INTRODUCTION
In recent years, empirical studies have attempted to assess the effect of endemic pathogens on host fitness and survival [Reference Burthe1–Reference Marzal4], contributing data that support the notion that parasites may play a role in regulating host abundance [Reference Anderson and May5]. Any conclusion that a pathogen reduces survival is based on the assumption that host condition preceding infection is comparable for both those that become infected and those that do not. However, individuals in poor condition may be more likely to contract an infection [Reference Beldomenico6].
Cowpox virus is a pathogen with zoonotic potential [Reference Vorou, Papavassiliou and Pierroutsakos7] that has been the subject of extensive research in wild rodent populations [Reference Burthe1, Reference Telfer2, Reference Bennett8–Reference Telfer12]. Field voles (Microtus agrestis L. 1761), bank voles (Myodes glareolus Schreber 1780) and wood mice (Apodemus sylvaticus L. 1758) are accepted as being reservoir hosts of cowpox virus [Reference Chantrey11–Reference Baxby, Bennett, Webster and Granoff13]. In M. agrestis populations, the virus is endemic [Reference Chantrey11], and may play a role in regulating abundance [Reference Burthe10]. Cowpox infection in rodents is acute and lasts about 4 weeks [Reference Bennett8, Reference Chantrey11], although it causes little obvious clinical disease [Reference Bennett8]. However, by extracting the host's resources and/or inducing a nutritionally demanding immune response, infections in wild rodents may have sublethal effects that affect population dynamics. It has been shown that cowpox is associated with significantly diminished reproductive potential in wood mice and bank voles [Reference Feore14]. Recently, a study reported a negative correlation between M. agrestis survival and cowpox infection in populations from Kielder Forest, UK [Reference Burthe1]. However, as acknowledged in that study, this association may reflect either a direct impact of cowpox infection on mortality rates and/or reflect lower survival in individuals of poorer health and increased susceptibility to infection [Reference Beldomenico15].
With this in mind, a nested case-control study (a retrospective longitudinal study using a subset of data from a prospective longitudinal study, the subset being a selection of observations following criteria that define cases and relevant controls) [Reference Ernster16], adapted to wildlife, was conducted to evaluate the relationship between seroconversion (becoming seropositive to cowpox virus antibody) and proxies of condition (haematological parameters and a measurement of body condition), in order to test the hypothesis that field voles in poorer condition are more prone to seroconvert in the near future (within the following 4 or 8 weeks).
MATERIALS AND METHODS
Sampling procedures
In Kielder Forest (Northumberland, UK), three sites with suitable habitat for M. agrestis were sampled (‘primary sessions’) every 4 weeks over a 2-year period (from April 2005 to March 2007) except for 8-week gaps between November and February [Reference Beldomenico17]. All sites were at the same stage of the multi-annual population cycle [Reference Beldomenico17]. At each site, a trapping grid measuring 50×50 m was established, with 100 Ugglan special live capture traps (Grahnab, Sweden), set at ~5-m intervals. In each primary session, the traps were checked for capture five times, at sunrise and before sunset (roughly 12-h intervals).
Individuals were uniquely and permanently marked on first capture with a small microchip transponder (Labtrac; AVID plc, Uckfield, UK). On first capture within a primary session, each vole was assessed for pelage (juvenile coat, first moult, adult coat), sex and body mass (to the nearest 0·5 g, using a spring balance). Body condition (BODYCOND) was evaluated by estimating by palpation the degree of fat and muscle cover over the vertebral column and the pelvic bones, giving a score between 2 and 10 [Reference Beldomenico17]. Blood was directly taken from the tail-tip into a heparin-coated capillary tube for haematology and into an Eppendorf tube to obtain sera for serology.
