INTRODUCTION
Since 2003, a distinct clone of methicillin-resistant Staphylococcus aureus (MRSA), related to the livestock reservoir has emerged in the human population [Reference Voss1]. As this clone was found to be non-typable (NT) by pulsed-field gel electrophoresis using the SmaI restriction enzyme, it was originally called NT-MRSA [Reference de Neeling2, Reference Bens, Voss and Klaassen3]. Multi-locus sequence typing revealed that all strains belonged to the clonal complex 398 (CC398) [Reference Huijsdens4]. At present, it is clear that people who have frequent contact with pigs or veal calves have extremely high MRSA CC398 carriage rates compared to national community prevalences (25–35% vs. 0·1% in The Netherlands) [Reference Broek5–8].
As a result of the elevated prevalences in this specific population, the ‘search and destroy’ policy in The Netherlands was adapted; persons in contact with live pigs and veal calves are added to the high-risk group and should be screened for MRSA upon hospital admission [9]. As a consequence, the number of MRSA CC398-carrying patients found in The Netherlands increased dramatically to nearly 30% of all newly detected MRSA strains in 2007 [Reference Haenen10], and 42% in 2008 [11]. The proportion of MRSA in S. aureus nosocomial infections remained very low (<2%), compared to other countries [12].
In a recent survey by the Food and Consumer Product Safety Authority in the Netherlands (VWA) MRSA was found in 11% of retail meat (with a minimum MRSA prevalence of 2% in game and a maximum of 35% in turkey) [Reference de Boer13]. Other studies also found MRSA in retail meat, in varying percentages (2·5% [Reference van Loo14], 19% [Reference Lin15], 0·7% [Reference Pereira16], 5% [Reference Pu, Han and Ge17], 0% [Reference Lee do18] and 17%, R. de Jonge, J. E. Verdier and A. H. Havelaar, unpublished observations).
In animal husbandry-dense areas, the majority of newly identified human MRSA carriers concerns this livestock-associated MRSA [Reference van Rijen, Van Keulen and Kluytmans19], and recently, the first hospital outbreaks of CC398 have been reported [Reference Wulf20, Reference Fanoy21]. Meanwhile, serious invasive infections due to CC398 have been observed [Reference van Loo22–Reference Lewis27]. Therefore, the emergence of this new livestock-associated clone poses a potential public health risk that warrants close monitoring.
The high prevalence of MRSA in meat products and in people working with livestock raises the question whether slaughterhouse workers, who are in contact with pigs (dead or alive) and meat products, are also at risk. Therefore, we performed a cross-sectional survey on nasal MRSA CC398 carriage in employees of pig slaughterhouses, and on the occurrence of MRSA in different slaughterhouse sections.
METHODS
Study population, questionnaires and human sampling
Three pig slaughterhouses were enrolled in the study on the basis of voluntary participation, from a complete list of 10 large pig slaughterhouses in The Netherlands. All were located in the south and the east of the country, in areas with a high pig density. By using a structured questionnaire, slaughterhouse-specific information was collected, e.g. number of employees, slaughterhouse capacity, specifics on lairages and the production process, information on microbiological contamination of the carcasses and working benches and hygiene measures.
Slaughterhouse workers were enrolled in the survey based on voluntary participation. A written consent was obtained from each participant. The survey contained questions on age, gender, country of birth, recent antibiotic use, job description, working in more than one section of the slaughterhouse (rotation), wearing plastic gloves, living on a livestock farm, and contact with family members working in healthcare or in livestock farming. Slaughterhouse workers were divided in three different categories according to their activities: contact with live pigs, dead pigs or other. When subjects indicated that they worked in more than one section, they were included in the category with the most intense contact with live animals.
Nasal swabs (Venturi Transystem, Copan Innovation, Italy) were taken from workers in order to determine the presence of MRSA. This study was approved by the Medical Ethical Committee of the University Hospital Utrecht (file no. 08/050).
