Introduction
Varicella infection during pregnancy has serious and/or difficult implications and in some cases lethal outcome. Varicella-associated pneumonia and death are more common among pregnant women [Reference Harger1]. Foetal varicella infection carries up to a 2% risk of congenital varicella syndrome (CVS) characterised by limb, eye and brain abnormalities [Reference Pastuszak2]. Varicella behaves as a childhood disease in temperate regions while it affects significant numbers of adolescents and adults in tropical regions. Large-scale seroepidemiological studies evaluating varicella susceptibility in India are lacking. In a study evaluating 778 health science students with a median age of 21 years, 25.8% were susceptible [Reference Arunkumar3]. In another study involving 5000 nurses with age between 21 and 30 years, 28% were susceptible [Reference Shah4]. An epidemic investigation in South India revealed that 24% of the people infected were 16 years and older. Similarly, 63% of the people infected in rural North India were at least 15 years with a mean age of 23.4 years [Reference Balraj and John5, Reference Sinha6]. Data from epidemics in various parts of India also highlight the high attack rate among age groups 16–25 years suggesting susceptibility [Reference Balraj and John5–Reference Venkitaraman7]. Hence, a high proportion of women of childbearing age likely remain susceptible in India. However, this proportion has not been studied so far.
Varicella in pregnancy poses some unique challenges. Avoiding exposure to varicella is difficult as the infectivity period starts a few days before the onset of the characteristic rash. Second, varicella immunoglobulin (VZIg), the treatment of choice, is not widely available in India and most developing countries. Finally, risk assessment for foetal anomalies involves the studies of the foetal blood or amniotic fluid and advanced sonological studies, which require considerable expertise and infrastructure.
An effective, safe, live attenuated Oka strain varicella vaccine has been available globally since 1995[Reference Woodward8]. Before the vaccine was widely available in the private sector, 3.4% and 31.1% among the urban and rural Indian population remained susceptible, suggesting significant epidemiological differences within the country [Reference Goh9]. Another study across four cities of India reported 31.8% of the population were susceptible to the age-related increase in seroprevalence rate from 1 to 40 years [Reference Lokeshwar10]. Epidemiological studies in developing countries reveal that a significant proportion (Sri Lanka – 34%, Iran – 10. 7%, Egypt – 11.7%) of patients may remain susceptible during pregnancy [Reference Premathilake11–Reference Ibrahim13]. As for our knowledge, varicella susceptibility rate and positive predictive value (PPV) of the history of chickenpox among pregnant women have not been evaluated in the Indian subcontinent. We attempted to address those questions in our study. Currently, India does not have a vaccine policy with regard to varicella-zoster virus (VZV) and vaccination against varicella is not included in the National Immunisation Program and findings from our study could provide newer insights.
Materials and methods
Study setting and participants
We conducted a cross-sectional study of pregnant women attending antenatal clinics in Bangalore Baptist Hospital, a tertiary care centre in Bangalore, Karnataka and Rural Development Trust Hospital, a rural secondary hospital in Bathelapalli, Andhra Pradesh between October and December 2019. The former conducts approximately 3000 deliveries with 7000 pregnant women registered for antenatal care in a year while the latter conducts approximately 4200 deliveries with 9000 registered pregnant women in a year. Considering the seroprevalence of 66% in Sri Lanka, the sample size was calculated as 430 (d – 5%, design effect – 1.2) [Reference Shah4]. Pregnant women with a confirmed pregnancy by either a positive urine pregnancy test or ultrasound with a period of amenorrhea for more than 4 weeks were recruited after informed consent.
Data collection and sample processing
Data were collected using a pre-tested interviewer-administered questionnaire during their routine visit to the hospital for antenatal care. A positive history of varicella was defined as a report of a generalised blistering rash with fever at least 2 weeks before sample collection. Positive vaccination history was defined as recalling having at least one dose of varicella-containing vaccine at least 2 weeks before sample collection.
The presence of anti-varicella IgG in serum samples was determined by the quantitative anti-VZV IgG ELISA (enzyme linked immunosorbent assay) from Euroimmun (Lübeck, Germany) using highly purified VZV antigens of strain Ellen coated on the microtiter wells. The optical density measurement and calculations were done on an automated ELISA equipment as per the manufacturer's instructions. As per the manufacturer's recommendation, the results were interpreted as positive when ≥ 110 IU/l and negative when <80 IU/l. Results between 80 and 110 IU/l were considered equivocal. Ten positive and 10 negative samples were tested in parallel in a reference laboratory for verification of accuracy.