Haematological parameters
As described in detail by Beldomenico et al. [Reference Beldomenico17], a haemogram was produced from blood collected in the capillary tube. Briefly, 2 μl of non-coagulated blood were diluted 1:20 in 4% acetic acid with 1% Crystal Violet and 1:5000 in PBS, to count white blood cells (WBCs) and red blood cells (RBCs), respectively, using Kova Glasstic® (Hycor Biomedical Ltd, Penicuik, UK) slides with grids and hence determine their concentration. The rest of the blood sample was used to produce blood smears for differential WBC counts, which allowed the proportion of each WBC type and their concentration to be estimated. Smears were air-dried, fixed with methanol and stained with Rapid Romanowsky Stain Pack – HS705 (HD Supplies, Aylesbury, UK). RBCs (as millions/μl) and lymphocytes (cells/μl) were the haematological proxies of condition. Low peripheral lymphocyte counts (lymphopenia) are an indication of immunosuppression or poor immunological investment, and low RBC counts (anaemia), an indication of poor aerobic capacity [Reference Beldomenico17].
Assessing seroconversion
Antibody to cowpox was detected in sera by immunofluorescence assay [Reference Crouch18]. Briefly, sera were diluted 1:10 and 1:20, and incubated on fixed, cowpox virus-infected Vero cell monolayers, which were then washed; bound antibody was detected using a commercial fluorescein isothiocyanate-conjugated anti-mouse antibody. The protocol was optimized using serially diluted known positive and negative sera, and is highly specific for antibody to orthopoxviruses [Reference Crouch18].
In rodents, antibodies to cowpox virus arise about 2 weeks after an initial infection and remain high thereafter [Reference Bennett8, Reference Chantrey11]. Infection with cowpox virus was approximated by seroconversion in a 4-week period (i.e. a seronegative vole becomes seropositive at a second sample taken 4 weeks later).
Selection of cases and controls
Field data consisted of 3494 observations from 1574 field voles. The analysis was restricted to data collected from April to November in 2005 and 2006 (4-week inter-sample interval). Juveniles (individuals with a juvenile coat and ⩽17 g in weight) were excluded as they may harbour maternal antibodies. The study unit was a ‘vole month’ (i.e. observations made on an individual vole two primary trapping sessions apart). The 372 observations that allowed the analysis originated from 293 repeatedly sampled voles. A ‘case’ was a vole which was caught seronegative after juvenile age, but then seroconverted in the following 4-week period. Because individuals that did not seroconvert in such 4-week periods (potential ‘controls’) were more abundant than cases in spring and early summer, but less abundant in late summer and autumn, a balanced design matching a single control to every case was not possible. Controls were therefore individuals caught simultaneously (at the same trapping session as cases, but not necessarily at the same site) that had not been found seropositive after juvenile age, but that did not seroconvert in the following 4 weeks. Multivariable analyses allowed adjustments to be made for the imbalances. Because control individuals might have been infected in the second sample despite still being currently seronegative, individuals that had seroconverted by their next sample were excluded when these data were available. Nonetheless, for 79 controls, no sample following the seronegatives–seronegative sequence was available, although these were included in the analysis in order to provide controls for all cases. In total, the number of observations designated as cases was 117, and there were 255 controls.
Statistical analysis
The hypothesis investigated was that individuals in poorer condition are more likely to contract cowpox virus infection, as measured by seroconversion. It takes 2 weeks for antibodies to rise, and therefore an individual that was seronegative at sample 1 and seropositive at sample 2 had, theoretically, a 50% probability of being recently infected (i.e. within the previous 2 weeks) at the time of the first sample [Reference Telfer2]. Hence, a good approach would be to investigate whether poor condition increased the probability of seroconverting between 4 and 8 weeks later, as this would effectively eliminate the possibility of a case being infected at sample 1. However, the small number of voles that were seronegative in two consecutive sessions and seropositive on a third (cases, n=44), and voles that were seronegative in three consecutive sessions (controls, n=130), did not allow the multivariable analysis needed. Therefore, we used a 4-week period from last-seronegative to first-seropositive to develop a final model, but the latter was then re-assessed and validated by checking for seroconversion 8 weeks later with the 174 individuals (44+130) that allowed such analysis.
