INTRODUCTION
Gonorrhoea (GC) remains a global public health problem, particularly in resource-poor populations [Reference Pultorak1]. GC is curable with administration of appropriate antibiotics (although antibiotic resistance is an increasing problem [Reference Bolan, Sparling and Wasserheit2]); however, in many populations, as many as 67–100% of all GC infections are asymptomatic [Reference Wilkinson3]. Asymptomatic infections are far less likely to be treated, making them an important driver of transmission. Moreover, GC symptoms can be mild and mistaken for self-limiting conditions [4] and treatment is not sought. Social pressures (e.g. stigma), culture (e.g. hygiene and sexual behaviour), and access to care all influence whether infected individuals are likely to seek treatment [Reference Wilkinson3].
We estimated the prevalence of GC, co-infection with chlamydia, and demographic and ecological correlates of positive GC status in the semi-nomadic, subsistence-living pastoralists of Kaokoland, Namibia [Reference Frank, Bollig and Gewald5–Reference Vedder7]. Anthropologists and local healthcare workers have long suspected that the GC burden is high in this region [Reference Talavera8] and in related tribes [Reference Pennington and Harpending9], because partner concurrency is common [Reference de la Torre10] – 51% report concurrent sex partners – condom use is rare (A. Hazel, unpublished observations), and treatment – syndromic management exclusively – is difficult to access.
METHODS
Field site and recruitment
Data were collected from April to November 2009 in 28 villages in Kaokoland, northwestern Namibia. Village sites were selected (based on a census conducted to develop an understanding of the variation in population density, subsistence behaviour, and tribal affiliation throughout rural Kaokoland) to represent the ecological, geographical and economic diversity of the most remote regions of Kaokoland. The Himba tribe, which dominates this area of Kaokoland, lives in close proximity with several other tribes that were also included in the study. Herein, we refer to them collectively as the ‘Kaokoland pastoralists’. There are no comprehensive survey data for population size in Kaokoland. Our census estimates that there are a little over 1800 adults overall in our village data collection sites. Previous anthropological work and projections from district-level census data suggest that there are about 20 000 Himbas [Reference Bollig, Bollig and Bubenzer11], but it is not clear if this number includes individuals from related tribes who live alongside Himbas. Data were collected over all three recognized seasons: rainy, winter, and dry seasons.
Population density varies across seasons as pastoralists move between the traditionally recognized Kaokoland regions, due to their semi-nomadic lifestyle. Participants were often recruited while living in areas separate from their ‘home’ village. Upon entering a village, we conducted a brief census; if fewer than two consenting adults were present, we did not collect data in that village at that time (if possible, we returned to the village later). We targeted highly clustered villages in order to aggregate them for analyses if sample sizes were too small. We set a threshold of two participants to minimize the possibility that a sole resident would feel unduly pressured. Permission to conduct this research was given by the University of Michigan's Internal Review Board (HUM00025104) and the Namibian Ministry of Health and Social Services (MoHSS). We also received permission from each local chief before establishing a village as a study site.
We recruited any male or female volunteer who was (a) a culturally recognized adult (i.e. women ⩾16 years; men ⩾18 years), and (b) who resided in Kaokoland for at least six, non-consecutive months of the year (this criterion was to address traders or cattle workers who often are transient inhabitants). We used a convenience sampling method and interviewed any willing adult who met the above criteria. It was very difficult to accurately estimate the response rate for this study. In the larger villages (n∼1000), we directly invited only a small proportion to participate, but word spread rapidly (as villagers were illiterate, posters and fliers were not used). This makes it difficult to determine the number invited to participate relative to those who did participate. By contrast, in small villages, the study team was able to visit all the homesteads and directly invite all adults; in these instances, the response rate is easier to estimate. The proportion of the village populations (based on our census) that participated ranged from 0·02 (largest villages) to 0·70 (mean 0·33, median 0·30). We are confident that our recruitment method did not bias our sample with positive volunteers seeking treatment because most of our participants, as discussed in the Results section, reported no current or recent symptoms nor did many seek treatment within the past 6 months. In accordance with the terms of MoHSS research approval, we took to the nearest clinic for treatment any village member who presented with symptoms of a bacterial sexually transmitted disease (STD) or who reported a partner with symptoms for treatment.
Sample collection
Our study focused on regions of Kaokoland that are so remote they are not connected to a power grid. Therefore, we developed a sample collection protocol that did not require cold storage of any materials.
