion waves due to C. burnetii in the spring of 2007. Ideally, more environmental samples are needed to fine-tune the highest-risk area, which was not feasible during the present investigation.
While all previously identified cases at the inception of the study reported having had fever, this symptom was reported by only a quarter of the recently infected individuals obtained from the random population sample. Similarly, all symptoms shown in Table 2 were systematically more frequent in the initial cases that had been reported through the mandatory notification system. The name ‘Q fever’ may better correspond to clinical cases and should not be considered a prerequisite when considering a C. burnetii infection.
Compared to other outbreak reports, full screening of IgG and IgM phases 1 and 2 was performed in order to improve diagnostic accuracy and detect past infections. As the study was a population survey in an outbreak setting, we used a single 1:64 dilution for screening of the population sample in contrast to diagnostic testing of individual clinical cases. The cut-off is a balance between increasing/decreasing sensitivity vs. decreasing/increasing specificity, which will thereby influence the positive and negative predictive value, which is further dependent on the prevalence in the study population; the latter is expected to be relatively high in an outbreak setting. There is a lack of standardization in interpretation of serology results [Reference Schimmer18]. The choice of 1:64 dilution is further justified by our current experience with the 2008 outbreak, which is still under investigation. Finally, IFA results of, among others, outbreak sera were compared with results of two commercially available ELISAs, and showed that the ELISAs had a lower sensitivity compared to IFA and that the IFA showed no substantial cross-reactivity (P. Schneeberger, personal communication).
Rodolakis et al. [Reference Rodolakis19] showed that goats excreted C. burnetii primarily in milk, and that sheep shed the bacteria primarily in faeces and vaginal mucus. Our results showed that goats also shed C. burnetii in faeces and vaginal mucus, which is in agreement with their observations that human Q fever cases are more often related to ovine (in our case caprine) than bovine flocks affected by Q fever. We found no C. burnetii presence in the milk samples, but the number of milk samples screened (four) was too small for a valid comparison with other studies.
Weather conditions in April 2007 were favourable for the spread of C. burnetii. The unseasonably warm and dry weather conditions, in addition to an unusually easterly component in wind direction, probably contributed to a wide spread of aerosols from contaminated farms to nearby residential areas. Daily maximum temperatures were up to 15°C higher than the average for April and hardly any precipitation had fallen during that month. The predominant wind direction was also in agreement with the location of area A.
The role of windborne spread is also indirectly supported by the lack of a gender difference in cases in the case-control study. Usually, more males are infected, mainly through occupational exposure. A windborne spread would not give a preference for either females or males; an even sex distribution was indeed observed in the outbreak discussed here.
Only 39/98 cases (39·8%) reported direct contact with, possibly contaminated, agricultural products such as manure, hay and straw. However, contact with hay or straw can cause aerosols and by that contaminate a wide environment. This may explain why no particular common exposures were found by the previously performed hypothesis-generating interviews targeted at the cases diagnosed in the regular medical circuit, which preceded this epidemiological outbreak investigation.
As stated in the first brief outbreak report [Reference Karagiannis14], the initial hypothesis was that the increase in pneumonia cases was caused by Mycoplasma pneumoniae. As Q fever is a relatively rarely notified disease in The Netherlands, clinicians usually do not test for C. burnetii infection in patients with an atypical pneumonia. Consequently, small or diffuse clusters of cases might easily be missed. This outbreak clearly stresses the added value of early warning by physicians based on the clinical picture only. Therefore, reporting of unusual numbers of cases with common symptoms to the local health authorities is strongly encouraged. The higher numbers of Q fever cases notified in the second half of 2007 (Fig. 1) and first quarter of 2008 may be partly attributable to the raised awareness among clinicians and increased diagnostic testing at laboratories following the outbreak.
Contact with animals and consumption of raw milk products were not significant risk factors in the multivariable analysis of our study. In theory, this could have been because inhabitants residing closer to area A tended to have more contact with animals. However, a closer inspection of our data showed no interaction between distance of residence to area A and contact with animals. Thomas and colleagues showed that exposure to cattle (but not sheep), cats, raw milk and hay, all reported sources of Q fever, are associated with C. burnetii IgG by univariate analysis, but this association was not independent from animal contact [Reference Thomas20].
No information on possible general exposures in the past was available from the present study. Individuals with a past infection were older than recent cases, which could represent the cumulative risk for acquiring a C. burnetii infection. This group worked more frequently in the agricultural (11·1% vs. 2·8%) and meat-handling (7·4% vs. 1·4%) sector than recent cases, but these differences were not statistically significant.