The multivariable analysis was conducted using generalized linear mixed effects models with random intercepts (GLMMs) and a binomial response, using the function ‘lmer’ in the ‘lme4’ library of R (The R Foundation for Statistical Computing; http://www.r-project.org/). The response was ‘seroconversion’ and the explanatory variables of interest were RBC counts (millions/μl), lymphocyte counts (cells/μl) and BODYCOND. The analysis was conducted using the ‘Laplace’ method for GLMM.
Given that a design in which cases and controls were balanced with regard to potential confounders and effect modifiers was precluded, the following variables had to be included in the analysis to correct for this unbalance [Reference Kleinbaum19]: seasonality, by using two sinusoidal components (SEASON[sin]+SEASON[cos]) [Reference Beldomenico17], SEX, YEAR, AGE and DENSITY, together with all pairwise interactions. DENSITY was estimated in the program mark [Reference White and Burnham20] using Huggins' closed capture models within a robust design [Reference Huggins21, Reference Kendall and Nichols22] and mixture models [Reference Pledger23] to allow heterogeneity in capture probabilities. AGE was approximated using a dichotomous variable. ‘Adult’ and ‘young’ individuals were distinguished on the basis of capture histories as being older and younger than 90 days, respectively. In the absence of sufficient trapping history, non-juveniles were classified as adult if they weighed ⩾22 g, and otherwise classified as young. Once classified as an adult, individuals remained so for every successive capture.
Based on a model that included all these terms, Akaike's Information Criterion (AIC) [Reference Akaike24] was used to determine the best density lag (time lags 0, 3 or 6 months) for inclusion in the maximum model. AIC was also employed to decide whether to use AGE or body weight as explanatory variables, as they were highly correlated. Subsequently, starting from this maximum model, terms were removed unless they reduced AIC by more than 2 U when included. The random effect SITE*SESSION (the interaction between site and month) was added prior to model restriction to control for lack of spatial and temporal independence of observations [Reference Telfer12]. However, due to the relatively low number of captures per animal, it was not possible to control for individual autocorrelation by including as a random effect in the maximum model the unique identification number given to each vole (VOLE_ID). VOLE_ID, nonetheless, was added to the restricted model to verify that accounting for non-independence of samples from the same animal did not alter the results. The validation of the model using individuals that seroconverted 8 weeks later excluded random effects, because of the small sample size.
RESULTS
The probability of seroconverting in the following 4 weeks was correlated with RBC counts and body condition prior to seroconversion (Table 1, Fig. 1). A poor body condition significantly increased the probability of seroconversion. Although RBC counts were not associated with the probability of seroconversion in females, or in males with good body condition, anaemic males in poor body condition had the highest probability of seroconversion. (When interaction terms were removed from the model, the effect of body condition remained significant, but that of RBCs disappeared.). Both RBC counts and body condition remained significant and their coefficients were similar when the model was reassessed using the probability of seroconversion in 8 weeks for the reduced dataset (Table 1). In some circumstances the influence of condition was great. When a susceptible male with good body condition (score=8) had a relatively low probability of becoming infected (around 20%), a susceptible male with poor body condition (score=4) was twice as likely (around 40%) to contract cowpox; if this male was also anaemic the chances of cowpox infection were almost quadrupled (around 75%) (Fig. 1). Including VOLE_ID did not have a substantive impact on the final model.
