INTRODUCTION
Clostridium difficile is the major cause of hospital-acquired infectious diarrhoea and one of the most common pathogens responsible for healthcare-associated infections [Reference Rupnik, Wilcox and Gerding1]. Over the past decades, a marked increase in the incidence of C. difficile infections (CDIs) has been reported from different countries and continents [Reference Freeman2–Reference Lessa, Gould and McDonald4]. In the United States, CDI hospitalizations/10000 population increased from 3·1 cases in 1996 to 6·1 in 2003, and from 5·5 cases in 2000 to 11·2 in 2005 [Reference McDonald, Owings and Jernigan5, Reference Zilberberg, Shorr and Kollef6]. A similar epidemiological picture has been observed in Europe [Reference Asensio7–Reference Burckhardt9].
In recent years, a relative increase in the number of severe cases of CDI has also been registered, possibly related to the increased use of wide-spectrum antibiotics and the emergence and spread of hypervirulent strains [Reference Freeman2, Reference McDonald10–Reference Kuijper12].
To reduce both the incidence of CDI and the proportion of severe cases the supranational data cited above should be considered together with the detailed epidemiology of every single hospital, aiming at identifying the most cost-effective measures to be used locally. As the first step in this process, we assessed the changes in the incidence of CDI over a 5-year period in a large teaching hospital in Liguria (the Italian region with the oldest population [13]), as well as the risk factors for developing severe disease and for dying within 30 days of infection.
METHODS
Setting and definitions
We carried out a retrospective study at IRCCS AOU San Martino–IST, a 1300-bed tertiary adult acute-care teaching hospital in Genoa, Liguria Region, Italy. From 1 January 2010 to 31 December 2014, all patients with hospital-acquired CDI were identified through the hospital laboratory database.
A CDI episode was defined as the presence of at least one unformed stool specimen positive for C. difficile toxin A and/or B (C. Diff Quick Chek complete®, Techlab, USA; Alere Medical Co. Ltd, USA), according to the National Health Safety Network (NHSN) definition [14]. For the purpose of the study, recurrences of disease (new episodes occurring within 56 days after the first positive sample) were excluded from the analyses [14]. A CDI episode was defined as hospital-acquired if occurring >3 days after hospitalization, or within 28 days after discharge [14].
A CDI episode was classified as severe if fulfilling at least one of the following criteria: (i) death within 14 days after the onset of symptoms; (ii) requirement of intensive care unit (ICU) admission; (iii) requirement of colectomy or other surgery procedures; (iv) intestinal perforation [Reference Sailhamer15, Reference Debast, Bauer and Kuijper16].
Study endpoints
The primary endpoint was the incidence of CDI/10000 patient-days over the study period. Secondary endpoints were: (i) proportion of positive tested patients; (ii) proportion of severe cases; (iii) development of severe CDI; (iv) 30-day all-cause mortality.
Data collection
For the computation of the annual incidences of CDI, the overall number of hospital patient-days was obtained from the hospital digital archives of patients' clinical charts.
For the assessment of risk factors for severe disease and mortality, the following data were collected from the medical records of patients with CDI: age; gender; baseline comorbidities (congestive heart failure, myocardial infarction, chronic obstructive pulmonary disease, cerebrovascular accident, solid malignancy, haematological malignancy, diabetes mellitus, chronic renal failure); Charlson comorbidity index score [Reference Charlson17]; chronic haemodialysis; previous surgery (within 3 months); previous ICU stay (within 3 months); previous mechanical ventilation (within 3 months); previous therapy with antimicrobials, proton pump inhibitors, and/or histamine 2 (H2) blockers (within 8 weeks before CDI); length of hospital stay before developing CDI; other infections at the time of CDI diagnosis (defined and classified according to CDC definitions [18]); serum haemoglobin, albumin, creatinine, and leukocyte count at the time of CDI diagnosis; type of antimicrobial treatment and complications of CDI (requirement of haemodialysis, requirement of ICU admission, requirement of surgery, intestinal perforation).
Statistical analysis
Annual CDI incidence rates with their 95% confidence intervals (CIs) were calculated as the number of events/10000 patient-days. They were also stratified according to the ward where the diagnosis of CDI was made (ICU, medical ward, haemato-oncological ward, rehabilitation ward, surgical ward). A χ 2 test for linear trend was used to assess the changes in the annual incidence of CDI in our hospital over the study period, as well as the changes in the proportion of positive tested patients and severe cases.
