INTRODUCTION
A number of environmental factors have the potential to drive the transmission of influenza and respiratory syncytial virus (RSV). Low absolute humidity appears to be the dominant driver of seasonal influenza and RSV epidemics in temperate climates [Reference Shaman and Kohn1–Reference Yusuf3]. This effect of low humidity in temperate winters is probably augmented by one or more of the following factors: low temperature, increased crowding, and low micronutrient levels (including low vitamin D levels) [Reference Yusuf3–Reference Lofgren5]. In tropical settings seasonal influenza and RSV epidemics often occur during the rainy season; however, the mechanisms driving this seasonal pattern are not clear [Reference Tamerius4, Reference Tamerius6, Reference Weber, Mulholland and Greenwood7].
RSV and influenza are structurally similar viruses: both are lipid enveloped, single-stranded RNA viruses. For this reason, the survival of these viruses in the environment is likely to be similar. However, the route of transmission appears to differ between RSV and influenza. Respiratory infections can be transmitted by three main routes [Reference Killingley and Nguyen Van Tam8]. Large respiratory droplets can travel short distances and may deposit directly on mucous membranes of the respiratory tract (direct contact). These same droplets will also deposit on surfaces where the virus may persist for long enough to be transferred to mucous membranes (indirect contact). Small respiratory droplets can form droplet nuclei, which are small enough to be inhaled, and can remain suspended in the air for hours (aerosol transmission). The relative importance of each of these three routes varies between different infections, and for a particular infection the relative importance of each route is also likely to vary according to the setting and ambient conditions. RSV appears to be predominantly spread by direct and indirect contact through large respiratory droplets [Reference Hall and Douglas9–Reference Leclair11], thus virus survival on surfaces is likely to be the more important factor. Influenza appears to spread by all three routes [Reference Killingley and Nguyen Van Tam8, Reference Tellier12], thus viral survival both in aerosol and on surfaces is important.
This paper reviews the effect of humidity upon influenza and RSV transmission – in particular, the different effects of humidity on aerosol and contact transmission. This paper also reviews the different seasonal patterns of influenza and RSV in temperate, subtropical and tropical climates. The results from these reviews are then combined to assess whether the differing effects of humidity on aerosol and contact transmission may be a plausible explanation for differences on influenza and RSV seasonality.
METHODS
A Medline search was performed using the following terms: (influenza OR ‘respiratory syncytial virus’) AND (persistence OR survival OR viability) AND humidity.
Search results were restricted to papers published in English. The Medline search was complemented by citation searches of the retrieved articles. Articles citing the retrieved articles were also searched using Google Scholar.
RESULTS
Humidity and aerosol transmission of influenza
Humidity can influence aerosol transmission via two mechanisms: the proportion of respiratory droplets becoming aerosolised and remaining in aerosol, and the survival of the virus within these aerosols.
Respiratory droplets are generated in the high humidity of the respiratory tract. On entering an environment with low humidity, respiratory droplets reduce in size within seconds due to evaporation. The resulting small droplets and aerosol nuclei settle slowly. At higher environmental humidity, respiratory droplets evaporate more slowly, and hence are larger and settle faster, and less aerosol nuclei are produced [Reference Xie13, Reference Yang and Marr14].
