Introduction
Mandatory notification of laboratory-confirmed cases is a widely utilised infectious disease surveillance approach, with the aim to understand disease occurrence, trends and epidemiology [Reference M'ikanatha1, Reference Weiss and Koepsell2]. It allows for assessing distributions over time, place and person, providing the necessary data for situational awareness and risk assessment, the triggers for response and hypothesis generation. Many surveillance systems often require reporting only the number of laboratory-confirmed cases, per a case definition, for a list of ‘notifiable diseases’ [Reference M'ikanatha1]. While not commonly reported, the number of tests performed is an important epidemiological indicator [Reference Dimech3], because notifications of infectious diseases are strongly associated with the number of tests conducted [Reference M'ikanatha1, Reference Chen4, Reference Cretikos5]. Sexually transmitted infections (STI) are particularly vulnerable to changes in ascertainment (i.e. surveillance or ascertainment bias indicating changes in ascertainment rather than a change in disease occurrence [Reference Weiss and Koepsell2, Reference German6]), as changes in screening policies, testing behaviours, clinic hours and other practices/behaviours can influence detections [Reference M'ikanatha1]; thus, using multiple surveillance approaches and data sources and ‘prevalence monitoring’ (i.e. assessment of STI test positivity among persons screened) has been recommended [Reference M'ikanatha1]. In fact, surveillance for certain infectious diseases (e.g. measles, polio, seasonal influenza) has required such denominator test data to be reported, to allow for more confident assessments of the epidemiological situation [7–Reference Adams10]. Surveillance data therefore need to be interpreted cautiously when testing data are unknown – lack of such denominators make it challenging to know if low notifications are due to low disease occurrence or lack of testing, and similarly, if a rise in notifications is due to increase in occurrence or increased testing.
In Japan, infectious disease surveillance is conducted through the National Epidemiological Surveillance of Infectious Diseases (NESID) system [11, Reference Hashimoto12]. As with many other developed countries [Reference M'ikanatha1], HIV/AIDS is notifiable, with laboratory-confirmed cases legally requiring notification to a public health agency. HIV testing is available at local public health centres (approximately 450 located throughout the country in each jurisdiction) and affiliated public facilities (23 sites as of 2014 [13]) (together, termed here as ‘PHC's), and at many medical facilities, such as hospitals (both private and public, including 382 hospitals designated as AIDS Core Hospitals; includes pre-surgery and entry-screening tests) and private STI clinics. Importantly, at PHCs, anonymous HIV testing is free and the number of HIV tests is known; these aspects make HIV a unique notifiable disease in Japan. Such PHCs, in fact, serve an important role in HIV detection in Japan – during 2007–2014, PHCs conducted annually an average of 144 510 HIV tests, detecting an annual average of 475 HIV-positive samples (during the same period, an average of 1521 HIV/AIDS cases were reported annually) [14].
Based on the surveillance data, HIV epidemiology is known to be similar to other industrialised settings – consistently, >90% of reported cases have been male, and nearly three-quarters men who have sex with men (MSM), as reported in the HIV ‘transmission category’ [Reference M'ikanatha1] on the case notification form. Female cases are uncommon, and with only seven cases of HIV infections detected in newborns in Japan during 2007–2014 – despite universal HIV screening among pregnant women – HIV transmission is believed to be predominantly among MSM in Japan [15].
Recently, the number of people living with HIV/AIDS was estimated to be 26 670 persons in 2015 [Reference Iwamoto16], indicating that Japan is a low prevalence country. Notifications of HIV-positive cases have plateaued since peaking in 2008 [15, Reference Suguimoto17], but a descriptive assessment accounting for testing has been lacking. We present these data to facilitate better interpretation of HIV surveillance in Japan, and iterate the importance of such denominator information.
Methods
Surveillance of HIV in Japan
Since April 1999, HIV/AIDS surveillance in Japan has been conducted under the Infectious Diseases Control Law [Reference Hashimoto12, Reference Yoshikura18]. Detection of a laboratory-confirmed HIV infection must be notified within 7 days of diagnosis to the local public health centre, using the uniform case notification form [19]. Public health staff check the reported data and enter verified data into the electronic NESID system. Separately, the annual number of HIV tests conducted at PHCs are reported to the Ministry of Health, Labour and Welfare (MHLW) and managed by the ‘National AIDS Surveillance Committee’.