For the assessment of risk factors for severe CDI and 30-day mortality, continuous variables were dichotomized and cut-off values were determined through receiver-operating characteristic curves. Then, demographic and clinical variables of patients with CDI were compared in univariate analyses by the χ 2 test or Fisher's exact test, as appropriate. These tests were performed for both comparisons (patients without severe CDI vs. patients with severe CDI, and survivors vs. non-survivors), all tests were two-sided. To assess the independent role of risk factors, variables with a P value < 0·05 in univariate comparisons were included in two logistic regression models (patients without severe CDI vs. patients with severe CDI, and survivors vs. non-survivors).
Statistical analyses were performed with Epi-Info v. 7.0 (CDC, USA) and JMP v. 10.0 (SAS Institute, USA).
Ethical statement
The study was performed within the institutional surveillance of healthcare-associated infections and involved the analysis of existing anonymized clinical and laboratory data. An informed consent for the use of anonymized data for scientific purposes is signed by all patients admitted to IRCCS AOU San Martino–IST and included in surveillance databases. The study was approved by the Regional Ethics Committee of Liguria Region.
RESULTS
During the study period, we identified 388 episodes of hospital-acquired CDI. These episodes occurred in 381 patients, of whom seven experienced at least one novel episode beyond 56 days after the previous CDI (1·8%). The complete demographic and clinical characteristics of patients at the time of CDI diagnosis are summarized in Tables 1 and 2. Of note, the median age of patients was as high as 81 years [interquartile range (IQR) 74–87] and as many as 81% and 89% of the patients had comorbid conditions and previous exposure to antibiotics, respectively.
Table 1. Demographic, anamnestic and clinical characteristics of the study population with C. difficile infection (CDI)
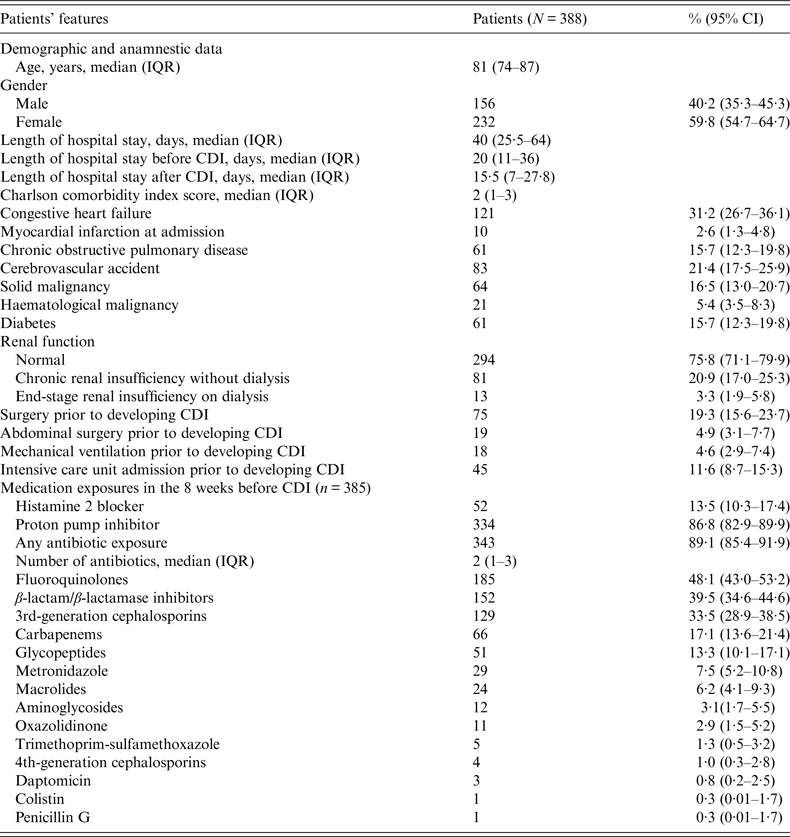
CI, Confidence interval; IQR, interquartile range.