Male participants submitted a self-collected urethral swab sample; females submitted a self-collected vaginal swab sample. Self-collected swabs are as reliable as clinician-collected swabs [Reference Knox12] and our participants appeared to understand collection instructions clearly. We ensured participants had not urinated within the hour prior to sample collection and that women had not dried their vaginal tissue – a common local practice that is performed for hygienic purposes as well as in preparation for sexual contact (A. Hazel, unpublished observations).
We stored sample material using FTA cards (Whatman, USA). Upon contact with the chemical matrix in the FTA card, cells are lysed, proteins are denatured, and DNA is held stable. FTA technology allowed us to store our samples for several months while data collection continued. At the end of the data collection period, the FTA cards were shipped to the University of Michigan for diagnostic qPCR. The long-term viability of bacterial DNA stored in FTA cards has been confirmed [Reference Rajendram13]. More recently, FTA cards have been demonstrated as an effective storage tool for molecular diagnosis of GC [Reference Chi14]. Prior to data collection, we tested the sensitivity and specificity of the FTA collection/storage system for GC with spiked and non-spiked vaginal and urethral swabs collected in the same manner as those from the study.
qPCR primer sets
For N. gonorrhoeae detection, we created a primer set that targets a conserved segment of the porin A pseudogene that is specific to Neisseria gonorrhoeae and does not have binding affinity for other species (Table 1). Logistic limitations prevented us from running a second assay or developing a multiplex assay, so it is possible that we underestimated the number of GC cases in our study by targeting a single genetic region.
Table 1. Primer sequences and qPCR conditions for in-house designed Chlamydia trachomatis and Neisseria gonorrhoeae assays
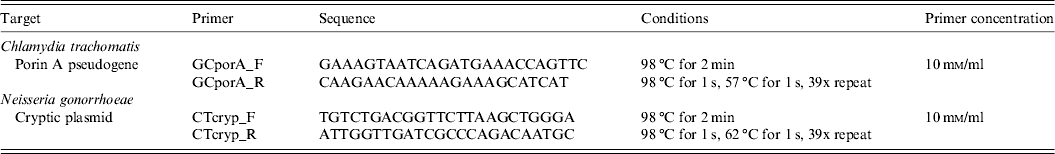
In light of the discovery of a new Chlamydia trachomatis (CT) variant in Sweden, wherein a deletion occurred at a highly conserved and common diagnostic target site in the cryptic plasmid [Reference Ripa and Nilsson15, Reference Ripa and Nilsson16], we designed a new primer set for our CT assay. We targeted an alternative conserved region of the cryptic plasmid (Table 1) based on sequencing results by Seth-Smith et al. [Reference Seth-Smith17].
Using positive and negative controls, we determined the sensitivity and specificity of the qPCR GC assay was 83% and 100%, respectively. For CT, the values were 100% (sensitivity) and 94% (specificity).
Finally, we ran all samples in an assay using universal bacterial primers [Reference Nadkarni18], to ensure that each sample contained bacteria, minimizing the possibility that a GC-negative sample was actually a false negative due to collection, storage or DNA purification failures. We were able to compare our total bacterial concentrations with the bacteria-specific concentrations for each participant sample to check that there was no relationship between total bacterial load (which includes commensal species and other pathogenic bacteria); an association would indicate that low GC or CT concentrations are due to poor sample collection and not a realistic picture of the infection load.
qPCR assays were performed using the CFX96 Real-Time System (Bio-Rad, USA) with SYBR Green master mix (Ssofast EvaGreen Supermix, Bio-Rad, USA) in a total volume of 20 μl. Optimal primer volume and qPCR conditions for our in-house primers are given in Table 1. For CT and GC assays, we ran standard curves from a tenfold dilution series (108–102) using genomic DNA (GC) or cloned target DNA (CT) to quantitate starting concentrations, which are equivalent to the values we used to determine infection concentration. All samples were run in triplicate from which we calculated the average starting quantity. Any samples with a mean starting quantity of GC or CT DNA below 7·5 × 101 copies (our limit of detection for both the GC and CT assays) were considered too low to reliably call positive. These, along with samples that did not amplify were determined as negative. Samples with a mean starting quantity ⩾7·5 × 101 were considered positive.
Interview data
In addition to providing a self-collected swab, participants answered questions in a closed-ended, oral interview. The entire interaction took 30–60 min. Participants answered questions about their STD symptoms and treatment history within the past 6 months, demographics (estimated age, tribal affiliation, marital status), residence (home village and region of Kaokoland in which the village is located), wealth (herd size: 0–10 cattle = poor; 11–50 = middle; >50 = wealthy), and the number of sexual partners they had in the past 6 months.
