INTRODUCTION
Toxoplasmosis is a zoonotic parasitic disease caused by an obligate intracellular protozoan, Toxoplasma gondii, which is distributed worldwide. The definitive host of the parasite belongs to members of the family Felidae, but several warm-blooded animals including humans serve as intermediate hosts for the parasite. The disease is important for its serious implications in immunosuppressed individuals such as HIV patients as well as its severe consequences for fetuses in congenital transmission [Reference Jones1]. Serological surveys demonstrate a wide variation in the prevalence of the infection in various geographical locations. Prevalence is known to vary with age, climate and socioeconomic status (SES) [2]. Higher prevalence has been observed in tropical countries with humid and warm climates, while lower prevalence is found for arid and colder countries [Reference Robert-Gangneux and Darde3]. Seroprevalence is higher in Central Europe, South America and Africa [Reference Berger4–Reference Zemene6]. In the USA and the UK, it is estimated that 16–40% of the population are infected with T. gondii. Lower prevalence rates of 4–39% have been reported in South East Asia, China and Korea and 11–28% in Scandinavia [Reference Robert-Gangneux and Darde3, Reference Dubey and Beathie7]. Seroprevalence in India has been reported to be 24·3% [Reference Dhumme8]. In Brazil, about 50–80% of the population is infected with T. gondii [Reference Khan9]. Testing of all pregnant women for T. gondii infection is routine in some European countries, including France and Austria [Reference Roberts, Murrell and Marks10]. In France, for instance, the existence of a national programme for the prevention of congenital toxoplasmosis since 1978 has seen seroprevalence in pregnant women fall from 84% in the 1960s to 54% in 1995 and to 44% in 2003 [Reference Berger4]. In Ghana, only two hospital-based data are available on T. gondii infection in humans [Reference Ayi11, Reference Ayeh-Kumi12]. Here we conducted the first ever population-based study on T. gondii seropositivity and its related infection risk factors in Ghana.
METHODS
The study areas
The Central Region, situated at the southern part of the country along the Atlantic Ocean, is one of the ten administrative regions of Ghana. The region can be broadly divided into two ecological zones: the coastal savannah with grassland along the seashore and a semi-deciduous forest, predominating the inland areas. The study was conducted in three communities, Moree, representing the coastal communities, Nkanfoa, located midway between the coastal and the forest zones, and Jukwa, the forest zone within the region. Moree is predominantly a fishing community located between latitude 5° 25′ N and longitude 1° 20′ W. A huge expanse of the community is along the coastal stretch of the area. Jukwa is mostly a farming community lying within a district with large areas of forest reserves located between latitude 5° 16′ 0″ N and longitude 1° 19′ 60″ W with an altitude of 75 m above sea level. Nkanfoa is located between latitude 5° 06′ 19″ N and longitude 1° 14′ 47″ W. The vegetation consists of shrubs, grass and a few scattered trees. The occupation of the people ranges from petty trading to farming/gardening and artisanship (masonry, carpentry, fitting).
Ethical approval
The study was conducted according to the Helsinki Declaration on Research regarding human subjects. The protocol for this study was reviewed and approved by the Ghana Health Service's Ethical Review Committee (ID: GHS-ERC: 21/11/12). Approval was also given by the respective District Health Directorates and the Chiefs and Elders of the selected communities. Volunteer participants signed consent forms attached to the questionnaires after the procedures had been explained to them in their own language. For participants aged <18 years whose assent was sought, their parents/guardians signed the consent forms on their behalf. Confidentiality and safety were assured at all times.
Selection of study participants
A community-based cross-sectional design was employed. A minimum sample size of 384 was calculated based on the expression
where N = minimum sample size, p = anticipated prevalence of Toxoplasma infection of 50%, b = desired error bound taken as 5% and Z = the standard score at 95% confidence interval (1·96). An inclusion criterion was for a participant to have lived continuously in the particular community for at least 2 years [Reference Portela13]. A pilot study had indicated that parents were reluctant in allowing their young children to donate blood samples; hence children aged <10 years were not included. The research team visited various households (on days that residents did not go to farms or go fishing) to explain the purpose of the study and to request their consent to participate. The purpose of the study and the examination procedures were explained to residents in their local language after which those who met the inclusion criteria and agreed to participate by signing the consent forms were interviewed by questionnaires and asked to donate a blood sample at the community health centres.