Table 2. Clinical characteristics, therapeutic management and outcomes of C.difficile infections (CDI)
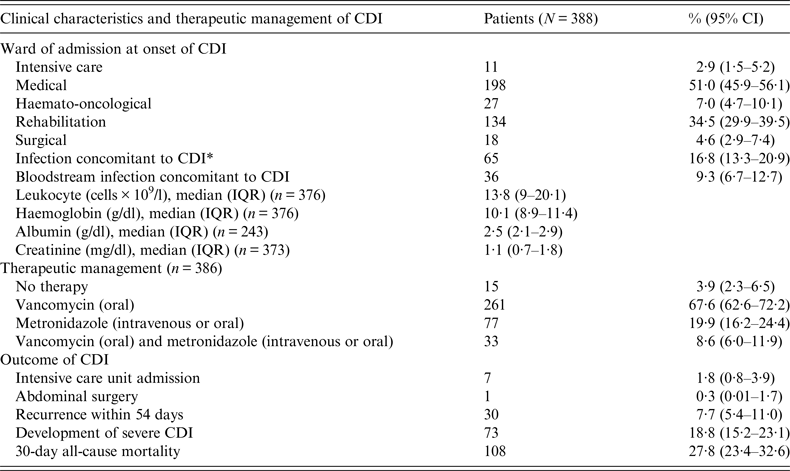
CI, Confidence interval; IQR, interquartile range.
* Bloodstream = 36 (55·4%); urinary tract = 21 (32·3%); lower respiratory tract = 7 (10·7%); surgical site = 1 (1·6%).
Table 3 shows that the trend in the annual incidence of CDI/10000 patient-days significantly increased from 0·54 in 2010 to 3·04 in 2014 (χ 2 for linear trend, P < 0·001). When the analysis was stratified according to the ward where the CDI diagnosis was made, statistically significant increases were observed in medical, haemato-oncological, and rehabilitation wards. The highest annual incidences of CDI were registered in rehabilitation wards, with a peak of 10·01 episodes/10000 patient-days in 2013. It should be noted that the proportion of positive tested patients significantly increased over the study period (χ 2 for trend, P = 0·002), while the proportion of severe cases apparently decreased (χ 2 for trend, P < 0·05).
Table 3. Number of samples and patients tested (total and proportion positive) per year and annual incidences of hospital-acquired C. difficile infections (CDI) in IRCCS AOU San Martino–IST of Genoa, Italy
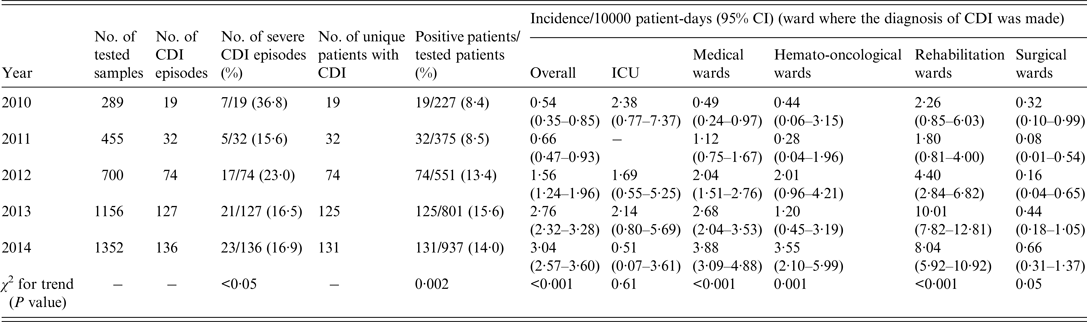
CI, Confidence interval.
Seventy-three of 388 CDI episodes met the criteria for severe CDI (18·8%). Results of the univariate and multivariate analyses of risk factors for severe CDI are shown in Table 4. In the univariate analysis, age ⩾72 years [odds ratio (OR) 2·79, 95% CI 1·31–6·93, P = 0·007], congestive heart failure (OR 1·98, 95% CI 1·17–3·34, P = 0·01), previous mechanical ventilation (OR 2·93, 95% CI 1·04–7·73, P = 0·04), previous therapy with H2 blockers (OR 2·15, 95% CI 1·10–4·09, P = 0·03), ward of stay at time of CDI diagnosis, and serum albumin ⩽2·5 g/dl (OR 3·30, 95% CI 1·76–6·43, P < 0·001) were associated with the development of severe CDI, while an intriguing protective role was suggested for diabetes (OR 0·26, 95% CI 0·08–0·67, P = 0·003). In the multivariate analysis, previous therapy with H2 blockers (OR 2·70, 95% CI 1·13–6·38, P = 0·03) and serum albumin ⩽2·5 g/l (OR 2·90, 95% CI 1·44–6·05, P = 0·003) remained significantly associated with the development of severe CDI, while diabetes was confirmed as a possible protective factor (OR 0·18, 95% CI 0·04–0·57, P = 0·002).