A native speaker conducted all interviews with assistance from the lead author. The survey was developed using previously validated questions as well as questions developed specifically for this study. The survey was administered interactively to allow for clarification if the participant had trouble understanding any questions. Privacy was a great concern and we reminded participants at several stages of the interview that all information provided was completely confidential.
Statistical analyses
We conducted bivariate analyses using contingency tables (reporting the estimated odds ratios and 95% confidence intervals) and χ 2 tests to explore associations between main effect variables and GC status. Fisher's exact tests were used to explore associations between GC status and self-reported symptoms, and to explore other associations when sample sizes were very small. Data were analysed using R v. 2.14.1 (R Foundation, Austria).
RESULTS
We recruited a total of 446 people. Three people refused to collect a swab and 12 were excluded because we determined that they did not reside in Kaokoland for at least 6 months, leaving a final sample size of 431 (males 222, females 209; sex ratio 1·06). Because people in the study population generally do not know their exact age, we grouped people into age categories. In our sample, 58% of men were aged ⩽35 years, and 69% of women were aged ⩽35 years.
GC status by qPCR
We found GC-specific DNA in the samples of 64% of participants, but there was high variability in the amount of DNA present in participant samples (ranging from 75 to 106 copies/20 μl reaction). We found no relationship between total bacterial concentration (estimated from the assays using universal primer) and GC concentration (t = − 0·87, d.f. = 433, P = 0·39), confirming that low GC concentration was not a result of ineffective collection or attenuation of genomic material during storage.
Given the very high prevalence of GC (64%), the fact that GC is often asymptomatic or mild in presentation, and that infection is known to result in partial immunity to additional infection [Reference Plummer19], we hypothesized that the number of GC cells identified might be associated with symptoms or epidemiological differences. Using the ID50 for GC – 103 organisms – as our cut-off point [Reference Todar20], we divided