Questionnaire
The questionnaire sought information on (a) demographic characteristics (gender, age, educational level, occupation); (b) possible exposure to and consumption of T. gondii oocysts: (i) contact with soil, working on a garden, (ii) contact with cats (owning cats, presence of cats around the house, disposing of cat litter); (iii) source of drinking water; (c) possible consumption of T. gondii cysts, type of meat consumed, state of meat consumed (undercooked meat or well cooked); (d) hygiene habits (hand washing, washing of vegetables/fruits or not before consumption).
Blood sample collection and serum preparation
Venous blood samples were collected by trained laboratory technologists. About 3 ml of venous blood was obtained from each participant using a sterile disposable hypodermal syringe fitted with a 23-gauge needle and dispensed from the syringe barrel into a sterile tube and allowed to clot. Sample tubes were centrifuged at 500 g to precipitate red blood cells. Clear sera were collected into Eppendorf tubes and stored at −20 °C until tested. All sample tubes were appropriately labelled with code numbers.
Detection of anti-T. gondii antibodies by commercial ELISA kit
Each serum sample was tested for the presence of anti-Toxoplasma IgG and IgM antibodies using a commercial ELISA kit (VEDALAB, France) according to the manufacturer's instructions. For IgG assay procedure, 100 μl of diluted (1:41) test sample, calibrator, positive control, and negative control were transferred into the precoated wells, covered and incubated at room temperature for 30 min. Wells were then washed five times with diluted wash solution after which 100 μl of horseradish peroxidase (HRP) conjugate was added to each well, covered and incubated for 30 min. The wells were again thoroughly washed five times with the diluted wash solution after which 100 μl tetramethylbenzidine (TMB) substrate was added to each well. The plate was covered, incubated at room temperature for 30 min and 100 μl of stop solution added to each well. Finally, a 450 nm set wavelength micro plate reader was used to measure the optical density (OD) of each well. IgG cut-off values were determined in enzyme units (EU)/ml and calculated using the formula:
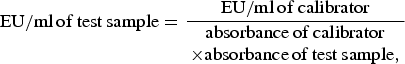 $${\rm EU/ml\,of\,test\,sample} = \displaystyle{{{\rm EU/ml\,of\,calibrator}} \over {\matrix{{\rm absorbance\,of\,calibrator} \cr \times {\rm absorbance\,of\,test\,sample},} }}$$
$${\rm EU/ml\,of\,test\,sample} = \displaystyle{{{\rm EU/ml\,of\,calibrator}} \over {\matrix{{\rm absorbance\,of\,calibrator} \cr \times {\rm absorbance\,of\,test\,sample},} }}$$
and interpreted as follows: a value of <40 EU/ml was considered negative for IgG antibody to T. gondii. A value between 40 and 50 EU/ml indicated the test result was equivocal and had to be retested. An EU/ml value >50 was considered positive for IgG antibody to T. gondii.
A similar assay procedure was carried out for IgM antibody detection except that the plate was incubated at 37°C and index calculations were determined by dividing the OD value of test sample by the OD value of the calibrator, and interpreted as: a calculated index value <0·90 was negative for IgM antibody to T. gondii. An index value between 0·90 and 0·99 was equivocal, and an index value ⩾1·00 was positive for IgM antibody to T. gondii. A serological criterion for systemic infection was a positive test result for any of the two anti-Toxoplasma IgG or IgM antibodies or a combination of both.
Data analysis
Data were analysed using SPSS version 16 (SPSS Inc., USA). χ 2 test was used to determine associations between categorical variables. P ⩽ 0·05 was considered statistically significant. Logistic regression analysis was used to predict the association between T. gondii seropositivity and risk factors.
RESULTS
A total of 390 participants of mean age 47·0 years (s.d. ±20·35, range 10–100 years) were studied. There were 118 (30·3%) males and 272 (69·7%) females, while 332 (85%), 52 (13%) and six (2%) of the participants were of lower, medium and upper SES, respectively. Table 1 shows T. gondii seropositivity rates in the study communities. The overall seroprevalence of T. gondii to IgG and/or IgM antibodies in the study population was 85% (333/390). Of this, IgG antibodies were detected in 84% (329/390) while IgM antibodies were present in 6% (25/390) of the study population. Twenty-one (5%) participants tested positive for both IgG and IgM antibodies. Four (1%) participants tested positive for only IgM antibodies while 79% (308/390) participants tested positive for only IgG antibodies. The remaining 15% (57/390) of the participants tested negative for all antibodies.