Environmental sampling
To determine the MRSA status of the different slaughterhouse sections, environmental wipe samples were taken from surfaces in each section (Fig. 1) at the beginning and at the end of the working day using Sodibox wipes (Raisio Diagnostics B.V. Nieuwerkerk aan den IJssel, The Netherlands). Sections of the slaughterhouse were divided in two different categories according to the cleanliness of the animal/carcass: dirty or clean areas. In the dirty area, the carcass surface is cleaned by scalding, depilation and singeing. In the clean area, the carcass is eviscerated and processed into meat products.
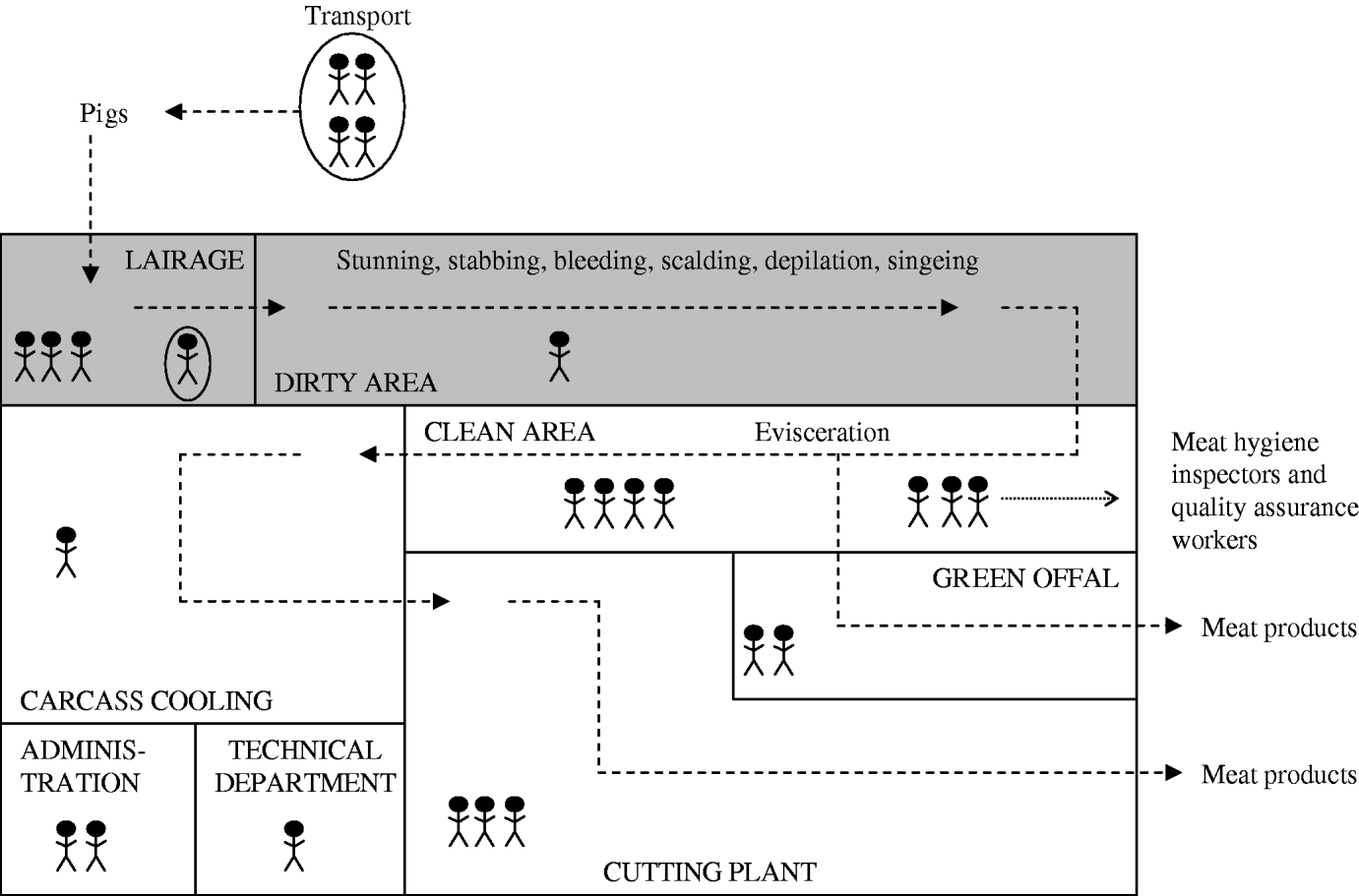
Fig. 1. Schematic representation of the sections of the production chain (dotted lines) in a pig slaughterhouse. The shaded area represents sections where live pigs are located (dirty area). Each human figure represents about 10 persons, circled persons are not actual slaughterhouse employees (livestock transport workers and official veterinarians and auxiliaries).