Notification of an HIV-positive case requires a positive result from a screening test (e.g. enzyme-linked immunosorbent assay, particle agglutination assay, immunochromatography) and a confirmatory test (antibody confirmatory test (e.g. Western blot assay, immunofluorescent antibody tests) or HIV antigen test (e.g. virus isolation, PCR)) [15]. For surveillance purposes, based on the laboratory criteria above, two categories are used: (1) ‘HIV case’ if HIV infection was detected before AIDS manifestation and (2) ‘AIDS case’ if infection was detected with AIDS. CD4 count or stage of HIV infection is not reported.
Data collection and analysis
Data assessed were from 2007 to 2014 when the surveillance system remained unchanged. We describe trends over time and place for the total number of reported HIV-positive cases, but in order to account for testing frequency, we restricted the primary assessment to notifications from PHCs, where the number of tests is available (for cases reported from other facilities such as hospitals, test data are unknown/inaccessible). Additionally, cases detected from tests conducted at PHCs are more likely to represent infections that are relatively less severe and more recent. This is because the modality of testing is self-initiated (i.e. voluntary counselling and testing (VCT)) and PHCs do not offer treatment. In fact, based on recent findings, among patients diagnosed with HIV infection, those found through VCT had higher median CD4 counts (338/microlitre) relative to other means such as pre-surgery or admission screening conducted at hospitals and other facilities (292/microlitre) [Reference Nishijima20]. Lastly, restricting to PHCs minimises the possibility of any case being counted twice (e.g. a case that receives testing at a PHC and later at a hospital).
Thus, focusing on the population that sought testing at PHCs, for the denominator (i.e. annual number of HIV tests performed at PHCs), data were obtained from the annual report of the ‘National AIDS Surveillance Committee’ [14], for both the national and prefectural levels. For the numerator (i.e. annual number of HIV-positive cases detected from tests at PHCs), data were obtained from two sources. For the aggregated national level, as with the denominator, numerator data were obtained from the National AIDS Surveillance Committee. For the prefectural level, numerator data were obtained from NESID, since only the national-level case data are available from the National AIDS Surveillance Committee (as the reporting source is recorded in NESID, those reported from PHCs were extracted from NESID).
We focused on Tokyo and Okinawa, two prefectures that have continued to report relatively high HIV notification rates per population in Japan [15, 21] and report more than 10 cases every year from PHCs. Population data for each calendar year were obtained from the ‘Portal Site of Official Statistics of Japan’ [22] and used to calculate notification rates and number of tests performed per 100 000 population per year. For each prefecture, the total prefecture population for the respective year was used; similarly, for the national level, the total national population for the respective year was used. Data were analysed using Microsoft Excel 2013 and R version 3.4.2.
Results
During 2007–2014, nationally, there was a total of 12 167 HIV-positive cases notified, ranging from 1449 to 1590 cases each year (annually n = 1500, 1557, 1452, 1544, 1529, 1449, 1590, 1546, respectively). During this period, approximately 70% were ‘HIV cases’ (without AIDS), and the largest number of ‘HIV cases’ were notified in 2008 (annually n = 1082, 1126, 1021, 1075, 1056, 1002, 1106, 1091, respectively). During this period, based on the 2010 population, the notification rate was 3.70/100 000 person-years for Tokyo, 1.73/100 000 person-years for Okinawa, and 1.19/100 000 person-years nationally.
During 2007–2014, nationally, 3798 HIV-positive cases were detected from PHCs, ranging from 442 to 508 cases each year (n = 508, 501, 442, 473, 462, 469, 453, 490, respectively). The largest number of cases was reported in 2007 (n = 508), followed closely by 2008 (n = 501), similar to the peak observed for all ‘HIV cases’. The annual number of samples tested in PHCs ranged from 80 to 115 (median = 85) per 100 000 population and the proportion of samples that tested HIV-positive ranged from 0.28% to 0.36% (median = 0.34%) (Fig. 1a). Overall, while there was an increase in the absolute number of HIV detections with an increase in tests (Fig. 1b), positivity showed an inverse relationship with the testing rate (Fig. 1c). In 2008, when test frequency peaked (n = 177 156), there was the second highest number of HIV detections but the positivity dropped to the lowest value (0.28%); conversely, when there were fewer tests performed in 2010–2012, there were correspondingly fewer HIV detections but positivity was at its highest (0.36%). There was a nearly linear inverse relationship between test frequency and positivity (Pearson's correlation coefficient of −0.88 (P = 0.004)) (Fig. 1c).