GC-positive samples into high-level (⩾103) and low-level (<103). The prevalence of low-level positive GC samples was 48%. The prevalence of high-level positive GC samples was 16%, for an overall prevalence of 64%. This dichotomization maps onto the distribution of DNA quantities from our samples because there is a marked drop off in the number of samples with quantities approximately more than 103 copies (Fig. 1). To check the validity of choosing 103 as our cut-off point for high- and low-level GC, we ran a sensitivity analysis wherein we tested the effect of a 10% increase or decrease in the value of our cut-off point on major associations. We found no changes in the direction of associations; thus, we maintain that 103 copies (i.e. ID50) is a reasonable dichotomy for further analyses.
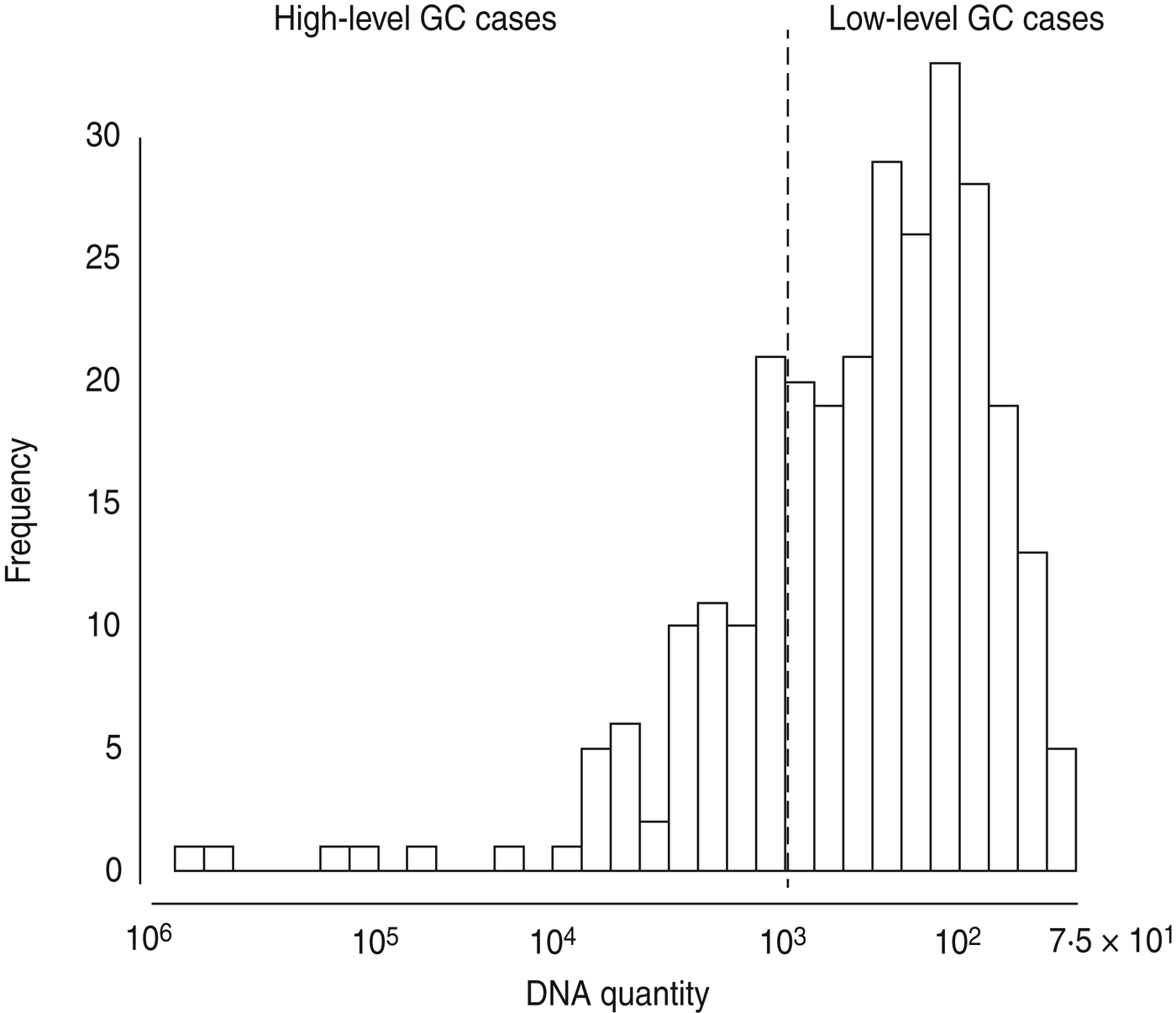
Fig. 1. Histogram of gonorrhoea (GC) DNA quantity in 431 urethral or vaginal samples from participants in Kaokoland, Namibia 2009. Quantities of ⩾1000 copies were categorized as ‘high-level’ infections, based on the definition of the ID50 dose for GC. This is very close to what appears to be a natural cut-off in our data.
We tested the strength of association between several variables and the likelihood of having GC at either a high level or low level. The results are presented in Table 2. We found a strong association between female gender and high and low GC status. Young adults were most likely to have high-level GC, although this result was only weakly significant. Low-level GC was distributed evenly across the population. Region of residence (high-level and low-level) and season of data collection (high-level only) were both significant correlates of positive GC status. Other variables, including tribal affiliation (Fisher's exact test, P = 0·2), marriage status (χ 2 = 0·3, d.f. = 2, p = 0·8), number of sexual partners in the past 6 months (Fisher's exact test, P = 0·9), and co-infection with chlamydia (Fisher's exact test, P = 0·2), were not statistically significantly associated with GC prevalence (Table 2).
Table 2. Crude associations between GC status and selected demographic and ecological characteristics for 431 participants in 28 village field sites in Kaokoland, Namibia, 2009
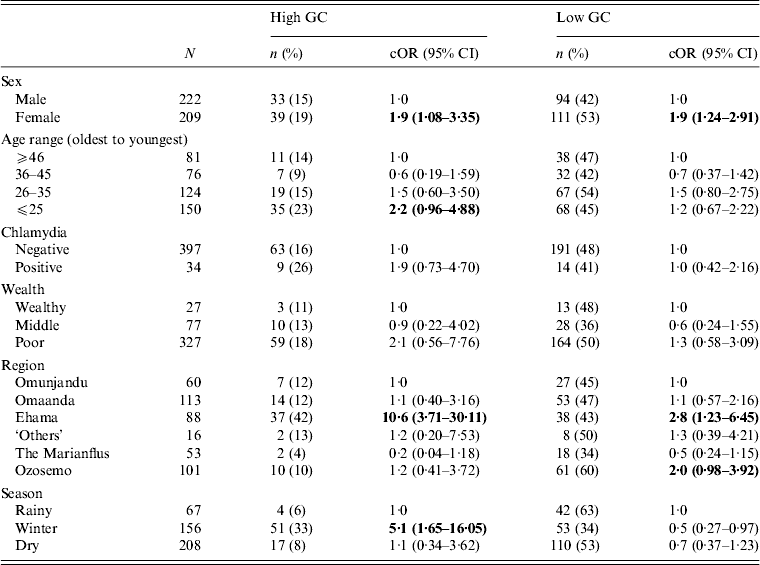
GC, Gonorrhoea; cOR, crude odds ratio; CI, confidence interval.