Table 4. Association between severe C. difficile infection (CDI) and potential independent variables: results of univariate and multivariate logistic regression
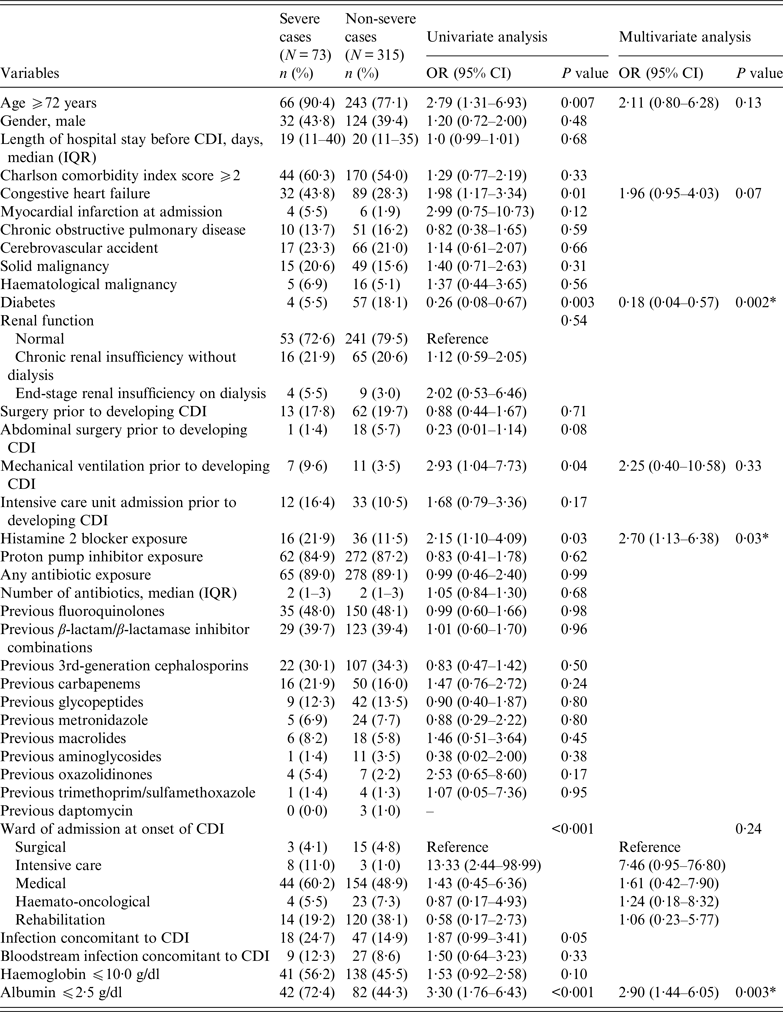
OR, Odds ratio; CI, confidence interval; IQR, interquartile range.
* Statistically significant (P < 0·05).
The all-cause 30-day mortality in patients with CDI was as high as 27·8% (108/388). In the univariate analysis of risk factors for mortality, age ⩾72 years (OR 2·52, 95% CI 1·34–5·08, P = 0·003), congestive heart failure (OR 1·92, 95% CI 1·2–3·05, P = 0·006), previous mechanical ventilation (OR 3·47, 95% CI 1·33–9·33, P = 0·01), ward of stay at the time of CDI diagnosis (P = 0·001, with the highest risk registered for patients hospitalized in ICU wards), other infections at the time of CDI diagnosis (OR 2·30, 95% CI 1·32–3·99, P = 0·004), leukocyte count ⩾20 cells × 109/l (OR 4·87, 95% CI 2·96–8·08, P < 0·001), serum albumin ⩽2·5 g/dl (OR 3·04, 95% CI 1·75–5·39, P < 0·001), serum creatinine ⩾1·6 mg/dl (OR 3·39, 95% CI 2·11–5·49, P < 0·001), and requirement of ICU admission (OR 16·41, 95% CI 2·76–311·9, P = 0·01) were unfavourably associated with outcome (see Table 5). Table 5 also shows the results of the related multivariate analysis, which confirmed the following variables as factors significantly and unfavourably associated with outcome: leukocyte count ⩾20 cells × 109/l (OR 2·74, 95% CI 1·39–5·46, P = 0·004); serum creatinine ⩾1·6 mg/dl (OR 1·94, 95% CI 1·0–3·79, P < 0·05); serum albumin ⩽2·5 g/dl (OR 2·19, 95% CI 1·11–4·37, P = 0·02).