The persistence of infectious influenza in artificially produced aerosols exposed to different levels of relative humidity (RH) was examined in several studies between 1940 and 1980. Virus persistence was assessed by culture of air sampled from experimental settling chambers. Some of these studies found a monotonic relationship, with decreasing influenza virus persistence in aerosol with increasing RH (tested up to 80%) [Reference Harper15–Reference Loosli17]. Figure 1 shows the results from one of these studies [Reference Harper15] which is representative of the results from all three studies. Other studies found a U-shaped function of viral persistence with increasing humidity, with maximum virus persistence at low RH, minimum persistence at 40–60% RH, and moderate persistence at higher RH (tested up to 80%) [Reference Schaffer, Soergel and Straube18–Reference Lester20]. Figure 1 shows the results from one of these studies [Reference Schaffer, Soergel and Straube18] which is representative of the results from all of these three studies. The reason for the differing patterns between 40% and 70% RH is not clear. It has been suggested that the low virus survival at 50–60% RH in the U-shaped pattern may be due to the low protein content of the solutions used in these studies, adversely impacting on virus survival at these levels of RH [Reference Yang, Elankumaran and Marr21]. In all of these older studies, influenza persistence in air may have been affected by aerosol settling as well as virus survival [Reference Hinds22]. A more recent study measured both the total amount of virus remaining suspended in aerosol (using quantitative PCR) as well as the amount that remained infectious (using viral culture) enabling a more direct measure of viral survival [Reference Noti23]. Figure 1 shows the results from this study [Reference Noti23]. All three patterns in Figure 1 indicate the steepest change in virus survival in aerosol occurs between 30% and 50% RH (at approximately room temperature).

Fig. 1. Influenza persistence in artificially produced aerosols. All studies measured influenza persistence after 1 h. All studies were performed at temperatures between 20 °C and 24 °C. Data from Harper [Reference Harper15], Schaffer et al. [Reference Schaffer, Soergel and Straube18], and Noti et al. [Reference Noti23].
Animal transmission studies may provide the most pragmatic evidence examining the effect of humidity on influenza transmission, because animal transmission studies examine the net effect of respiratory droplet settling and virus survival, and droplet composition is more physiologically relevant than for artificially produced solutions. At both 5 °C and 20 °C, aerosol transmission of influenza between guinea pigs was reduced with increasing RH, with the lowest transmission occurring at 80% RH (Fig. 2) [Reference Lowen24]. Influenza transmission between mice showed a similar relationship with humidity in an earlier study: influenza transmission reduced as RH increased from 47% to 70% [Reference Schulman and Kilbourne25].
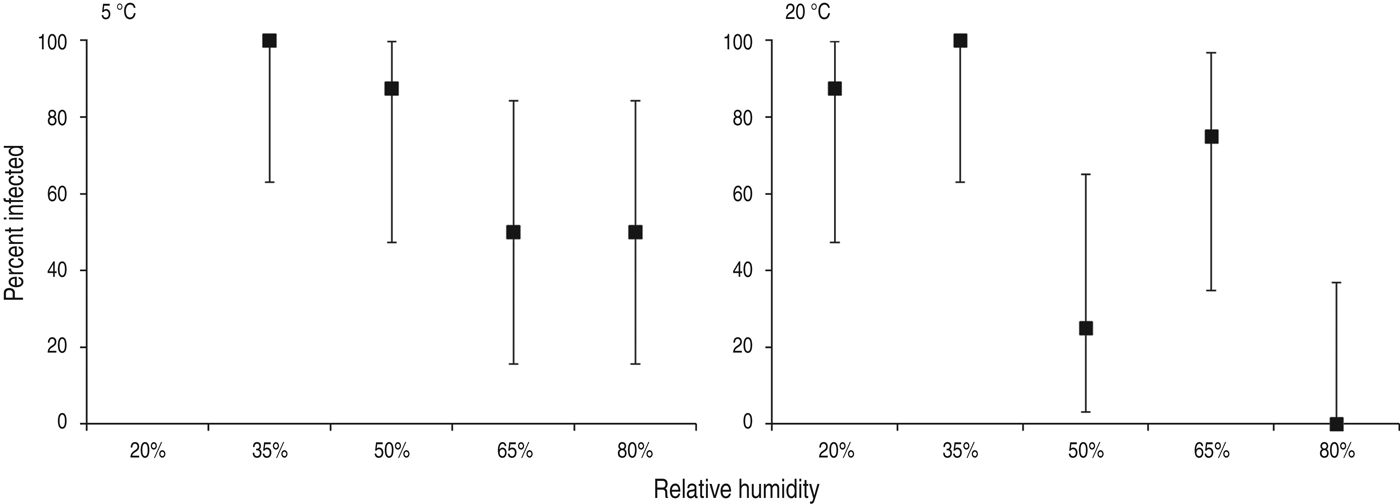
Fig. 2. Percentage of guinea pigs infected via aerosol transmission (with 95% confidence intervals) at different levels of relative humidity and temperature. Data from Lowen et al. [Reference Lowen24].