Microbiological methods
Nasal swabs were incubated in Mueller–Hinton enrichment broth (Becton Dickinson, USA) with 6·5% NaCl, for 18–48 h at 35°C. Then 10 μl of the broth was plated onto a MRSA-ID culture plate (bioMérieux, France), and incubated overnight at 35°C. Suspect (green) colonies were identified as S. aureus by a latex agglutination test (Staphaurex Plus; Murex Diagnostics Ltd, UK) and tested for cefoxitin sensitivity by the disc diffusion method [28]. The obtained MRSA isolates were subsequently stored at −80°C.
Environmental sample wipes were soaked in 100 ml Mueller–Hinton enrichment broth with 6·5% NaCl and incubated for 18 h at 37°C. Next, 1 ml of the broth was transferred into 9 ml Phenol Red mannitol broth with 5 mg/ml ceftizoxime and 75 mg/ml aztreonam (bioMérieux) and incubated for 18 h at 37°C. Subsequently, 10 μl of the suspension was transferred onto a Columbia agar plate with 5% sheep blood. In parallel, Brilliance MRSA culture plates (Oxoid, UK) were inoculated with 10 μl suspension and incubated for 18 h at 37°C. Colonies were subcultured until pure.
Confirmation of the isolates was done by a multiplex PCR specific for S. aureus [Reference Martineau29], the mecA gene [Reference de Neeling30], and the Panton–Valentine leucocidin (PVL) toxin genes [Reference Lina31]. Isolates were defined as MRSA on the basis of their mecA gene presence. Staphylococcal protein A (spa) typing was conducted according to Harmsen et al. [Reference Harmsen32]. On all MRSA-positive environmental and human samples, antimicrobial susceptibility was tested using the Vitek system (bioMérieux SA, France) according to the manufacturer's instructions.
Sample size and statistical analysis
The prevalence of MRSA nasal carriage in the general population in The Netherlands was assumed to be <0·5%. A nasal carriage rate of ⩾2% in slaughterhouse workers was considered as a significant increase. The required sample size was calculated as 450 subjects (α=0·05, β=0·10).
Prevalence of MRSA in slaughterhouse workers was calculated as a percentage of the total amount of samples in general and specified per category and job description. Wilson confidence intervals (CI) were calculated. Univariable exact logistic regression was performed using SAS, version 9.1 [33]. Odds ratios (OR) were determined by comparing different categories and job descriptions within those categories. In order to calculate the association between the human and environmental samples and because of the skewed distributions of the percentages of positive persons and environmental samples per section, Spearman's rank correlation was used.
RESULTS
Slaughterhouse characteristics
In the three selected slaughterhouses, the total number of employees varied between 80 and 260. The total number of slaughtered pigs per day varied between 3800 and 5000, all pigs originated from farms in The Netherlands. In one slaughterhouse, cattle were slaughtered as well, but in separate rooms in the same building.