Fig. 1. (a) Number of samples tested for HIV at health centres and affiliated centres (PHCs) and the percentage of samples that tested positive for HIV (positivity) by year, (b) number of HIV-positive cases by number of samples tested for HIV at PHCs, (c) positivity by number of samples tested for HIV at PHCs, Japan, 2007–2014.
During 2007–2014, based on these notifications from PHCs, Tokyo consistently reported higher annual HIV notification rates (median = 0.98; range = 0.89–1.33 per 100 000) compared with Okinawa (median = 0.61; range = 0.42–1.09 per 100 000) (Fig. 2). While Okinawa's notification rate was relatively low during 2010–2013 ranging from 0.42 to 0.50, it increased to 0.92 in 2014. In Tokyo, while the notification rate declined from 1.33 per 100 000 in 2007 to a low of 0.89 per 100 000 in 2009, an increase was observed in 2010 (1.09 per 100 000).
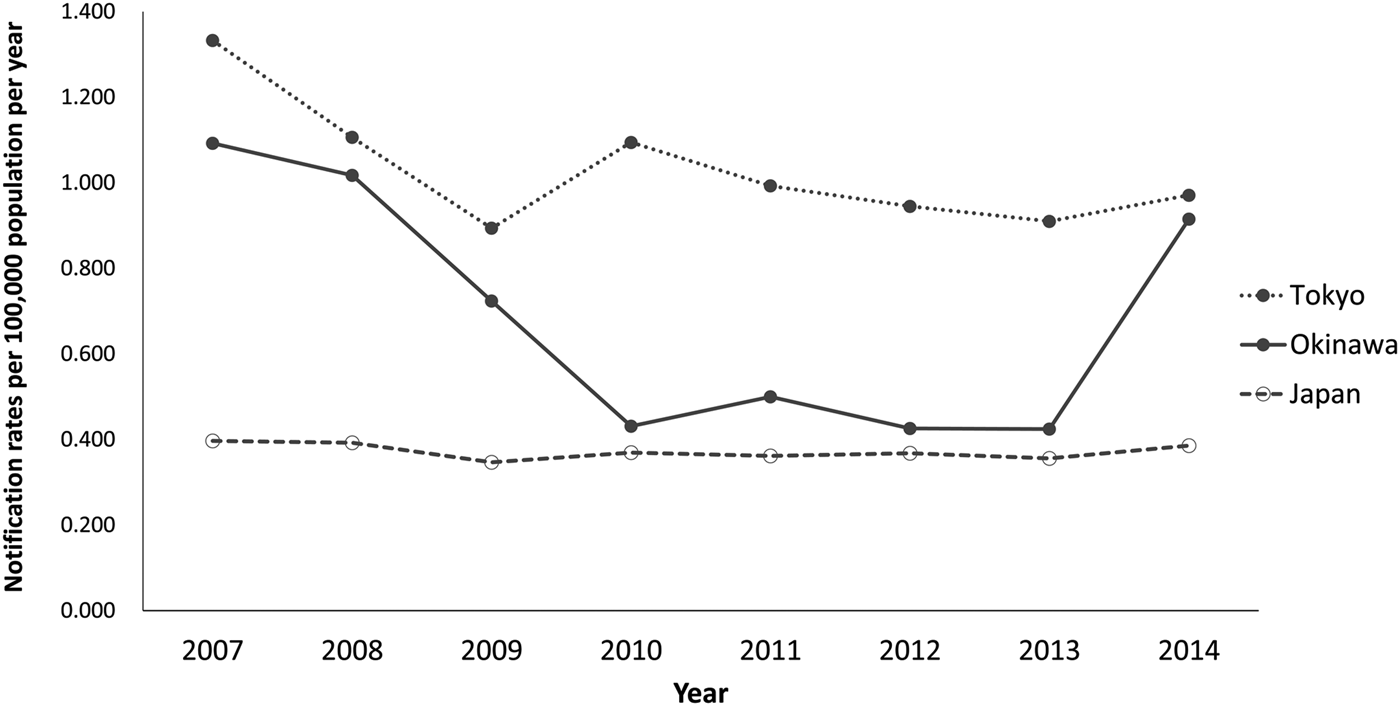
Fig. 2. Number of HIV-positive cases notified from health centres and affiliated centres per 100 000 population (notification rate) in Tokyo and Okinawa prefectures, by year, 2007–2014.