Table 5. Association between 30-day all-cause mortality of C. difficile infection (CDI) and potential independent variables: results of univariate and multivariate logistic regression
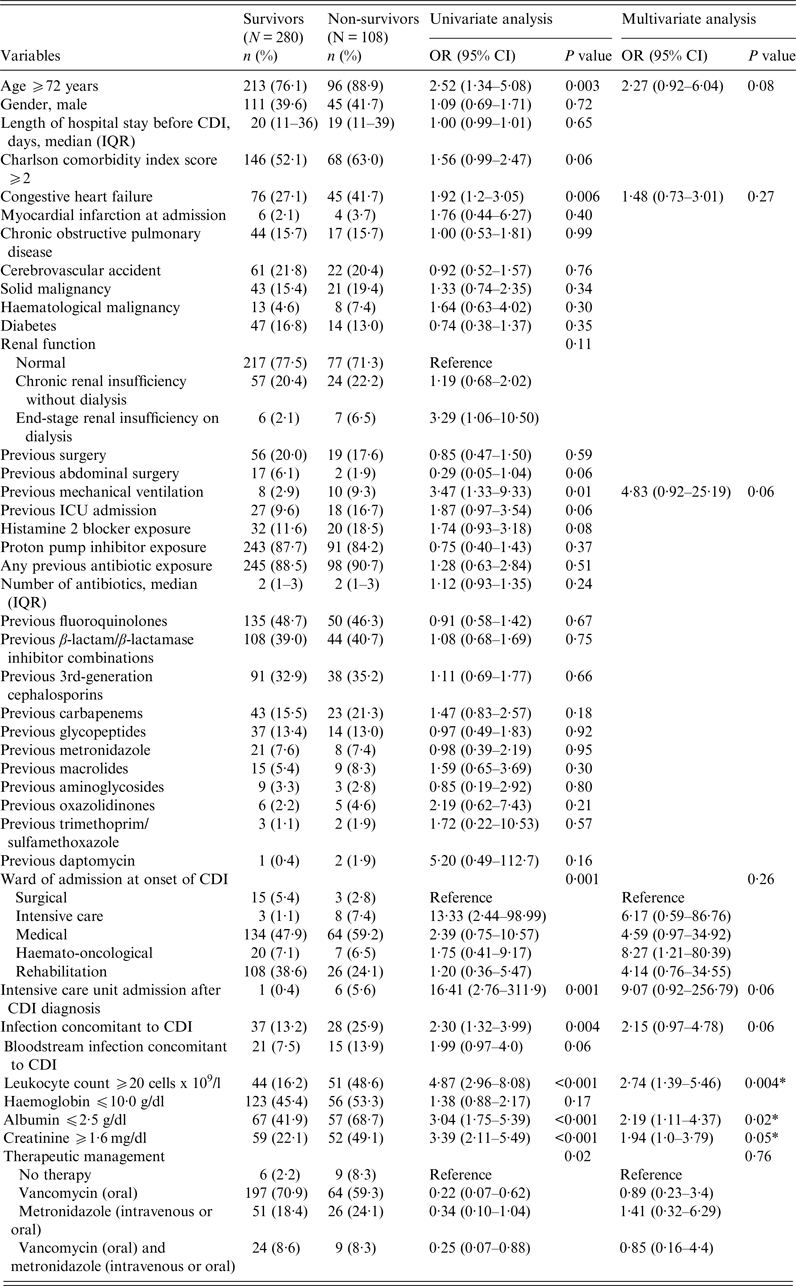
OR, Odds ratio; CI, confidence interval; IQR, interquartile range.
* Statistically significant (P < 0·05).
DISCUSSION
From 2010 to 2014, we observed an increase of nearly 600% in the incidence of hospital-acquired CDI in our facility, from 0·54 to 3·04 CDI episodes/10000 patient-days. These episodes mostly occurred in elderly patients, with very high rates of baseline comorbidities and previous antibiotic use.
Limited information is available on the incidence of CDI in Italian hospitals [Reference Di19, Reference Bassetti20]. Our results are consistent with those of Di Bella and colleagues, who reported an increased incidence of CDI, from 0·3 cases in 2006 to 2·3 cases/10000 patient-days in 2011 in five hospitals in Rome [Reference Di19]. Similarly, Bassetti et al. observed an increase in the incidence of CDI from 1·7 cases/10000 patient-days in 2009 to 3·0 cases in 2012 in a teaching hospital in Udine [Reference Bassetti20]. As in our research, the results of Di Bella et al. and Bassetti et al. relied on enzyme immunoassay (EIA) tests. Importantly, the change in the incidence of CDI in these studies might have been overestimated because of the increase in the number of tested patients over years [Reference Di19, Reference Bassetti20]. Although this bias was also present in our study, the simultaneous increase in the proportion of positive tested patients over the study period seems to confirm the role of C. difficile as an important and increasing cause of healthcare-associated diarrhoea. Of note, any change in CDI incidence due to reduced or enhanced adherence to infection-control practices appears unlikely to have occurred, since routine audits to guarantee a high level of compliance were performed in our hospital during the whole study period.