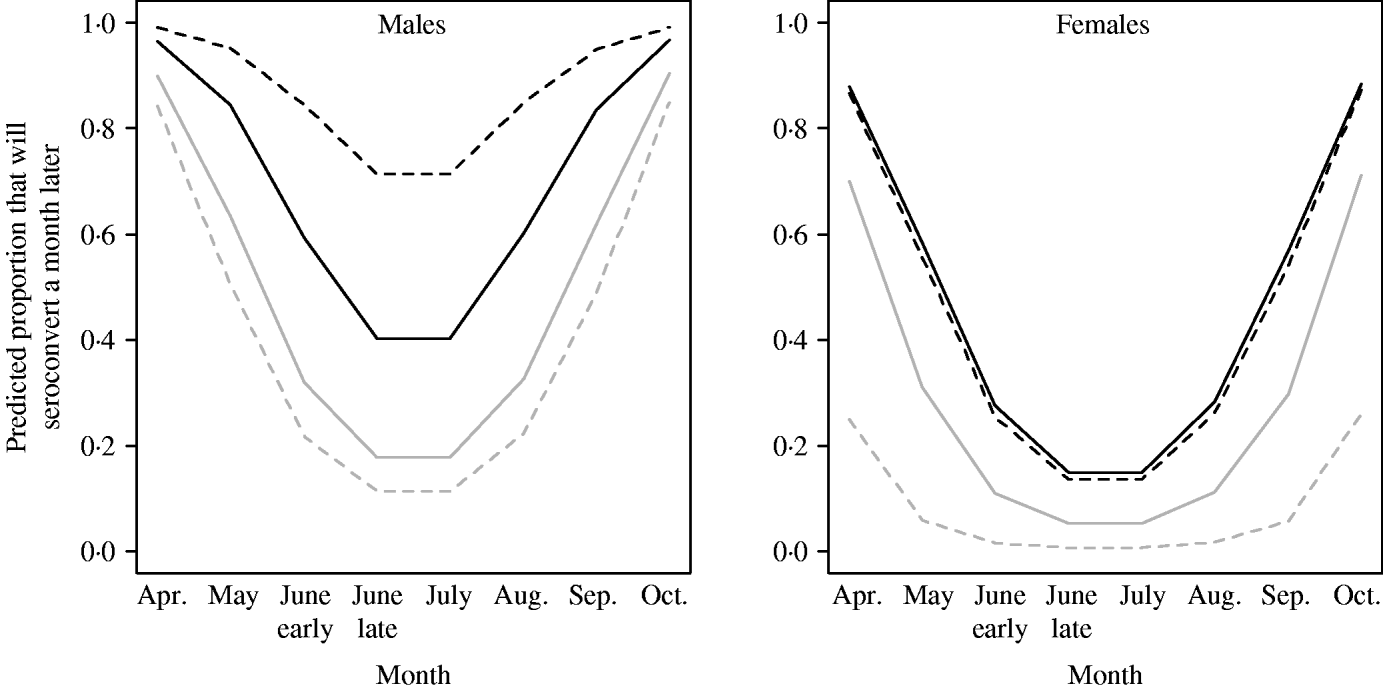
Fig. 1. Predicted probability of seroconverting as simulated by GLMM for 2005 (the seasonality was different in 2006). Variation by sex, month, body condition score (4=black lines; 8=grey lines) and RBCs (past density fixed at 50). In the simulation, anaemic (- - -) represents individuals with 3 million RBCs/μl, and normocytic (–––) represents voles with 8 million RBCs/μl.