For the numbers of samples tested per population, relative to the national testing rate (median = 85; range = 80–115 per 100 000), both prefectures had substantially higher levels, with Okinawa showing slightly higher test frequency (median = 187; range = 158–274 per 100 000) compared with Tokyo (median = 172; range = 163–210 per 100 000) (Fig. 3). In Okinawa, testing was low during 2010–2013, ranging from 159 to 177 per 100 000, but increased in 2014 to 204 per 100 000 (Fig. 3). In Tokyo, testing peaked in 2008 (210 per 100 000), but then declined, decreasing in 2010 to 164 per 100 000 (Fig. 3).
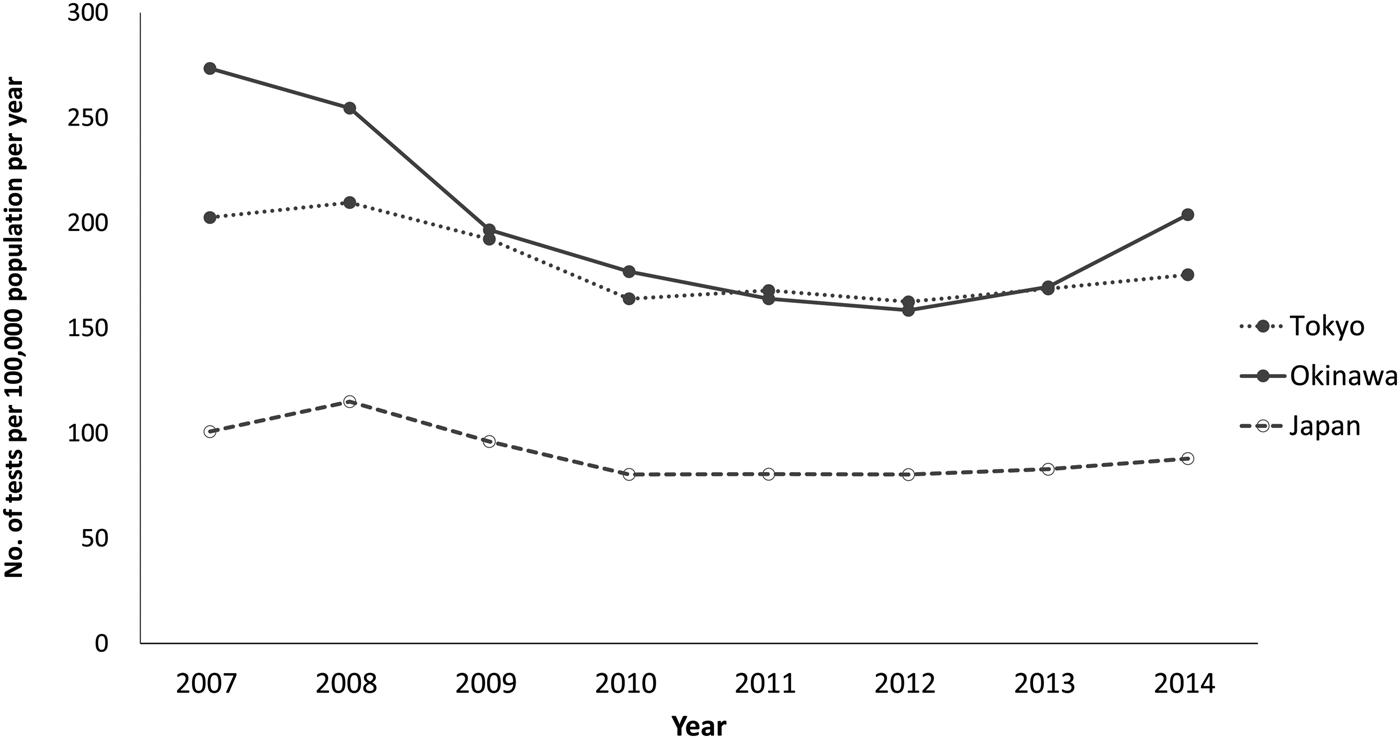
Fig. 3. Number of samples tested for HIV at health centres and affiliated centres per 100 000 population (testing rate) in Tokyo prefecture, Okinawa prefecture and Japan, by year, 2007–2014.
For positivity, Tokyo consistently reported higher values (median = 0.57%; range = 0.46–0.67%) relative to Okinawa (median = 0.34%; range = 0.24–0.45%) and to the national level (Fig. 4). While Okinawa had relatively lower positivity throughout the period, at times lower than the national level (0.24–0.31% during 2010–2013), it rose to 0.45% in 2014 (Fig. 4). In Tokyo, on the other hand, there was no such rise in 2014, and the positivity peaked in 2010 (0.67%) (Fig. 4).