Humans
Of the total of 497 slaughterhouse workers 195 (39·2%) agreed to participate. An additional 41 livestock transport workers and 13 official veterinarians and auxiliaries (i.e. persons from the VWA, who monitor and assist the meat hygiene inspectors) were included, yielding a total of 249 study subjects, including 16 female participants. Mean age was 43 years (range 19–73 years), and the mean working week was 41 h (range 7–80 h).
We found an overall nasal MRSA prevalence of 5·6% in slaughterhouse workers (14/249, Table 1). MRSA carriage was found exclusively in persons having contact with live pigs (15·1%), compared to subjects not working with live pigs (0·0%, OR 38·2, Table 2).
Table 1. Prevalence of nasal MRSA carriage in slaughterhouse workers
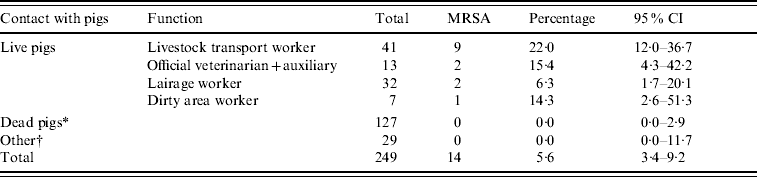
CI, Confidence interval (data from three slaughterhouses combined).
* Clean area worker, carcass cooling and cutting plant worker, green offal worker, meat hygiene inspector, quality assurance worker.
† Administrative and technical personnel.
Table 2. Univariable exact logistic regression analysis
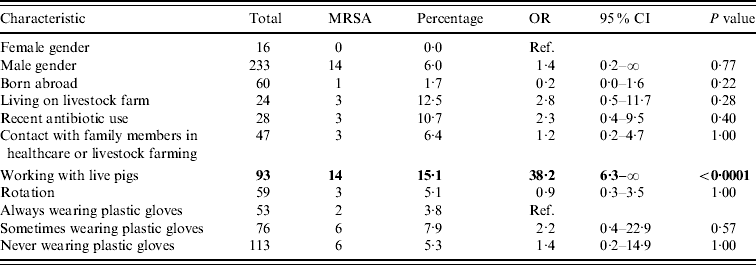
OR, Odds ratio; CI, confidence interval; ref. reference category.
Boldface values belong to characteristics that are significantly related to MRSA, when comparing the presence of the relevant factor vs. the absence of it.
Nine of the 41 (22%) livestock transport workers were MRSA positive, as well as 2/13 (15%) veterinarians and auxiliaries. In total, 3/195 (1·5%, 95% CI 0·5–4·4%) employees of slaughterhouses (excluding livestock transport workers and official veterinarians and auxiliaries) were MRSA positive; these were all working in the dirty area of the slaughterhouse. No specific slaughterhouse function proved to be a significant risk factor, when comparing different activities within the clean and the dirty areas. Twenty-three persons indicated working in both dirty and clean areas and only one of these was found MRSA-positive.
Regarding potential determinants and confounders, no significant difference in persons with and without MRSA was found (Table 2). Furthermore, no significant differences in MRSA prevalence in humans between slaughterhouses were found.
Environment
At the start of the day MRSA was only found in environmental samples from the lairages (10/12) (Table 3, Fig. 1). At the end of the day MRSA was found in the lairages (11/12), the dirty (5/12) and clean (3/12) areas and green offal (1/3). Spearman's correlation coefficient, a measure for the correlation between MRSA status of the environmental samples and the humans working in these areas, is 0·75 (P=0·002). The squared correlation (0·75×0·75=0·56) gives the coefficient of determination; 56% of variance in percentage of positive persons can be explained by environmental contamination.
Table 3. MRSA in environmental samples taken at start and end of working day
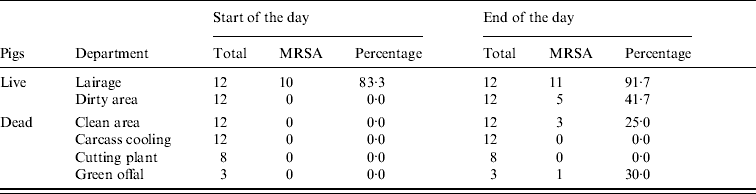
Data from three slaughterhouses combined.