Both influenza persistence in aerosol and transmission via aerosol are reduced by increasing temperature. The effects of temperature and humidity appear to be additive, as shown in Figures 2 and 3. Aerosol transmission of influenza between guinea pigs was completely blocked at 30 °C despite viral shedding from infectious individuals [Reference Lowen26].
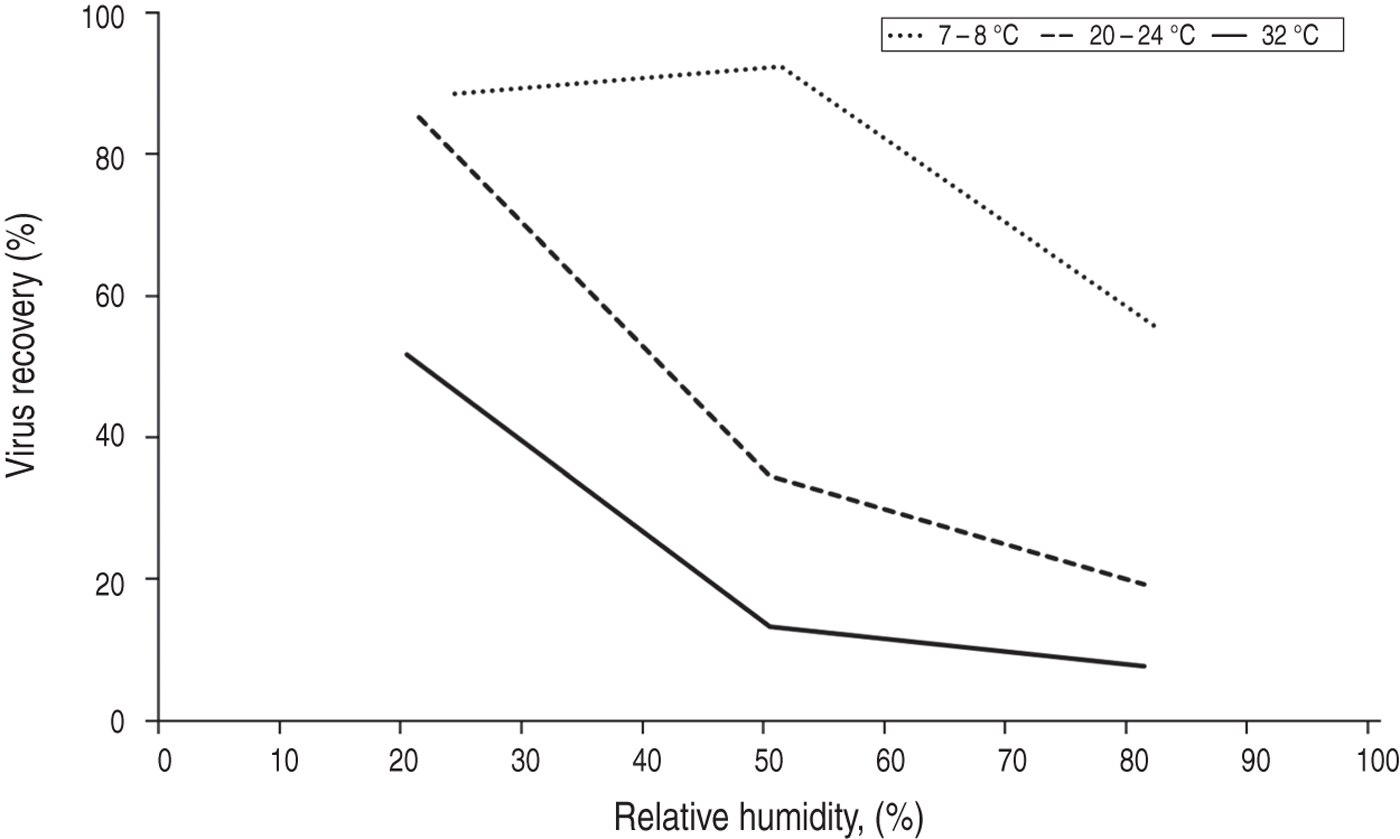
Fig. 3. Influenza persistence in artificially produced aerosols after 1 h, showing the effects of humidity and temperature. Data from Harper [Reference Harper15].