For added quality assurance of the ELISA runs, 10% random sample repeat of the entire specimen collection was performed within runs. Samples that initially tested equivocal were re-tested. The samples which remained equivocal again were considered susceptible for statistical analysis.
Statistical analysis
Data were entered into Microsoft Excel 2010 version and analysed using SPSS version 20.0 (Chicago, Illinois, USA). The terms ‘susceptibility’ and ‘immunity’ are used here to represent the absence or presence of varicella-specific IgG, respectively. Seroprevalence was calculated as the number of cases positive for varicella IgG divided by the number of examined sera. Socio-demographic factors were tested for their association with susceptibility using χ 2 test. Based on the bivariate analysis, we assumed that a variable with P < 10% was a confounder. A binary logistic regression was done to adjust for potential confounders such as age (≤30/>30 years), place of residence (rural/urban), education (lower/higher), husband's education (lower/higher), history of chickenpox (yes/no), centre for receiving antenatal care (urban tertiary/rural secondary) with susceptibility and reported as adjusted prevalence ratios with 95% confidence intervals. General assumptions of logistic regression were considered and we did not assume any interactions or collinearity. P < 0.05 was considered significant. Goodness of fit was assessed using Hosmer–Lemeshow statistic, Cox and Snell R 2, and Nagelkerke R 2, which are described at the bottom of Table 2. The presence of varicella IgG was considered as gold standard and sensitivity, specificity, negative and positive predictive values were calculated for a positive history of infection and reported with 95% confidence intervals.
Results
We prospectively recruited 430 pregnant women from 18 to 39 (25.3 ± 4.3) years of age. Almost half of them (54.7%) resided in rural areas, and 56.7% were primigravida. Half of the women (49.8%) had completed higher education (above high school), and 84.9% were housewives. More than half (51.6%) gave a positive history of previous chickenpox, and 8.1% were uncertain about chickenpox infection in the past (Table 1).
Table 1. Factors associated with VZV serological status
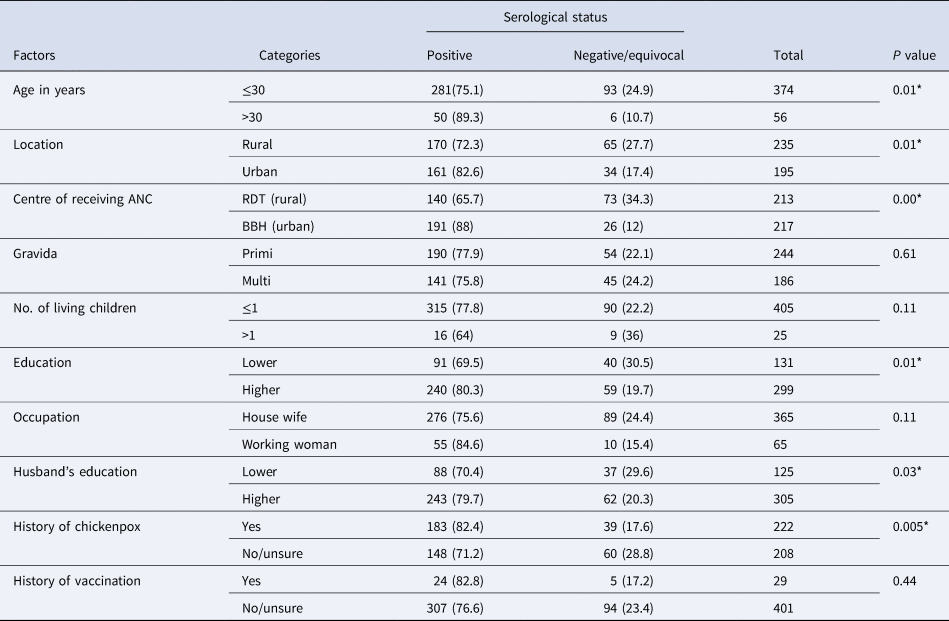
*Significant P value.