All ORs and CIs are from comparisons with the reference group and estimate the risk for having high- or low-level GC compared to being uninfected.
To check for any confounding relationships between age, sex, region and season, we built a multivariate multinomial model and found that all relationships between main effect variables and GC status remained (Table 3). We also ran a multinomial regression model to test for an interaction effect between age and sex on GC risk but found none (range of P values for interaction effects = 0·15–0·94).
Table 3. Multivariate, multinomial logistic regression with all variables that were significant in bivariate analyses for GC risk in 431 participants in 28 village field sites in Kaokoland, Namibia, 2009
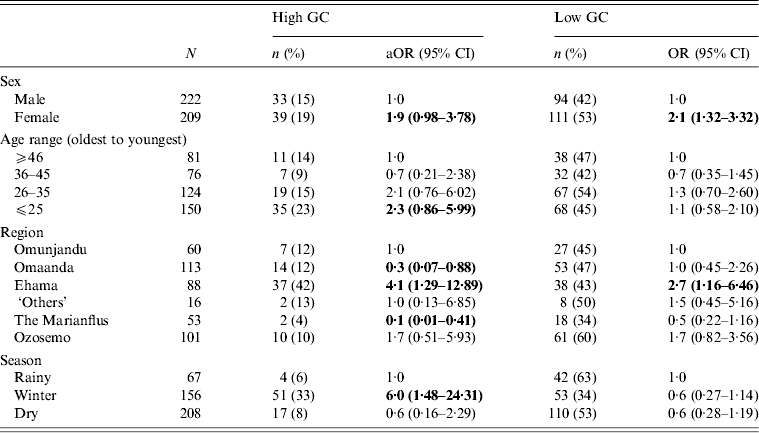
GC, Gonorrhoea; aOR, adjusted odds ratio; CI, confidence interval.
All ORs and CIs are from comparisons with the reference group and estimate the risk for having high- or low-level GC compared to being uninfected.
Sex differences in prevalence
GC prevalence was higher in women than men for both high-level and low-level GC (Table 2). However, men were far more likely than women to report abnormal discharge (χ 2 = 13·9, d.f. = 1, P = 0·0002) and burning or itching during urination (χ 2 = 12·1, d.f. = 1, P = 0·0005). Of people who reported having had GC-like symptoms in the past 6 months, there was no difference between men and women in their likelihood to seek treatment at the clinic (χ 2 = 0·03, d.f. = 1, P = 0·9); 8% of men and 2% of women reported having attended a clinic within the past 6 months to receive treatment for GC/chlamydia-associated symptoms.
GC status and self-report of symptoms
We explored the relationship between having high- or low-level GC and reporting GC-like symptoms (Table 4). We asked all participants about abnormal discharge separately from questions about dysuria. However, since participant responses regarding these symptoms were highly correlated (χ 2 = 156·7, d.f. = 1, P < 2·2−16) we grouped them when examining the relationship between symptom reporting and GC status.
Table 4. Association of chlamydia (CT), gonorrhoea (GC), and CT & GC infections* with self report of abnormal discharge or dysuria by sex in the past six months in 28 village field sites in Kaokoland, Namibia, 2009
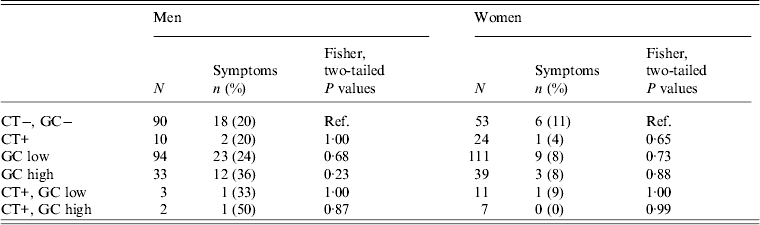
* Each row represents a separate analysis of a different selection of the participant pool. Thus, the sum of N for men or women is larger than the actual sample size.
We found no relationship between being positive for GC and having symptoms (Table 4). The percentage of men or women with high- or low-level GC who reported symptoms was not significantly different than the percentage of GC-negative men or women who reported symptoms.