Humidity and indirect transmission of influenza and RSV
Virus deposition on surfaces
Humidity can influence indirect transmission via two mechanisms: the mass of respiratory droplets accumulating on surfaces, and the survival of the virus on surfaces. While increased humidity reduces the number of droplet nuclei formed, the same mechanisms (reduced droplet evaporation and faster droplet settling) mean a greater mass of respiratory droplets is deposited on surfaces [Reference Xie13, Reference Yang and Marr14].
Influenza survival on surfaces
A study examining influenza survival over 2½ h in 0·1 μl droplets placed on glass slides at room temperature found survival was lower at 84% RH compared to 24% RH, but survival was greatest at 100% RH [Reference Buckland and Tyrrell27]. At 100% humidity, the influenza virus suspension placed on the slides was still wet after 2½ h, suggesting this was why the virus remained viable. At 24% and 84% humidity, the slides were dry, suggesting that when dry, influenza virus viability appears greater at lower humidity. A similar experiment assessed the survival of influenza in droplets of human mucus placed on culture plates, and found similar results. The experiments were performed over 3 h at room temperature, and the droplet size used was 1 μl. The results show progressively reduced influenza survival with increasing RH over the range from 27% to 84%, with an increase in survival at 99% RH (Fig. 4) [Reference Yang, Elankumaran and Marr21]. The authors hypothesize that at high RH the low evaporation from droplets leaves solute concentrations within droplets relatively unchanged, protecting the virus.
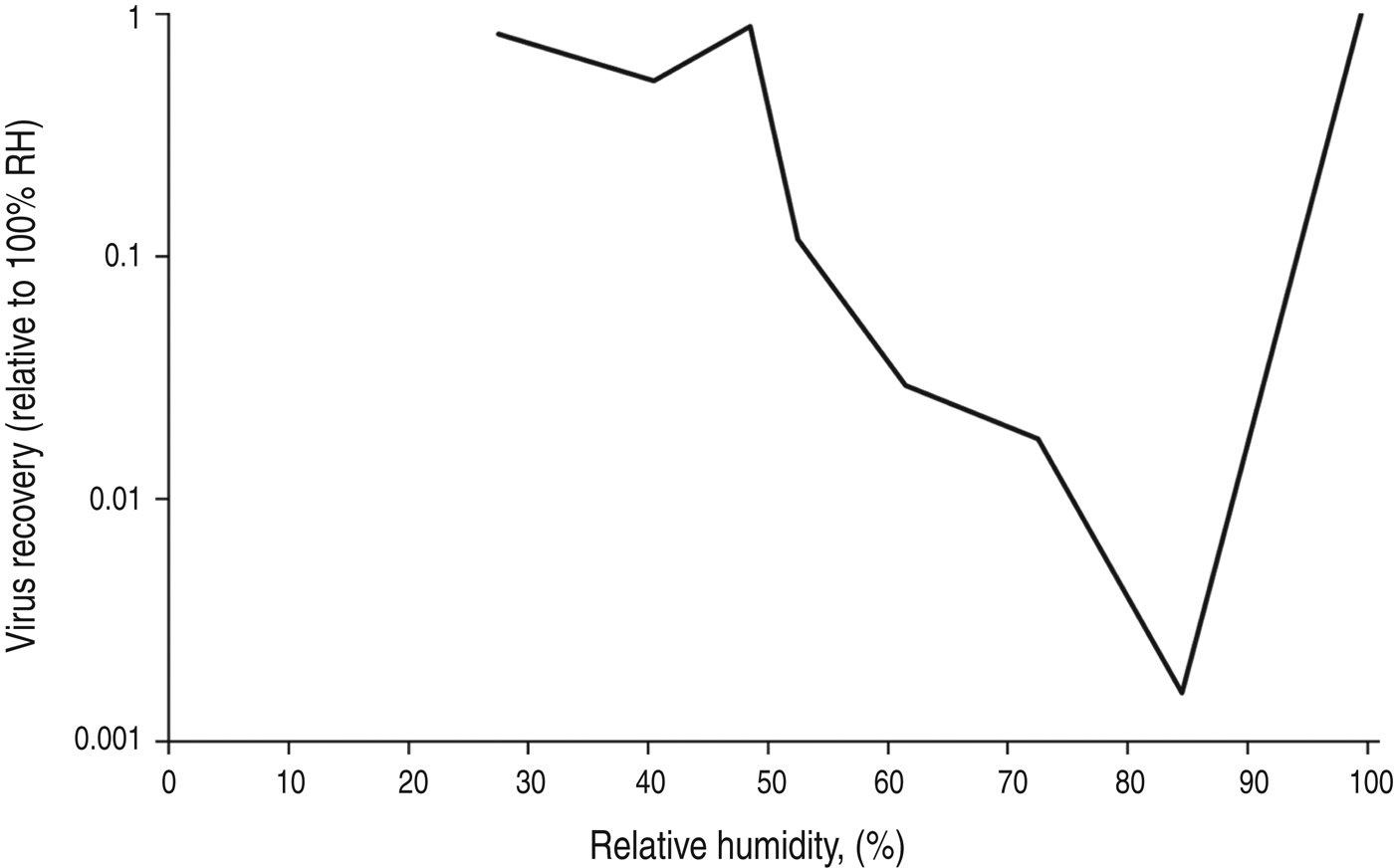