VZV IgG was present in 331 (76.9%, 95% CI 72.7–80.9) participants and three (0.6%) had equivocal results. History of chickenpox in the past had 55.3% (95% CI 49.9–60.6) sensitivity, 60.6% (95% CI 50.9–70.2) specificity, PPV of 82.4% (95% CI 77.4–87.4). Of the 222 women with a positive history of varicella, 39 (17.5%) were VZV IgG-negative. A small proportion (6.7%) of the women reported having taken the varicella vaccine in the past.
Susceptibility was 1.85-fold (adjusted prevalence ratio −1.85, 95% CI 1.15–3.0, P = 0.01) greater with a negative history of chickenpox. Similarly, antenatal care from rural secondary hospital was associated with four times increased rate of susceptibility (adjusted prevalence ratio 4.08, 95% CI 2.1–7.65, P = 0.00) compared to those who received antenatal care from a tertiary care urban hospital (Table 2). We also found that the proportion of women with susceptibility was highest in the age group of 21–25 years (47.4%), decreasing with age (Fig. 1). Among those who are susceptible, one-fifth of them (20.2%) gave a history of exposure to varicella sometime in the past and 2.02% of them during the current pregnancy. Only a quarter (24.4%) of the study population reported that they have heard of vaccines for varicella.
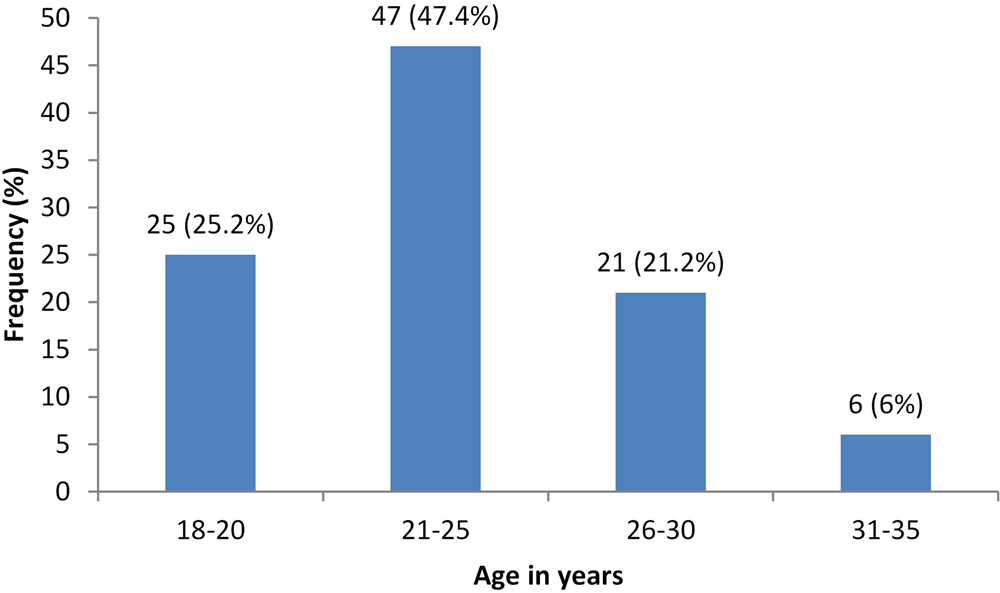
Fig. 1. Age specific susceptibility rates for varicella in our study population.
Table 2. Adjusted prevalence ratio for the susceptibility of varicella infection among pregnant women
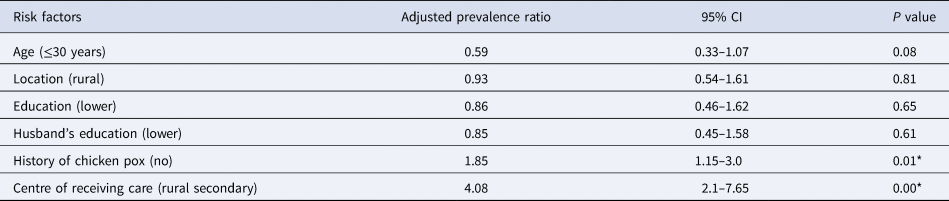
R 2 = 0.09 (Cox and Snell), 0.31 (Nagelkerke), Model χ 2(6) = 40.73.
*Significant P value.
Discussion
Our study highlights high VZV susceptibility, especially among younger, rural pregnant women in southern India. It also mirrors data from others regarding the high proportion of the susceptible population in India and other South Asian countries [Reference Premathilake11–Reference Lee14]. This epidemiological pattern is also reflected by the high burden of varicella-zoster among young adults during epidemics [Reference Balraj and John5, Reference Sinha6, Reference Meyers15].