Even after we excluded individuals who had been to the clinic within the last 6 months for treatment (n = 32), in order to remove people who received medical interventions and who were, thus, less likely to still have a GC infection, we still found no relationship between having symptoms and having a GC infection, with the exception of men with high-level infections. Most people who report having symptoms and attending the clinic are men (78%), so when the treated men are excluded, the relationship between reporting symptoms and having a high-level GC infection becomes significant for men (P = 0·02).
Seasonal variation
Nomadism in Kaokoland leads to variability in population density across seasons. For every season in which recruitment took place, we selected villages in areas where there were at least 8–10 eligible individuals present. Consequently, there is a strong association between region and season of collection in our dataset (χ 2 = 14·6, d.f. = 1, p = 0·0001). Despite the confounding effects of season on region, when we stratified by region, most cases of high-level GC were collected during the winter (Fig. 2). This is particularly true for people in the youngest age group (⩽25 years), living in the Ehama region (Fig. 2).
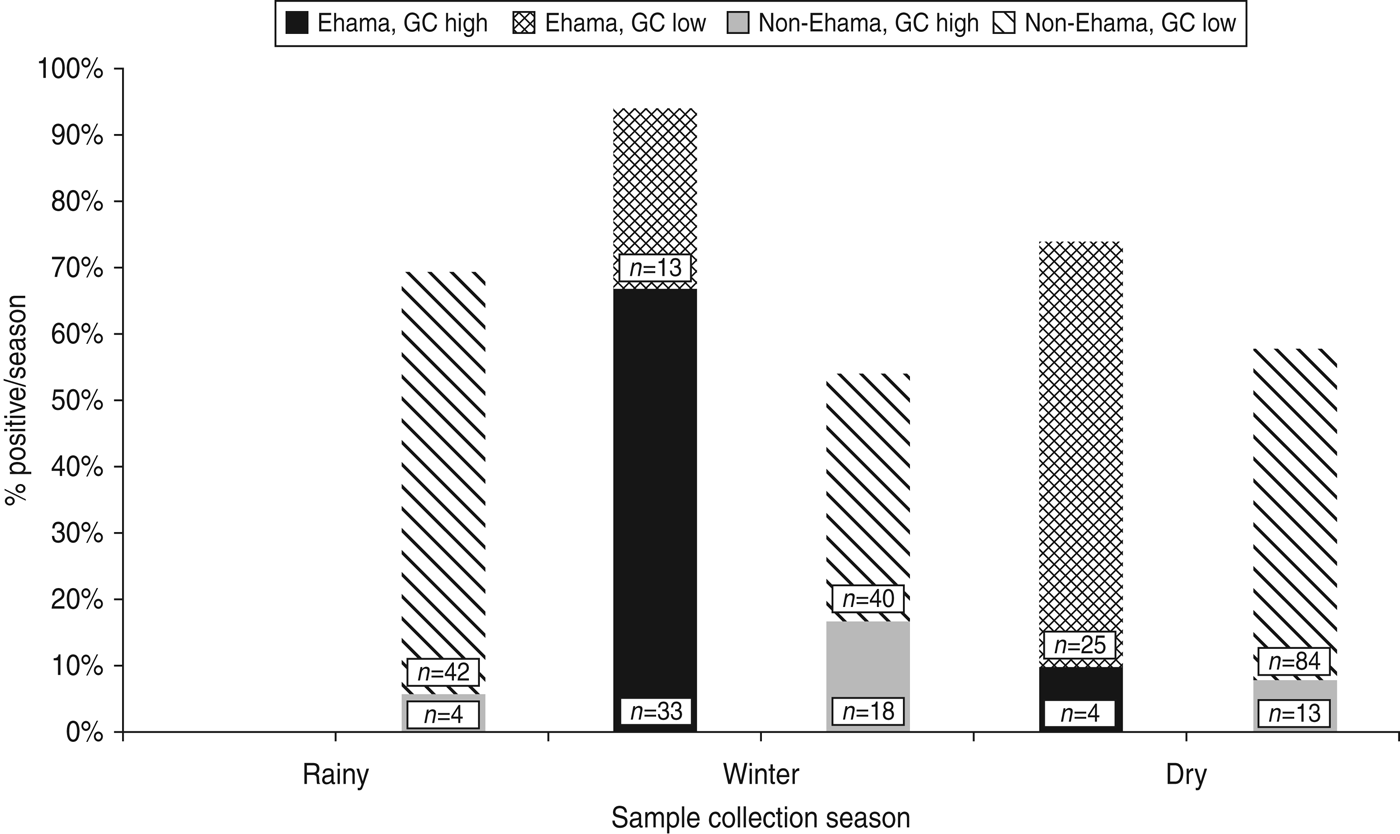
Fig. 2. Prevalence of high-level gonorrhoea (GC) cases by season and region in Kaokoland, Namibia 2009. Most cases occurred in Ehama (blue bars) and data collection in Ehama was most intense in the winter. However, winter is still significantly associated with high-level GC, when bivariate analyses are stratified by region (Ehama: OR 21·6, 95% CI 3·71–180·6; non-Ehama regions: OR 1·9, 95% CI 0·89–3·89), although this association is not as strong for non-Ehama regions. Numbers indicate the total number of samples collected in either Ehama or non-Ehama regions for each 2-week period.