Spa typing and antimicrobial susceptibility testing
In total, 14 human and 32 environmental MRSA strains were collected. The predominant spa type was t011 in both human subjects (11/14) and environmental samples (21/32). Spa type t108 was only found once in a human nasal sample, and also once in an environmental sample from the corresponding slaughterhouse. An additional 10 environmental isolates from the other slaughterhouses were typed as t108. Spa type t571 was only found once in environmental samples, and t034 and t1451 were found only once in humans, not in environmental samples of the corresponding slaughterhouse. From two environmental samples two different spa types were isolated, in both cases t011 and t108. PVL-positive strains were not found.
Antimicrobial susceptibility testing revealed that all MRSA isolates from humans and the environment are resistant against tetracycline (Table 4), and 19/46 isolates show combined erythromycin and clindamycin resistance. Furthermore, all isolates are sensitive for mupirocin and vancomycin (only human isolates tested). Spa type t108 appears to have less combined erythromycin+clindamycin resistance (0/11=0·0%) than t011 (17/32=53·1%, P=0·002). No clear difference in resistance pattern between the human and environmental isolates was determined.
Table 4. Antimicrobial susceptibility profiles of all human and environmental MRSA isolates
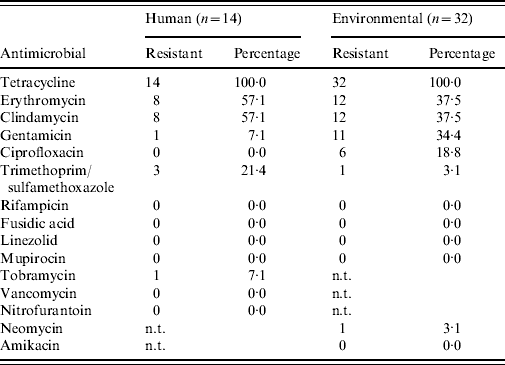
n.t., Not tested.
DISCUSSION
To our knowledge, this is the first study on the prevalence of nasal MRSA in pig slaughterhouse workers. Working with live pigs is the most important determinant for nasal CC398 carriage, justifying the present hospital infection control guidelines in The Netherlands, which indicate that contact with live pigs is a risk factor for MRSA carriage. Working with dead pigs does not seem to be a risk factor for MRSA carriage.
The prevalence of 15·1% in persons working with live pigs is comparable to data found elsewhere, e.g. 26% and 14% in pig farmers and 12·5% in veterinarians attending an international pig health convention [Reference Voss1, Reference Broek5, Reference Wulf34]. A low prevalence was found in Danish veterinarians (3·9%) [Reference Moodley35], but higher nasal prevalences were found in German pig farmers on MRSA-positive farms (86%), German pig veterinarians (45%) and USA pig farmers (45%) [Reference Cuny36, Reference Smith37].
The overall MRSA prevalence in all subjects in the current study is 5·6%, which is significantly higher than the general population prevalence reported in The Netherlands (0·1%) [Reference Wertheim7, 8, Reference Donker and Stobberingh38]. The higher prevalence in livestock transport workers compared to lairage workers might be explained by the less intense physical contact with pigs by lairage workers, who often use sticks to herd the animals. Transport workers earmark all animals at pick up and often herd the animals with their bare hands. Second, high-pressure spray cleaning of the truck may result in formation of MRSA aerosols, which can be inhaled by the transport worker. Insight into these mechanisms may give more information on the transmission route of MRSA.
During the day MRSA accumulates, particularly in the first stages of the production process, which predominantly deals with live pigs. Since pigs were loaded into the lairages at night, the lairages were not clean at the time of sample collection at the beginning of the day. Moreover, the lairages are cleaned every day, but not disinfected.