Ongoing varicella transmission, through early adulthood, ensures near-complete protection by around 35 years, as reflected in our study. Unfortunately, this age group is interspersed by periods of pregnancy. The mean age of mothers at the time of first childbirth is around 22 years in most countries of South Asia, and according to the National Family Health Survey-4, 27% of women aged 20–24 married before the age of 18 in India [16, Reference Bongaarts and Blanc17]. A significant number of susceptible participants (22.2%) in our study reported varicella exposures during the current pregnancy or in the past.
The poor PPV of self-reported history is an important finding from our study. Typically, the PPV is more than 95% among pregnant women across the studies done in the temperate regions [Reference Premathilake11, Reference Watson18]. Many authors have concluded that maternal history can be used safely to exclude patients from serological testing [Reference Coyle19, Reference Holmes20]. This is not only true in temperate regions with low susceptibility rates but also in countries like Sri Lanka [Reference Premathilake11]. This poor PPV (82.4%) noted in our study has important implications in vaccination strategies. Serological testing may be required to identify women who may benefit from the vaccine. While patient factors such as education may play a role, the relative prevalence of diseases that can mimic varicella is also significant. Viral exanthems such as measles and rubella remain prevalent in the Indian setting [Reference Sarkar, Mishra and Garg21]. India has also emerged as a hotspot of Rickettsial illnesses such as a spotted fever which can be mistaken for varicella [Reference Dincy22].
In the context of India and other developing countries, VZIg is not routinely available. While human intravenous immunoglobulin can be used as a substitute, it is expensive, requires multiple doses and often its varicella IgG titres are not known. Ruling out foetal infection after exposure is also difficult in developing countries as the facilities for cordocentesis or amniocentesis for varicella DNA or antibodies and imaging expertise for diagnosing CVS are frequently not available.
Varicella vaccine can overcome these challenges, and has been shown to decrease CVS burden [Reference Khandaker23]. The cost-benefit of varicella vaccination against VZIg is established in the context of pregnancy [Reference Troughton24]. However, being a live attenuated vaccine, it cannot be used during pregnancy and should be administered in the susceptible population in the preconception period. Similarly, the knowledge and uptake of varicella vaccine were also poor among the study population. It implies the need for community sensitisation and periconceptional counselling on varicella vaccines.
To our knowledge, this is the first study to document the susceptibility of varicella among pregnant women in the Indian setting. While the inclusion of participants from an urban and rural setting with an adequate sample size was the strength of our study, it has some limitations. Our study could potentially underestimate varicella exposure during pregnancy, as it shows a point estimate. A follow-up through the period of pregnancy will estimate the risk better. A repeat serology at the end of pregnancy in the susceptible population and documenting foetal outcomes would also be useful. Detailed data on the type of exposure and outcomes following exposure were also not collected.
Conclusion
Many Indian young pregnant women remain susceptible to VZV. Our data show that self-reported maternal history of varicella cannot be relied on as a surrogate for protection in this high-risk group of patients. We suggest that serologic testing is essential to recognise patients who would benefit from the vaccine in the pre-pregnancy period. We hope these preliminary data, in addition to others, will enable the adoption of the varicella vaccine in the National Immunisation Schedule to protect this high-risk group of patients.
Data
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
We would like to acknowledge the assistance of Mary, Sasikala and Dr Srinithya in data collection, Tata Rao, Ayisha Ali and Harini in data entry, Mahalakshmi and Pradeep in sample processing. We extend our thanks to Dr Bhagteshwar Singh for his critical comments to improve the content of this paper.
Author contributions
LR conceived the idea, developed the study instrument, conducted the analysis, interpreted data, prepared the manuscript. SC conceived the idea of the study, supervised sample processing and analysis in the laboratory and reviewed the manuscript. NA and JS conceptualised and assisted with the design of the study, tool development, collected the data and reviewed the manuscript. AM participated in the conception of the study, tool development, interpretation of the data and preparation of the manuscript. All authors read and reviewed the final manuscript.
Financial support
Research grant, Bangalore Baptist Hospital.
Conflict of interest
None.
Ethical standards
This study was approved by the Institutional Review Board of Bangalore Baptist Hospital. Written informed consent was taken from all the participants of the study.