For the most part, low-level GC cases were distributed evenly across populations, regions and collection season; however, their odds of having a low-level infection were significantly greater in Ehama and Ozosemo (Table 2).
DISCUSSION
GC occurs frequently in Kaokoland pastoralists, affecting the majority of those screened (64%). Individuals with high bacterial loads (⩾103 cells) clustered demographically, regionally and seasonally, whereas those with lower bacterial loads were more homogeneously distributed.
Most participants with GC reported no genitourinary symptoms. The prevalence of asymptomatic infections in our study is consistent with other studies conducted in high sexual activity populations in rural Africa. Among five high-risk populations, Detels et al. [Reference Detels21] found that between 66·7% and 100% of participants reported no symptoms. Wilkinson et al. [Reference Wilkinson3] found that rural South African women recognized symptoms in about 50% of cases (including C. trachomatis and Trichomonas vaginalis as well as N. gonorrhoeae). Of women who reported symptoms, only 2% sought treatment.
Due to expense and infrastructure requirements of laboratory-based diagnostics, most developing countries, including Namibia, use syndromic management [22] to treat a suite of symptoms that are commonly caused by bacterial STDs. Although syndromic management has very limited efficacy in populations with high percentages of asymptomatic infections [Reference Wilkinson3], the bigger problem in Kaokoland is, perhaps, poor access to care. Even when symptoms are present, seeking treatment is not trivial for people living in remote areas. The burden of accessing healthcare is entirely on the patient, who must travel to the clinic and pay for treatment and any other basic needs while away from home. The few clinics in Kaokoland are a long and expensive journey from many villages. This is especially true for women, who must leave children and put aside crucial provisioning duties to attend the clinic.
Our results suggest that syndrome-based care could be more efficacious if people could access treatment more reliably. GC symptoms are easily confused with other genitourinary tract infections [4] and, in our participants who reported symptoms, GC- and CT-positive participants were just as likely to report genitourinary discomfort as participants who were negative for GC and/or CT (with the exception of men who had high GC loads). This might be related to a problem with access to care. When getting to the clinic is too difficult, individuals endure uncomfortable symptoms longer; eventually having genitourinary discomfort can begin to seem normal and might not be perceived as a sign of infection. Furthermore, there are probably several reasons why people in Kaokoland – especially women – might experience discomfort that is not related to GC or CT infection, including herpes outbreaks (Hazel et al., unpublished data) and vaginal tissue irritated by dry sex. This would make it hard to identify potentially infected people by their current discomfort levels. However, if healthcare strategies for rural Kaokoland relied more on mobile clinics – which already exist, but are limited in their activities – than standing clinics, the burden of access would shift away from patients and reduce the tradeoffs they face when deciding to seek treatment. With adequate investment in a mobile clinic fleet, this system could also be more reliable. With less costly, more accessible treatment, people can get treated more frequently, which will decrease disease burden and disrupt the sense of normalcy associated with genitourinary discomfort.
Local conditions and behaviours may also account for the high prevalence of GC in Kaokoland. Partner concurrency and dry sex (where vaginal fluids are intentionally reduced to achieve the sensation of greater tightness during sex) are almost ubiquitous practices in the regions of Kaokoland where this study was conducted [Reference Talavera8, Reference Scelza23]. Other studies have found frequent partner exchange and concurrency to be associated with higher GC transmission risk for both men and women [Reference Hooper24, Reference Platt, Rice and McCormack25], and other non-STD conditions in women [Reference Fethers26], although we did not find a significant relationship between GC risk and number of partners, even when we stratified by number of partners. This may be because concurrency is so common. Therefore, people who do not have concurrent partners may still be likely to share their partner with others and, with GC so highly prevalent, this could indicate that avoiding concurrency does not offer much protection from exposure in this population. There is evidence that dry sex increases transmission probability for non-HIV STDs for men [Reference Beksinska27] and HIV in women [Reference Auvert28].