Table 1. Toxoplasma gondii seropositivity rates in the study communities

Test of association between T. gondii seropositivity and variables assessing risk factors is presented in Table 2. Although there were more female participants than males there was no significant association between seropositivity and gender (P = 0·7). Again, no item relating to meat consumption was significantly associated with T. gondii seropositivity (Table 2). There was also no significant association between Toxoplasma seropositivity and washing of hands or washing of vegetables/fruits before consumption (P = 0·48, P = 0·07, respectively).
Table 2. Test of association between Toxoplasma gondii seropositivity and variables assessing the risk of infection
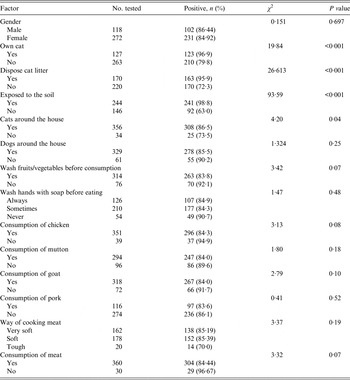
From Table 2, variables with a significant difference between groups by the χ 2 test were included in a multivariate logistic regression analysis to identify independent risk factors for the disease. Table 3 shows a multivariate logistic regression analysis between T. gondii seropositivity and demographic characteristics of the participants. T. gondii infection was found to increase with increasing age: 10–19 years (55·8%), 20–29 years (66·7%, OR 1·59, P = 0·3), 30–39 years (86·2%, OR 4·95, P = 0·008), 40–49 years (88·7%, OR 6·25, P < 0·001), 50–59 years (93·5%, OR 11·42, P < 0·001), 60–69 years (100%, OR 9·9 × 107, P < 0·001), >70 years (97·0%, OR 25·38, P < 0·001). With respect to level of formal education, participants who had no formal education, basic school education and second cycle education were respectively 19, three and two times more likely to be seropositive compared to those who had tertiary education. SES was significantly associated with seropositivity, with participants in the low SES group being eight times more likely to be seropositive than those in the high SES group. In terms of occupation, the least seroprevalence value of 59% was observed for students/pupils while the higher seroprevalence values were observed for jobs that exposed the individuals to the soil, e.g. farming, fishing and fishmongering.
Table 3. Multivariate logistic regression analysis between Toxoplasma gondii seropositivity and demographic characteristics of participants

aOR, Adjusted odds ratio; CI, confidence interval.
Table 4 presents a multivariate logistic regression analysis between T. gondii seropositivity and risk factors. There was a statistically significant relationship between Toxoplasma seropositivity and responses relating to the presence of cats. The highest risk factor was to have contact with the soil (OR 38·4, P < 0·001), followed by owning a cat (OR 7·76, P = <0·001), disposing of cat litter (OR 6·85, P < 0·001) and having cats around one's house (OR 2·31, P = 0·04). Source of drinking water was also found to be significantly associated with T. gondii infection.
Table 4. Multivariate logistic regression analysis between Toxoplasma gondii seropositivity and risk of infection

aOR, Adjusted odds ratio; CI, confidence interval.
Table 5 shows a multivariate logistic regression analysis between various occupations of participants and their exposure to the soil. Participants who were engaged in petty trading, fishmongering, farming and fishing were at higher risks of having contact with the soil.
Table 5. Multivariate logistic regression analysis between various occupations of participants and their exposure to the soil

aOR, Adjusted odds ratio; CI, confidence interval.
Analysis of soil samples from the study areas indicated that the soils were slightly alkaline for all the areas as follows: Moree (pH = 7·9, Na+ = 1·69); Nkanfoa (pH = 7·3, Na+ = 1·36); Jukwa (pH = 7·8, Na+ = 0·41).