Fig. 4. Influenza survival in 1 μl drops of mucus on a non-porous surface, after 2 h at room temperature. Data from Yang et al. [Reference Yang, Elankumaran and Marr21].
Increased temperature probably reduces influenza survival on surfaces. The studies examining this directly found reduced influenza survival with increasing temperature; however, these studies did not hold humidity constant, so it is difficult to separate out the effects of temperature and humidity [Reference Dublineau28, Reference Edward29]. It is worth noting that influenza survival in water is reduced at higher temperatures, which suggests that increasing temperature will reduce influenza survival in droplets independently of humidity [Reference Dublineau28]. No studies have examined the effect of high temperature combined with high humidity on influenza survival on surfaces.
Animal transmission studies
Contact transmission of influenza between guinea pigs appeared unaffected by increasing humidity and temperature. Four out of four susceptible guinea pigs were infected at 20% RH and 20 °C, while three out of four were infected at 80% RH and 30 °C [Reference Lowen26]. Because infectious and susceptible animals were kept in the same cages in these experiments, it is not possible to tell whether transmission was direct or indirect. Much of the transmission in this study may have been due to direct contact rather than indirect contact. A later study examining indirect contact transmission (by removing infectious animals from cages before replacing them with susceptible animals) found lower transmission rates: only three out of 16 exposed guinea pigs were infected [Reference Mubareka30]. These latter experiments were performed at constant humidity.