We observed a seasonal pattern in the high bacterial load GC cases, similar to those observed elsewhere [Reference Grassly, Fraser and Garnett29], which in our case, corresponded to the timing of ceremonies. A series of weddings and funerals occurred in July in the Ehama region, associated with a spike in GC-positive samples. The dual problem of remoteness and mobility make the Kaokoland pastoralists a particularly difficult group to provide treatment and outreach to, increasing their vulnerability to poor health outcomes.
Given infrastructural and resource limitations to intervention efforts in Kaokoland, outreach by a well-equipped mobile unit, as described above, could be concentrated in the winter using easily accessible local information networks to identify the timing and location of ceremonies in order to bring treatment access to high-risk locations at the time of year when transmission is most frequent. This is essentially the same strategy we used to identify densely populated areas for data collection. Not only could seasonally driven mobile clinic schedules streamline outreach efforts, it could also combine syndromic management with health education and disease prevention efforts.
Using qPCR as a detection method for GC had two advantages that shaped the results of this paper. First, it enabled us to detect the presence of GC on samples collected and stored in very remote regions where no electricity was available. Second, as qPCR is more sensitive than conventional PCR, our detection limit was 75 copies/20 μl. This enabled us to describe a wide range of infection, which proved to be valuable considering that we found an association in men between high bacterial loads and symptoms. Bacterial load appears to modify the association with some epidemiological correlates, but this needs to be confirmed in future studies. We found no other studies in the literature using qPCR for GC detection that examined the association of high and low bacterial loads with epidemiological correlates.
One set of limitations of our study, which were difficult to avoid working with remote nomadic populations, was our limited sample size per village and a lack of infrastructure, necessitating collection and storage protocols for testing later. While this limits the range of results we can produce from our specimens, it does not limit the value of our results, which give insight into GC epidemiology in vulnerable populations. Our study also highlights the value of Whatman FTA cards for data collection in highly remote populations where there is no laboratory infrastructure. Another limitation is a tradeoff associated with using a qPCR-based assay: qPCR can detect DNA at such a low concentration that it is possible that some of our lowest concentration cases are actually no longer infectious; rather the assay may be detecting DNA from a cleared infection. This would only account for a small number of low-level infections and, thus, would not change the arc of our findings. It is also possible that some of our GC- and CT-positive cases were due to our primer sets having binding affinities with other bacteria species. However, given that we designed primers that did not have affinities for other known species and that we did not have any problems with false positives in our negative controls, we do not anticipate this being an explanation for our results.
Although our study was not designed to identify adverse sequelae associated with GC, there are hints from our fieldwork that endemic GC is adversely affecting fertility in Kaokoland pastoralists – an observation that is consistent with findings in related populations [Reference Pennington and Harpending9]. Perhaps more troubling, the same behaviours that facilitate GC transmission put this population at high risk for a future HIV epidemic as the local culture and environment undergoes drastic transformation [Reference Fratkin, Roth and Nathan30, Reference Friedman, Miescher and Henrichsen31]. It will be critical for the health and survival of remote communities for public health outreach groups to be able to make accurate predictions of future risk and transmission pathways and design more effective interventions.
ACKNOWLEDGEMENTS
We thank the Namibian Ministry of Health and Social Services for permitting our research, Kemuu Jakurama for his role as an interpreter in the field, Beatrice Sandelowsky for administrative support in Namibia, and all the study participants in Kaokoland. This work was supported by funding from the Interdisciplinary Program in Infectious Diseases NIH training grant (B.F., T32 A1049816); Robert Wood Johnson Health and Society Small Grant Program (A.H.); American Philosophical Society's Lewis & Clark Fund for Exploration and Field Research (A.H.); Wenner-Gren Dissertation Fieldwork Grant (A.H.); University of Michigan's Centers for the Study of Complex Systems and Social Epidemiology (A.H.); Rackham Graduate School, University of Michigan (A.H.); and the School of Natural Resources and Environment, University of Michigan (A.H.). We also thank two anonymous reviewers for their comments on this manuscript.
DECLARATION OF INTEREST
None.