With regard to single departments, it should be noted that in our study the highest peaks in CDI incidence were registered in rehabilitation wards. This possibly occurred because of the high median age, median length of stay, and rate of previous exposure to antimicrobials of patients hospitalized in these wards (they were indeed mostly transferred to the rehabilitation unit after acute care in medical or ICU wards), all well-recognized risk factors for the development of the disease [Reference Evans and Safdar21]. However, it should also be noted that the overall median age of patients with CDI in the entire hospital was as high as 81 years. To the best of our knowledge, this is the highest median age observed in similar research, and it is probably related to the demographic characteristics of the population in the hospital catchment area [13]. Other important aspects worth reporting are the high frequency of baseline comorbidities (80·7% of patients with CDI had at least one chronic condition) and that the overall rate of previous use of antibiotics (mostly fluoroquinolones, β-lactam/β-lactamases inhibitor combinations, and third-generation cephalosporins) was as high as 89·1% in our cohort of CDI patients, among the highest reported in the literature [Reference Bassetti20, Reference Honda22, Reference Rodríguez-Pardo23].
As many as 18·8% of episodes were considered as severe CDI, in line with rates reported by others [Reference Abou Chakra24]. Of note, several variables associated with the development of severe CDI (i.e. prior acid suppression, baseline serum albumin ⩽2·5 g/dl) have already been described by other authors, and testify to the role of concomitant medications and nutritional status in influencing the course of the disease, as well as to the possible protective effect exerted by albumin [Reference Abou Chakra24, Reference Di25]. On the other hand, diabetes was apparently protective against severe CDI in our cohort. This result might be explained by the fact that diabetes treatment with metformin might have a protective effect against the development of CDI, according to some literature data [Reference Eliakim-Raz26]. However, this association has not been confirmed by other researchers, who conversely reported a harmful association between diabetes and CDI, thus this warrants further investigation [Reference Wenisch27].
The 30-day all-cause mortality registered in our study (27·8%) is consistent with rates reported in the literature [Reference Abou Chakra24]. However, we did not register any of the well-known associations between mortality and age, and between mortality and underlying comorbidities (e.g. malignancy, chronic renal failure, etc.) [Reference Abou Chakra24]. This is possibly related to the advanced age and the high frequency of chronic conditions in our study population. As regards laboratory variables, we found that a baseline serum leukocyte cell count ⩾20 × 109/l, a baseline serum albumin level ⩽2·5 g/dl, and a serum creatinine level ⩾1·6 mg/dl were associated with increased mortality, in accord with previous studies [Reference Abou Chakra24]. This is consistent with the association of unfavourable outcome of CDI and altered laboratory values, conceivably indicating a severe course of the disease [Reference Abou Chakra24].
The present study has some limitations. First, this was a single-centre study, and our results might not be reproducible in other Italian hospitals. In addition, under-reporting possibly occurred in some cases because of the retrospective nature of the study. Another major limitation is the lack of molecular typing data. Indeed, despite an intriguing decrease in the proportion of severe cases observed over the years, the absence of molecular data prevented us from assessing whether this was associated with any possible change in the proportion of hypervirulent strains in our institution during the study period, or if other factors might perhaps better explain these findings. Finally, we were not able to retrospectively conduct a reliable in-depth analysis of possible changes in the type of antibiotics prescribed in our hospital during the study period.
In conclusion, from 2010 to 2014 we observed a marked increase in the incidence of hospital-acquired CDI, highlighting the need for more efficacious preventive interventions, focused on strict adherence to infection control measures and on appropriate antibiotic use. This is of the utmost importance in our hospital, because of the advanced age of our patients and their very high frequency of chronic conditions and use of antibiotics, which readily predispose them to the development of hospital-acquired CDI.
ACKNOWLEDGEMENTS
The authors thank Monica Zacconi for assistance with data collection.
DECLARATION OF INTEREST
None.