Fig. 4. Percentage of samples tested for HIV at health centres and affiliated centres that tested positive for HIV (positivity) in Tokyo prefecture, Okinawa prefecture and Japan, by year, 2007–2014.
Nationally, assessing the three indicators together, years with high notification rates tended to be those with high test frequency but low positivity (e.g. 2008). In Okinawa, when a large increase in notification rate was observed in 2014, this occurred concurrently with increased testing and increased positivity. As for Tokyo, when the notification rate increased in 2010, this occurred when test frequency actually decreased, resulting in increased positivity.
Discussion
Overall, years with high HIV notifications tended to be those with more tests but lower positivity. Given these trends, and because frequency of HIV testing in Japan has been considered to be associated with the level of awareness/concern for HIV [23], notification trends need to be interpreted carefully. This is because increased notifications occurred in the context of more testing, but a proportionately lower increase in case detections. In the absence of any change in the true population prevalence, this would be in the expected direction of increased ascertainment, as increase in testing is likely accompanied by a proportionate increase in those with a lower risk profile seeking out testing, leading to a decrease in positivity. This inverse trend has also been observed in Spain, where declining HIV positivity among heterosexual testers over time was linked to more tests and the possibility of testing more people at lower risk [Reference Diez24]. Prevalence, in fact, often depends on the subgroup population being tested [Reference Weiss and Koepsell2]; for example, HIV positivity has been found to be high among several STI clinics located in urban Japan, ranging from 0.50% to 0.68% during 2004–2014, hypothesised to be due to the high-risk profile of this population [13]. Changes in the risk profile distribution of testers would thus affect the overall likelihood of testing positive given testing.
Thus, even if the true population HIV prevalence remains stable, changes in notifications may occur due to changes in testing activity. Notably, trends in HIV notifications – for all reported cases, those restricted to detections from PHCs, and those detected from select STI clinics – have all remained relatively stable in Japan, with years of increased notifications associated with more tests and lower positivity while years with fewer notifications having fewer tests and higher positivity for both PHC and STI clinics [13]. Taken together, these trends suggest that the recent fluctuations in HIV notifications may be more a function of ascertainment rather than any large changes in the population prevalence. On the other hand, even if notifications remain stable, we should be concerned if accompanied by a continuous decline in tests and increased positivity; if the same level of testing was performed, additional cases might have been detected (assuming that testing was not increasingly targeting high-risk MSM [Reference Smith25]). In fact, in England, declining testing accompanied by increased positivity has been interpreted as a concern for this reason when assessing chlamydia surveillance data [26].
These assessments, albeit based on expected directionality from certain assumptions, only become possible with testing data. Notably, after a rapid HIV testing system (i.e. results obtained on day of testing) was introduced at some PHCs around 2003, it gradually expanded nationwide, and along with testing on evenings/weekends at some PHCs, test frequency increased until peaking in 2008 [23, Reference Nakasone27]. Over the years, some jurisdictions have also coordinated with local organisations to enhance awareness for HIV testing, including for gay men [15, Reference Nishijima20, Reference Nakasone27]. As a programmatic indicator, HIV testing is encouraged, per the United Nations Programme on HIV/AIDS/World Health Organization 90-90-90 targets; accounting for changes in testing and the resultant positivity can add value when interpreting notification trends.
Comparing across prefectures, it has been reported that Tokyo consistently reports the most HIV cases [14, 15, Reference Nishijima20, 21, Reference Kihara28]. Results were the same in our restricted analysis, and Tokyo also had high testing activity and positivity, both considerably higher than national levels. As higher prevalence in the population would be expected to increase the likelihood of testing positive – while more testing would be expected to lower positivity – the high positivity despite more testing suggests high prevalence rather than high ascertainment. The high levels in all three indicators, taken together with other information (e.g. a well-established gay district in an urban setting, high notifications of other STIs such as syphilis [Reference Ishikane29]), help infer that Tokyo is likely a true HIV hot spot, and provide supporting information for public health decision-makers to focus on Tokyo.