RSV survival on surfaces
One study examined the effect of humidity on RSV survival in 1 μl droplets of tissue culture medium on polythene at room temperature [Reference Kingston31]. Over the first 5 h, RSV survival was highest at the highest humidity, while over the next 67 h, RSV survival was highest at the lowest humidity (Table 1). The explanation of these findings may lie in the droplet drying time in this study. Droplets exposed to 77% RH were still wet at 18 h (no data were given for drying times at 32% or 52% RH). The relatively high survival at higher humidity over the first 5 h was probably due to the fact that the droplets remained wet in these conditions. The survival over the final 48 h (when all droplets were dry) was progressively reduced with increasing humidity. Consistent with this explanation, only 1% of RSV was lost over the 72 h when stored in liquid culture medium, and in addition, the authors noted that RSV survival was increased with increased droplet size. Similarly, in another study the survival of RSV on countertops was reduced if the virus was in droplets that were dried quickly [Reference Hall, Douglas and Geiman32]. These results are consistent with the studies examining influenza survival on surfaces, suggesting that while the virus remains ‘wet’ in droplets, high humidity prolongs its survival, by reducing evaporation.
Table 1. Survival of respiratory syncytial virus (RSV) on surfaces at room temperature according to relative humidity (RH). Data from Kingston et al. [Reference Kingston31]

* Relative to survival at 32% RH.
Humidity and the seasonality of influenza and RSV
The reviewed studies suggest that aerosol transmission of influenza decreases with increasing RH, due to a progressive reduction in the amount of aerosol nuclei produced, and a reduction in virus survival in aerosol. Aerosol transmission of influenza also appears to be greatly reduced at temperatures above 30 °C. These results indicate that aerosol transmission would be low during the high temperature, high humidity conditions of tropical rainy seasons. High humidity appears to promote increased survival of influenza and RSV while they are in droplets on surfaces, by slowing the evaporation of the droplets (however, once the droplets are dry, higher humidity appears to reduce survival). In addition, the deposition of respiratory droplets is increased at higher humidity. Transfer of virus from surfaces to hands also appears increased at higher humidity (although this has only been tested up to 65% RH) [Reference Lopez33]. These results suggest that the high humidity found in tropical rainy seasons could favour indirect transmission, however the net effect of high humidity and high temperature in on indirect transmission in these settings needs to be resolved.
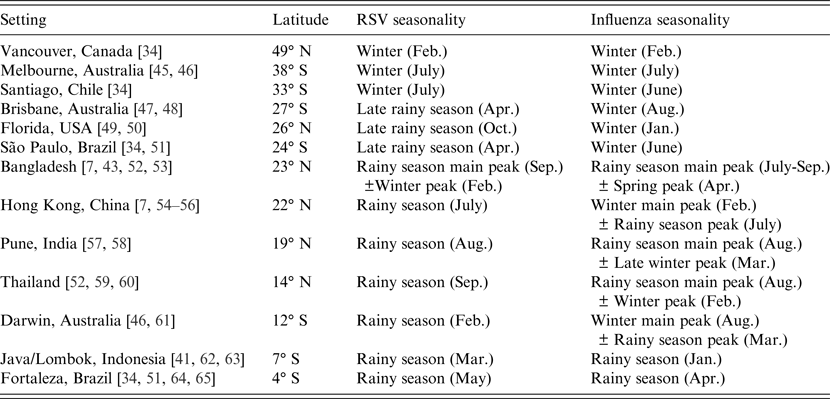
One notable observation of RSV and influenza epidemiology is the differing seasonal patterns in tropical and temperate climates, which have been reviewed in detail previously [Reference Tamerius4, Reference Weber, Mulholland and Greenwood7, Reference Bloom-Feshbach34, Reference Stensballe, Devasundaram and Simoes35]. The general trend of the change in seasonality with latitude is illustrated in Table 2. In the temperate settings (Vancouver, Melbourne, Santiago) both RSV and influenza incidence consistently peak in winter. In the middle latitudes of the subtropical climates (Brisbane, Florida, São Paulo) the different seasonality of RSV and influenza is evident: while influenza still occurs in winter, RSV occurs during the rainy season, several months before the winter influenza peak. In the tropical settings, RSV incidence is usually maximal during the rainy season, while the influenza peak is sometimes associated with the rainy season, and sometimes during winter (and sometimes both).