Table 1. GLMM showing variables associated with the probability of seroconversion in a 4-week period, and the same model without the random effects and non-significant terms, run with individuals that were seronegative for two consecutive months and seroconverted or not in a third sample
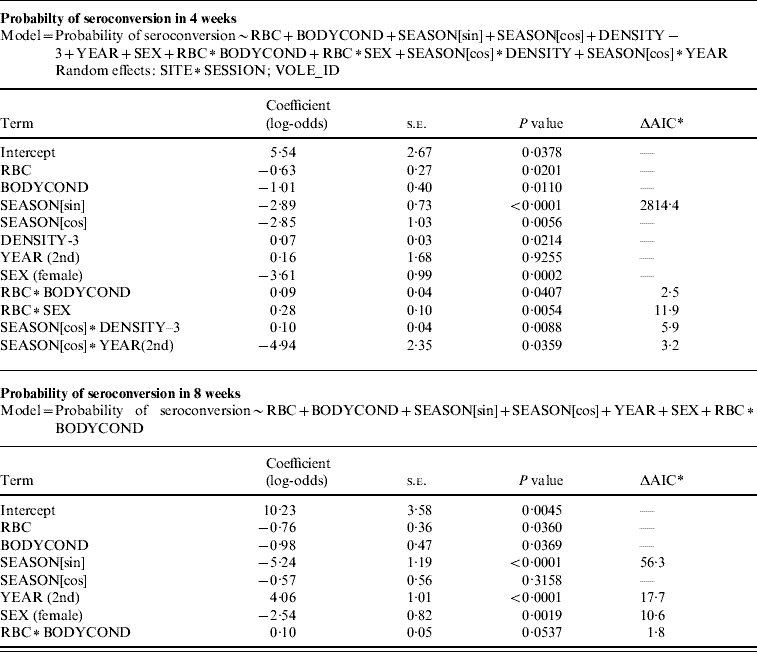
* AIC value increment if the single term is dropped.
DISCUSSION
The association of poorer condition with higher probability of infection observed here probably represents an increased vulnerability to infection, rather than an early effect of cowpox in some of the cases, as the same significant associations, with similar magnitudes, were found with the probability of seroconverting 8 weeks in the future. Therefore, our results support the hypothesis that field voles in poor condition are more prone to acquire cowpox infection.
Lochmiller's hypothesis that an underlying dynamic in immunocompentence is key to explaining the natural regulation of animal populations [Reference Lochmiller25] has previously been supported by empirical data generated from M. agrestis populations: by the observation that high past densities and poor body condition were associated with lower immunological investment and decreased immune response [Reference Beldomenico17], by lower levels of indicators of condition being followed by increases in indicators of infection [Reference Beldomenico6], and by the finding that poor condition preceded lower survival rates [Reference Beldomenico15]. The present study further supports this hypothesis by showing that the dynamics of infection by a specific endemic pathogen are dependent on the condition of the hosts. Host vulnerability to infection, therefore, seems likely to play a key role in infection dynamics.
Lochmiller's hypothesis would be further supported if it could be shown that this increased vulnerability to infection originates from a decreased immunocompetence, especially as condition may also affect infection through behavioural differences, e.g. individuals in poor condition may be at increased risk of contact with infected conspecifics because they need to forage more. This link between immunocompetence and infection has been demonstrated for humans and domestic animals [Reference Chandra and Kumari26, Reference Louria27], but it has not been established for wildlife. Here, the failure to find an association with lymphocyte counts suggests that immunocompetence may not be playing a role. However, lymphocyte counts in voles may have ambiguous meanings [Reference Beldomenico17]. Apart from being a measure of immunocompetence, lymphocyte counts also tend to rise in some chronic infectious diseases. Thus, for example, it was found that lymphocyte counts were not associated with field vole survival when other indicators of chronic infection (monocytes) were high [Reference Beldomenico15], but they were positively correlated with survival when monocyte levels were normal. Hence, further studies (perhaps with experimental approaches) are required to identify the proximate causes behind the proneness to infection of those in poor condition, or to rule out alternative explanations for the association between condition and likelihood of becoming infected.