There is a significant association between the presence of MRSA in different sections, and the percentage of MRSA-positive persons working in these relevant sections. It is possible that acquisition of MRSA occurs through contaminated surfaces [Reference Boyce39]. However, presence of MRSA on different surfaces does not necessarily imply that there is an increased risk of human MRSA acquisition via the environment: where the lairages have a high percentage of MRSA-positive samples at the end of the day (92%), a relatively low percentage of lairage workers had acquired the bacterium (6·3%). It is plausible that animals spread MRSA to both humans and the environment, and human acquisition of MRSA seems to be more likely by contact with MRSA-positive animals than through environments with MRSA in dust or aerosols.
All spa types found in our study were previously confirmed as belonging to the CC398 livestock-associated MRSA clone [Reference Huijsdens40]. The most predominant spa types in both human and environmental isolates were t011 and t108, which is in accord with previous studies in pigs and pig farmers [Reference Voss1, Reference Huijsdens4, Reference Broek5, Reference van Loo22, Reference Armand-Lefevre, Ruimy and Andremont41, Reference van Belkum42]. The subject with t034 was an official veterinarian and the spa type t1451 came from a livestock transport worker, these persons often have more animal contacts than in the slaughterhouse alone. Antimicrobial susceptibility, in particular tetracycline resistance was comparable to profiles found in other studies for livestock-associated MRSA [Reference de Neeling2, Reference Broek5, Reference van Loo22].
The prevalence of MRSA found in retail meat in other studies is considerable, the prevalence of MRSA found in employees of pig slaughterhouses in this study is low. The role of slaughterhouse employees in transmitting MRSA to the meat products thus does not seem to be large. Especially as persons working with meat products were all negative in this study. This finding is in accord with an unpublished study (R. de Jonge, J. E. Verdier and A. H. Havelaar, unpublished observations), where none of 101 employees from the cold-meat processing industry and institutional kitchens carried MRSA. It is probable that another transmission route to retail meat is involved here. Contamination of meat with MRSA by the environment (surfaces) and/or equipment, or from animals to carcasses/meat products is more likely to occur. This kind of cross-contamination has already been demonstrated for Salmonella spp. in pig slaughterhouses [Reference Prendergast43].
Our study has a few limitations. As with every questionnaire, survey recall bias, selection bias, and language bias may have occurred. Next, the low number of slaughterhouses visited (n=3) yields little power to find significant differences between slaughterhouses. Nevertheless, we assume that these results are representative for all Dutch pig slaughterhouses, because the working conditions in all pig slaughterhouses in The Netherlands are comparable due to automation and the strict legislation on hygiene and animal handling. Despite a smaller sample size than calculated beforehand, the number of subjects is still sufficient to confirm previous findings on the risk of acquiring MRSA for people in contact with live pigs. Possibly more risk factors could be found if the number of slaughterhouse workers was larger, e.g. country of birth, recent antibiotic use, amount of hours worked per week, and contact with healthcare. Furthermore, no pigs were sampled in our study, but in a previous study on MRSA at Dutch slaughterhouses MRSA was detected in 81% of the Dutch slaughter batches and 39% of the individual pigs [Reference de Neeling2]. Environmental samples are considered to be a good proxy for animal MRSA carriage, concerning the association found between environmental and animal samples in other studies (OR 27·5, κ=0·68) [Reference Broens44]. Longitudinal information on duration of MRSA carriage and the possibility of transient colonization is not yet available; this will be our group's next study subject.
In conclusion, nasal MRSA CC398 is found in pig slaughterhouse workers in significantly higher percentages than the general population prevalence in The Netherlands. It is found exclusively in persons working with live pigs. In addition to contact with live pigs, environmental contamination might also play a role in the acquisition of MRSA, but exact transmission routes from animals to humans remain to be elucidated in order to enable application of targeted preventive measures.
ACKNOWLEDGEMENTS
We thank the three slaughterhouses and in particular their quality assurance workers for their participation and help in the data collection in this study.
DECLARATION OF INTEREST
None.