DISCUSSION
This is a report of a population-based study on T. gondii from the West African state of Ghana. The overall seroprevalence of 85·4% found in the present study was quite high but lower than an earlier hospital-based study in pregnant women in Accra, Ghana. That study of pregnant women of mean age of 28·1 years had a seroprevalence of 92·5% [Reference Ayi11]. However, another hospital-based study in patients of mean age 30·2 years visiting the Korle Bu Teaching Hospital in Accra reported a lower prevalence rate of 49·7% [Reference Ayeh-Kumi12]. The differences of prevalence rates in these studies may be due to the different populations studied (pregnant women, sick people and the general population) and the age differences of the study populations. Studies on T. gondii infection in farm animals in Ghana have indicated seropositivity of 39% in pigs, 26·8% in goats, 33·2% in sheep, and 64% in chickens [Reference Arko-Mensah14–Reference Dubey16]. The infection rate in the present study was comparable to findings from southern Brazil and some European countries. A seroprevalence of 84% was found in a lower SES populations in Brazil [Reference Bahia-Oliveira5]. Similar findings have been reported in Holland and Germany [Reference Kortbeek17]. The current finding is also similar to reported seroprevalence rates of 83·7% and 60% in two different studies from the East African country of Ethiopia and 66–88% from Togo in West Africa [Reference Zemene6, Reference Negash, Tilahun and Medhin18, Reference Deniau19]. The finding is, however, higher compared to reported cases from other West African countries of 63·1% in São Tomé, 32·4% and 23·9% in Nigeria, 25·4% in Burkina Faso, and 37·2%, 55·6% and 70% in the Ivory Coast [Reference Fan20–Reference Dumas24].
Consistent with many reported cases of T. gondii infection, the current study found no significant association between gender and seropositivity. A study in Egypt in 250 human samples found no significant difference between males (34·7%) and females (35·8%) (P > 0·05) [Reference Aboelhadid25]. Similar results have been reported in Ethiopia, Nigeria, and Tanzania [Reference Negash, Tilahun and Medhin18, Reference Kamani22, Reference Swai and Schoonman26]. However, a higher risk of infection for men than women was observed for the hospital-based study in Accra, Ghana [Reference Ayeh-Kumi12]. The higher risk of Toxoplasma infection for men has been attributed either to contact with soil or to improper hygiene and thus a difference between men and women might appear in populations with high exposure to soil [Reference Fromont, Riche and Rabilloud27]. In the present study, however, both men and women were at risk of being exposed to the soil as indicated by their professions (Table 5). Thus, fishmongering (for women), farming and fishing (for men) were the highest risk factors for the infection (Table 3).
In the present study we found an increasing pattern of seropositivity with increasing age group which was also observed in Egypt where seropositivity increased from 11% in participants aged <20 years to 42% in those aged >50 years [Reference Aboelhadid25]. Similarly, a study from Ethiopia found a seropositivity of 64·0% in the 15–19 years age group and 94·1% in the 30–35 years age group [Reference Zemene6]. Another report from Tanzania found that T. gondii infection increased by 1·4% for a 1-year increase in age [Reference Mwambe28]. From the city of Maiduguri, Nigeria, there was a positive correlation between the mean antibody titre and the age of subjects, with seroprevalence being highest in subjects aged 51–60 years and lowest in subjects aged <21 years [Reference Kamani22]. The same trend was also observed in The Netherlands and Japan [2], Rio de Janeiro state, Brazil [Reference Bahia-Oliveira5], France [Reference Fromont, Riche and Rabilloud27], and the UK [Reference Allain, Palmer and Pearson29]. The increase in seropositivity with age may be attributed to the fact that since T. gondii infection is strongly associated with contact with soil, the longer an individual lives the more likely they are to be exposed to the soil. It might also be attributed to the reason of decreased cell-mediated immunity with advanced age. Surprisingly, a study in a rural area of the Minas Gerais state of Brazil found no significant difference between seropositivity and age groups. That finding suggested that a significant proportion of the population acquired toxoplasmosis at an early age [Reference Portela13]. We also observed an increasing trend of seropositivity in the lower SES group in the present study. Such a trend was reported in the northern Rio de Janeiro state of Brazil, where the age-adjusted seroprevalences were 84%, 62% and 23% in the lower, middle and upper SES levels, respectively [Reference Bahia-Oliveira5]. In the USA, Toxoplasma infection was considered an infection associated with poverty [Reference Jones1, Reference Munoz-Zanzi30]. It has been suggested that individuals of lower SES may be related to occupations with greater soil exposure and therefore be at higher risk of becoming infected [Reference Jones1].