Smoking was found to be an important risk factor for the acquisition of a C. burnetii infection. A possible explanation could be outdoor smoking habits, resulting in smokers being more exposed to outdoor contaminated aerosols than non-smokers. No information on smoking habits was collected to confirm this. Alternatively, smoking might represent an increased risk by more hand–mouth contact or an increased risk for respiratory infections in general because of alterations in structural and immune defences, as suggested by others [Reference Carolan21]. Smoking was not associated with development of symptoms in seropositive participants in the present study, although a slight tendency towards more symptoms for smokers was observed. In contrast, McCaughey and colleagues in a study in Northern Ireland concluded that smoking was not a significant risk factor for the acquisition of infection, but suggested that it was only associated with developing symptomatic disease [Reference McCaughey22]. No clear explanation can be given for this discrepant result, but different laboratory tests were used and sera in the study in Northern Ireland were 20 years old. Moreover, that study [Reference McCaughey22] refers to general population sera, where the infection risk is multifactoral and scattered in place and time, while the present study is targeted at an outbreak setting with an overall more homogeneous risk of exposure.
The present study shows that small ruminants were most likely responsible for the outbreak. This group of animals has been implicated in Q fever outbreaks previously [Reference Lyttikäinen5, Reference Sawyer, Fishbein and McDade7, 23–Reference Bamberg25]. It should also be noted that contact with one's own animals was not found to be a significant risk factor in the present study, which might assume partial immunity. The percentage of a past Q fever infection was 10·6% in those who had animals and 4·5% in those who did not. The goat density in the south of the country is the highest (about 38·1 goats/km2 while the national average is 9·2 goats/km2) and the goat population is still increasing. Public health education and control measures are, hence, of great importance to avoid future similar outbreaks.
Although not included in the final model, some of the significant univariate associations deserve further attention, as they might have played an intermediate or minor role in the outbreak. Having touched horses and ponies and frequent horse-riding are among these possible risk factors, associated only univariately. This may either indicate Q fever being transmitted from an infected horse, as Coxiella infection in horses has been documented previously [Reference George and Marrie26], or the activities related to horse-riding such as cleaning stables, handling straw or hay and brushing the crest might have lead to exposure to contaminated dust or air from the environment. Second, having seen rodents, rabbits and reptiles was univariately found to be associated with Q fever, although the latter was reported by a very small group of cases only. This is of particular interest as Q fever has been shown to be rodent-associated in some cases [Reference Gardon27]. In further analyses, having seen rodents, including rabbits, was strongly associated with having had contact with unhandled animal products, such as hay, straw or dung; of those that had contact with these animal products, 84·6% had also seen rodents in the recall period. Adjusted for age and sex, having seen rodents had a positive interaction with distance from ‘area A’ (OR 1·001, 95% CI 1·000–1·002). Interestingly, this seems to suggest that windborne transmission was most likely for those living in proximity to ‘area A’, while rodents, possibly infected through the environment near area A, might have facilitated the transmission beyond the reach of the wind.
Our study had several limitations which need attention. Participants were asked about possible exposures that had taken place 5 months before. Consequently, some participants may have answered based on their usual habits rather than their behaviour in the time period they were asked about. However, we believe that recall bias did not occur, as our analysis was restricted to a population sample which included mainly cases with milder symptoms. Moreover, self-reported symptoms might have been underreported, especially the ones that are mild and non-specific, although the proportion of asymptomatic infected individuals was quite similar to others [Reference Raoult, Marrie and Mege1].
Just before and during our study, media reports were released promoting the idea of goats being a plausible cause of the outbreak (Q fever was called ‘goat flu’). We were expecting a priori that this would cause reporting bias for questions about goats. However, univariately, not only contact with goats but also with other ruminants were associated with infection and both subjectively calculated distances of their residence to goat farms and GIS-based distances were associated with infection. In the present study, only the latter objective distances were used in the multivariable analyses.
This first documented outbreak of Q fever in The Netherlands received plenty of attention from both the public health and the veterinary authorities. It was an excellent chance for these parties to cooperate with each other and facilitate long-term communication channels. Further, it was a unique opportunity to test and improve diagnostic assays for C. burnetii in humans, animals and environmental samples. Unfortunately, immediate implementation of control measures was hampered because of the failure to identify the exact source of the outbreak. In future similar outbreaks, an earlier start of the epidemiological investigation combined with more intensive environmental sampling should improve the quality of data, provide more detailed exposure and contamination data and, by that, enhance adequate control.
ACKNOWLEDGEMENTS
Financial support for this study was provided by ZonMW, The Netherlands Organization for Health Research and Development, which is predominantly funded by the Ministry of Health, Welfare and Sport (VWS) and The Netherlands Organization for Scientific Research (NWO). We thank Frederika Dijkstra for providing surveillance data and assistance during the preparation of the study; all staff from the Municipal Health Authority (GGD) ‘Hart voor Brabant’ for their help in collecting sera and questionnaires of participants and responding to questions following study results; Maurice Hamans, Peter Wielinga and Jasper Bok for sampling at the two goat farms; the municipality of Oss for their help in the mailing of the eligible study population; Ineke Weers-Pothoff for testing the sera of all participants; Daan Notermans for his helpful comments on the manuscript; Laurens Zwakhals for providing the maps; Barbora Kostalova for providing constantly updated maps in the course of the outbreak; Olaf Stenvers, for his help in communication between public health and veterinary parties and for providing data on ruminant farms in the cluster area.
DECLARATION OF INTEREST
None.