For Okinawa, also known for a relatively high HIV notification rate, testing was found to be in fact slightly higher than in Tokyo. However, the positivity was considerably lower, at times lower than the national level. In fact, Okinawa has been proactively implementing HIV prevention and testing campaigns [Reference Nakasone27], which may be contributing to the high level of testing. Okinawa's high notification rate should thus be interpreted within this context. The need for such caution when making comparisons to other prefectures become apparent thanks to the test information, and provide contextual information for public health decision-makers.
Importantly, however, the increase in notifications from Okinawa in 2014 is concerning, as testing and positivity both increased, a direction opposite to that expected from increased ascertainment. As far as we are aware, there were no changes in testing policy or campaigns in Okinawa around that time, which could have influenced the population of testers (e.g. high-risk MSM increasingly getting tested). In fact, while a health centre in Okinawa newly initiated a free ‘HIV test day’ targeting MSM, this was in 2013, and all test results were negative [Reference Taira30]; in 2013, Okinawa did see a slight increase in tests, but the positivity and notification rate declined (Figs 2–4). Thus, when there is an increase in all three indicators, in the absence of other information which could explain such trends, further investigation and interventions are warranted. Notably, when Tokyo experienced a rise in the notification rate in 2010, there was actually a decrease in tests. While this situation could arise from more efficiently targeted campaigns, in the absence of such information, this scenario should also raise a flag, since there were more cases despite fewer tests.
Conversely, situations where there is a decline in all three indicators would provide reassurance – reduced ascertainment would not easily explain this scenario (assuming high-risk persons are not increasingly avoiding testing). Or, as reported recently from the UK, a decline in HIV diagnoses, even with more testing, would also suggest a true decrease, which was in line with the results from the estimated incidence model and other information [31].
Limitations
There are important limitations in our assessment. First, data for HIV cases and samples tested at medical facilities were not included. However, our interpretations are relative in nature, and comparing the total notifications to our restricted dataset, the order of notification rates (i.e. Tokyo > Okinawa > national) and the temporal trends seen for the national level, Tokyo and Okinawa were the same. As PHCs consistently make up nearly a third of all notifications, have testing data available, and are capturing cases at an earlier stage of disease, restricting to PHCs is in fact appropriate for HIV situational analysis in Japan (especially since CD4 data are unavailable). Second, as the same individuals could have contributed multiple negative tests per year, such repeat testers could affect our interpretations. Data on test history are not available in Japan, and the frequency of repeat testing is unknown. However, even if repeat testing had increased in Okinawa in 2014, it would still warrant concern as positivity had increased. Ideally, it would also be helpful to have testing rates by gender and age group [Reference Rao32]. Given the presumed low HIV prevalence in Japanese women, for instance, assessing the gender distribution of testers would help with interpretations (even more useful would be specific transmission categories [31]). Such data could also be useful in assessing testing behaviours by age group/birth cohort – if, for instance, testing was infrequent among older persons but positivity high, they would be an important group for outreach. More rigorous assessments for HIV surveillance exist, such as including CD4 count data, ‘late presenter’ data [Reference Likatavicius and Van de Laar33], second-generation HIV surveillance, stratification by risk groups [Reference Diez24] and laboratory methods (e.g. Lag avidity tests) that discriminate past from recent infections [Reference Duong34], especially useful for modelling incidence [35]. However, despite these limitations and in the absence of such data, our simple approach utilizing existing testing data allow for interpretations that are more reassuring than numerator data alone, and together with other information, provide a more comprehensive assessment [Reference Adams10, 31]. Also, such basic testing data may be more available/accessible when assessing across areas/regions.
Conclusion
Accounting for the number of tests and the proportion positive provide insights otherwise not possible from notification rates alone. Whenever feasible, we encourage incorporating such data for surveillance, as a practical approach to improve preliminary situational analysis and for better-informed public health assessments and response.
Acknowledgements
We thank all the staff at local public health centres and prefectural and municipal public health institutes in Japan who dedicate their time and effort to surveillance. The contribution from reporting physicians and local governments through NESID is valuable for infectious disease prevention and control in Japan. We also thank Dr Takuya Yamagishi, Dr Tadashi Nakasone and Dr Yoshiyuki Sugishita for their valuable comments and insights from the field.
Financial support
This research was supported by Research on Emerging and Re-emerging Infectious Diseases and Immunization (H27-shinkougyousei-shitei-001), a grant from the Ministry of Health, Labour and Welfare of Japan.
Conflict of interest
None.
Ethical standards
No ethical approval was necessary because this study was conducted for public health purposes using national surveillance data. Only aggregate level data were used in analysis.