We found a statistically significant association between T. gondii seropositivity and all variables assessing the presence of cats with a 2·3- to 38·4-fold increased risk of infection. The role of the cat in the transmission of toxoplasmosis has been established and many studies around the world have associated T. gondii infection with cats [Reference Robert-Gangneux and Darde3]. Ayi et al. [Reference Ayi11] found exposure to cat faeces to be the major risk factor for T. gondii infection in the study of pregnant women in Accra, Ghana. A study to assess seroprevalence and risk factors of T. gondii in pregnant women in southwestern Ethiopia significantly associated having cats at home with T. gondii antibodies [Reference Zemene6]. Another study from Ethiopia observed that individuals with a known history of association with cats were 5·3 times more likely to be seropositive than those with no history of such association [Reference Negash, Tilahun and Medhin18]. In the Democratic Republic of São Tomé and Príncipe, children who had a history of raising cats showed significantly higher seroprevalence than those who did not [Reference Fan20]. With respect to occupation, we observed that jobs which tended to expose the individuals to the soil were strongly associated with the infection. Such an observation was also made in the USA where soil-related occupations were associated with T. gondii seropositivity [Reference Jones1]. Other reports have associated the infection with certain occupations. Swai & Schoonman [Reference Swai and Schoonman26] reported from Tanzania that seroprevalence of Toxoplasma antibodies was significantly higher in livestock keepers and abattoir workers. Again, in the São Tomé study, children whose parents were unskilled workers showed significantly higher seroprevalence than those of semi-skilled and skilled workers. Fishermen after their fishing expedition normally sit by the seashore to mend their nets, exposing themselves to a greater contact with the soil. This might account for the observation that fishermen are more susceptible to T. gondii infection in the present study. Fishmongers were also at a high risk of being exposed to the soil as our analysis showed that fishing, farming and fishmongering were, respectively, the jobs that had the highest risk of exposing individuals to the soil (Table 5).
In the present study, we found that higher level of education was significantly associated with consistent reduction in the risk of infection. Lower levels of education have been associated with increased risk for toxoplasmosis in many epidemiological studies. In the USA and Chile, two separate studies found that participants with less than college education were significantly associated with T. gondii infection [Reference Jones1, Reference Munoz-Zanzi30]. Lower levels of education are usually associated with lower SES and may be related to employment in jobs with greater soil exposure. Our finding suggested that meat consumption was not a potential source of T. gondii infection in this study population. There was no significant association between seropositivity and participants who responded ‘yes’ to consumption of meat (P = 0·10) nor was there any significant association between seropositivity and any variables relating to meat consumption (Table 2). The reasons for this observation may be attributed to the exhaustive way of cooking meat by the participants. Nearly 94% of participants who reported consuming meat also reported that they always cook the meat until it is at least soft before consumption. However, Toxoplasma tissue cysts contained in meat or meat-derived products have been shown to serve as important sources of infection for humans [Reference Munoz-Zanzi30–Reference Kapperud33], and that the risk of acquiring the infection via meat sources depends on cultural and eating habits in different human populations [Reference Cook31].
In conclusion, T. gondii seroprevalence in the study population was high. The major risk factors associated with T. gondii seropositivity in the present study were old age, contact with soil, presence of a cat, lower level of formal education, and lower SES. Consumption of meat was not associated with the infection and seemed to suggest that contamination by sporulated oocysts may be the major source of transmission of toxoplasmosis in the study population. Fishing and fishmongering were highly associated with T. gondii seropositivity suggesting that the seashore may serve as a good ground for sporulation and survival of Toxoplasma oocysts. Further studies are needed to verify the viability of the parasites at the seashore.
ACKNOWLEDGEMENTS
The authors acknowledge all staff at the main laboratory of the School of Medical Sciences, University of Cape Coast. Special thanks go to Mr Johnson for his immense contribution towards this work. This study received no funding.
DECLARATION OF INTEREST
None.