Males in this study were at a higher risk of becoming infected, as previously observed [Reference Burthe10]. This may be due to behavioural differences, or could be related to the immunosuppressant effect of testosterone [Reference Grossman28], which may predispose them to infections. In rodents, plasma levels of testosterone are substantially increased during the breeding season [Reference Schradin29], and sex ratios turn from being male biased in winter to female biased in summer [Reference Beldomenico15, Reference Pusenius and Viitala30, Reference Agrell31]. This suggests a coincidence between high testosterone levels and high male mortality. This could be explained by aggressive behaviour when testosterone is high, although, to the contrary, it has been observed in voles that spring mortality is least pronounced when there are more signs of fighting and most pronounced when wounding levels are lower [Reference Boonstra and Boag32]. Alternatively, therefore, this poor peri-mating survival of males, especially prominent in species with short breeding seasons [Reference Lee and Cockburn33, Reference Boonstra, McColl and Karels34], may result from a differential trade-off between immunocompetence and reproduction, making males more susceptible to infection [Reference Boonstra, McColl and Karels34]. This is in agreement with the findings of the present study, which mostly correspond to the breeding season: April–November. The risk of cowpox infection was greater for males, and it was only in males that anaemia seemed to have an effect on susceptibility of infection. However, it should be acknowledged, that dispersion may carry additional risks and therefore contribute to the differential mortality of males. Further studies should be carried out to confirm the proximate determinants of male mortality.
The delayed effect of field vole density on cowpox incidence, which has been documented in detail by a previous study of the same populations over a different time period [Reference Burthe10], is important, as host density also has a delayed negative effect on host condition in vole populations [Reference Beldomenico17, Reference Huitu35]. The implication of this is that elevated host abundance may not only be followed by higher cowpox infection due to increased contact rates between infectious and susceptible hosts, but also due to an increased vulnerability to infection, as previously proposed [Reference Burthe1].
The previous study of cowpox infection risk [Reference Burthe10] failed to find an association with either body condition or season but suggested that risk increased with weight, contrary to the present findings. However, besides differences in data subset selection criteria, period of the year investigated, and model restriction procedures, in that study it was body condition at the time of the first seropositive record that was investigated. Hence, the impact of body condition preceding infection was not considered. The differences in seasonality seem likely to be related to the cycles of abundance exhibited by these populations [Reference Begon9], since that previous study took place immediately before an abundance peak (late 2003), the next peak occurred in 2007 (1 year after the present study), and the seasonal dynamics of cowpox vary with past density [Reference Begon9]. Phase-related differences may also account for the differences with regard to body weight (body weight was added to the final model in this study, finding no effect).
Our results highlight the special care needed when interpreting the findings of observational wildlife disease studies on the effects of pathogens, as infected individuals might also be those that are more vulnerable and would have a decreased longevity regardless of the infection. Longitudinal studies that allow demonstration of putative causes preceding the effect investigated are a priority. Moreover, infection with one pathogen may increase the likelihood of being infected by other pathogens [Reference Telfer36], such that the impact measured could be that of many parasites combined. Further still, the health status of an individual may result from the synergistic effects of infection and poor condition [Reference Beldomenico6]. Hence, establishing the impact of a specific pathogen poses a challenge that warrants the exploration of novel approaches. Whenever possible, a measure of host condition prior to infection should be included. The results here, and those of previous studies [Reference Beldomenico15, Reference Beldomenico17], show that even simple and inexpensive measures may be useful in estimating host condition. To our knowledge, this work is the first in directly adapting the nested case-control study design to wildlife disease investigation (but see a similar approach in Burthe et al. [Reference Burthe37]). Future studies should consider increasing the trapping effort to allow a more rigorous assignment of controls. We believe that other initiatives using similar adaptations would be beneficial.
ACKNOWLEDGEMENTS
We thank Gill Telford, Roslyn Anderson, Jenny Rogers and Gemma Chaloner for their assistance in the field, Gill Hutchison, Cathy Glover and Chris McCracken for the cowpox diagnostics, Sue Jopson for her work in the animal house, and especially Sarah Burthe for her comments on the manuscript. This work was funded by Wellcome Trust grant 075202/Z/04/Z to M.B. and a NERC Dorothy Hodgkin Award to P.M.B., and licensed under Home Office project licence PPL40/1813.
DECLARATION OF INTEREST
None.