Table 2. Seasonal patterns of respiratory syncytial virus (RSV) and influenza in temperate, subtropical and tropical settings
The overall trend of these seasonal patterns could be explained by variations in humidity. In the low humidity and low temperature conditions of temperate winters, both RSV and influenza will survive better in the environment, whether in aerosols or on surfaces. In the subtropical settings, the high humidity during the rainy season may improve the transmission of RSV, because RSV is spread predominantly by direct and indirect contact. Influenza, on the other hand, is also spread by aerosol, and this may explain why influenza does not have a clear advantage during the subtropical rainy season, maintaining its peak incidence during the cool winters. In the tropics, the more humid rainy season (combined with warmer winters) may tip the balance, making the rainy season more favourable than the winter for influenza transmission as well as RSV transmission.
This hypothesis offers a parsimonious explanation for the overall trend of the seasonal patterns of RSV and influenza, consistent with the observation that the rainy season appears to encourage RSV transmission more than influenza transmission. However, a number of other environmental factors are known to increase the risk of respiratory infection, and seasonal variations in these may be influencing RSV and influenza transmission as well as (or more than) humidity. These factors may explain a number of exceptions to the overall trend described in Table 2. For example, malnutrition is a known risk factor for respiratory infection [Reference Caulfield36–Reference Paynter38]. In settings with substantial seasonal malnutrition, it is plausible that malnutrition may be a stronger driver of seasonality than climate [Reference Paynter39]. This may explain the seasonality of RSV in settings such as Kenya and Nigeria, where RSV epidemics occur outside the rainy season [Reference Nokes40, Reference Robertson41]. Singapore has high rainfall all year round, with little variation in humidity (the average monthly RH varies between 77% and 82%) [42]. The timing of influenza epidemics is irregular in Singapore: in some years influenza circulates for most of the year, while in others one or two distinct epidemics occur, at different times from year to year [43]. This irregular pattern could be due to the lack of a dominant environmental driver, consistent with the small variation in humidity.
Although there is enough existing data to hypothesize that high humidity may be driving influenza and RSV transmission in tropical settings via indirect contact transmission, more research is required to fill in the gaps in our knowledge of how climate affects the transmission of these viruses. No studies have examined influenza transmission above 80% RH. Few studies have examined transmission at high temperatures, and very few studies have provided data to assess indirect contact transmission. In particular, the interaction between high temperature and high humidity on indirect contact transmission needs further exploration. More can be learned by contrasting the differing epidemiology of influenza and RSV, and transmission models of these viruses incorporating environmental effects will be required to explain the different seasonal patterns of these two viruses.
CONCLUSIONS
For infections with such a high burden of disease, very little is known about how tropical climates affect the transmission of influenza and RSV. Predicting the timing of peaks in RSV and influenza activity can improve disease control. Considerable effort is put into predicting and detecting increases in influenza and RSV activity in temperate climates, where the onset of seasonal epidemics generally varies by a few weeks from year to year. In contrast, in tropical climates influenza epidemics can occur in completely different seasons from year to year, and methods to predict oncoming epidemics will be particularly valuable. Improved knowledge of the drivers of transmission in tropical settings will also enable improved prediction of the consequences of environmental change. Recent projections from the Intergovernmental Panel on Climate Change indicate monsoon systems will intensify, last longer and cover a larger geographical area than currently [44]. If high humidity is driving contact transmission of influenza and RSV, this will clearly have implications for future disease burdens. Finally, improved knowledge of the effects of humidity and temperature on influenza and RSV transmission can be used to modify indoor environments to improve infection control. Our current state of ignorance concerning the environmental drivers of influenza and RSV transmission in tropical settings, and the role of humidity in particular, needs addressing.
DECLARATION OF INTEREST
None.









