INTRODUCTION
Leptospirosis is one of the most prevalent zooanthroponoses worldwide. The agents of leptospirosis are the pathogenic species of Leptospira [Reference Levett1]. Leptospirosis is a disease affecting multiple organs, and the clinical symptoms and signs are often non-specific influenza-like symptoms. In the most severe cases, the disease is known as Weil's disease, a syndrome characterized by multi-organ failure and a mortality of 5–15% [Reference Levett1]. The disease is probably heavily underdiagnosed in humans, due to the often non-specific symptoms, low accessibility of rapid diagnostic methods and lack of awareness among clinicians [2].
Infected animals excrete Leptospira in the urine, and the primary route for further transmission of the infection is through contact with urine or water contaminated with urine of infected animals [2–Reference Twigg, Cuerden and Hughes4]. Rodents are an important reservoir of Leptospira, and the commensal brown rat (Rattus norvegicus), which is closely linked to human activities, is believed to be the reservoir host of the pathogenic Leptospira interrogans serovar Icterohaemorrhagiae known to cause severe leptospirosis in humans and dogs [2, Reference Faine5–Reference Borg-Petersen and Jacobsen7].
In cities of the UK and in Denmark, 70–90% of complaints concerning rats are related to defective sewers and the number of reported rat problems in cities is increasing [Reference Mayer8, Reference Heiberg9]. Through defective sewers, rats can enter people's homes, factories, have contact with foodstuffs, etc., thus presenting a risk of transmission of Leptospira to humans. Despite these facts, only very limited information is available on the biology of rats living in sewers, their role as carriers of zoonotic agents in general and Leptospira spp. in particular. The present study on R. norvegicus caught in sewers is the first since Seguin et al. [Reference Seguin10] tested 91 rats sampled in sewers in Lyon, France. They found that 17% had positive titres against Leptospira spp. using the microscopic agglutination test (MAT), while only 7% were found infected by culturing of kidneys. Our study is the first of its kind to use polymerase chain reaction (PCR) to detect Leptospira spp. in rats living in sewers.
Earlier studies of surface rodent populations have primarily been conducted in tropical regions, where the burden of leptospirosis is severe [Reference Levett1, Reference Faria11–Reference Dalu and Feresu17]. Some of the most recent surveys from temperate climates have been performed in the UK [Reference Webster, Ellis and MacDonald18], Switzerland [Reference Adler19] and Baltimore, USA [Reference Easterbrook20]. Webster et al. [Reference Webster, Ellis and MacDonald18] tested rats from farms while Adler et al. [Reference Adler19] and Easterbrook et al. [Reference Easterbrook20] tested rodents and shrews from city areas. These studies showed varying proportions of infected animals, ranging from 13% [Reference Adler19] to 65% [Reference Easterbrook20], partly explained by different methods (ELISA, MAT, culture, silverstaining, IFA, PCR). In 1986, the latest prevalence study on Danish rats was undertaken. This was conducted on surface rats and the seroprevalence of L. interrogans serovar Icterohaemorrhagiae was found to be as low as 3% [Reference Lund21]. The observed differences in prevalence in the different studies may reflect geographical and habitat variation, but may also be due to the variation in sensitivity and specificity of different diagnostic techniques used [Reference Webster, Ellis and MacDonald18–Reference Lund21].
MATERIALS AND METHODS
Sampling of rats
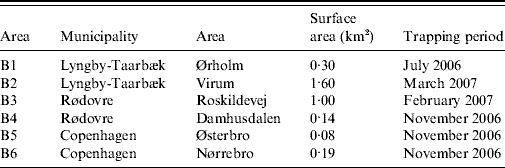
From the summer of 2006 until spring of 2007, rats (R. norvegicus) caught in sewers in six locations (B1–B6) in Copenhagen city and its suburbs were sampled (Table 1). Sewer locations were selected based on signs and reports of recent rat activity in the sewers. Metal wire-cage live-traps (61×21×24 cm or 46×18×20 cm) were placed in sewer manholes in a deactivated state 1–2 weeks prior to trapping, in order to make rats accustomed to the traps. Traps were placed free from the water flow on wooden platforms. Balls of bird seed, coated in tallow, were used as bait. After the acclimation period, traps were activated and checked daily. The exact position of trapping was noted for each individual rat. Trapped rats were transferred to the laboratory, weighed, sexed and caged singly in wire-bottomed steel cages (31·5×19×26 cm) suspended 7 cm above trays which allowed collection of faeces and urine in the trays below the cages. Rats were, therefore, never in contact with droppings from other rats. For descriptive reasons and in order to investigate possible differences in infection rates related to age, rats were divided into subgroups based on body weight: juveniles were defined as rats weighing <100 g, sub-adults weighing between 100 g and 200 g and adults with a weight >200 g as described by Webster et al. [Reference Webster, Ellis and MacDonald18]. Rats were fed a standard rat and mouse laboratory diet (5 mg vitamin K3/kg; Altromin No. 1324 Fortified; Chr. Petersen A/S, Denmark) with tap water available ad libitum. As the rats were originally collected for the purpose of screening for resistance to anticoagulant rodenticides, the kidneys were sampled at autopsy, after anticoagulant resistance testing (bromadiolone blood-clotting response test combined with a VKORC1 ARMS–PCR assay) [Reference Heiberg9]. It should be noted that all rats were submitted to the same resistance testing protocol, although there is no suggestion that the resistance testing could affect the level of Leptospira infection.
Table 1. Location and period of trapping of rats
Rats were killed with CO2 and the kidneys were removed under sterile conditions using sterilized scissors and tweezers. Both kidneys were stored at −80°C until DNA purification.
Precautions to avoid PCR product carry-over
Strict physical separation between sample preparation, PCR setup and analysis laboratories was maintained. All procedures in the DNA extraction and PCR preparation were performed in bio safety level II laboratory benches under sterile vertical laminar air flow (LAF bench).
Handling of tissues until proteinase K treatment was done in a separate building. The rest of the DNA and PCR preparations were done in a separate LAF bench in a separate building. DNA preparation and PCR were performed on separate days. The subsequent gel-electrophoresis was performed by laboratory technicians not involved in the DNA and PCR preparation, and in a separate building. Sterile filter tips (ART; SDS, Sweden) were used in all sample manipulations, and all surfaces in the PCR setup laboratory were regularly wiped with a 0·5% hypochlorite solution and exposed to UV light between sessions with the purpose of destroying contaminating DNA.
Positive controls had low copy numbers, containing 20 and 200 genome copies of L. interrogans, respectively. At least two negative controls were included in each run. Furthermore, all PCR reactions were performed with dUTP instead of dTTP, allowing for enzymatic prevention of PCR product carry-over with uracil-N-glycosylase.
Purification of DNA
For DNA extraction, a 145–160 mg transverse section including all functional layers of the kidney was isolated from either the left or right kidney and DNA was purified according to the manufacturer's protocol (DNeasy blood and tissue kit; Qiagen Ltd, UK). Since we used a larger amount of starting material than recommended by the manufacturer, the volume of lysis buffer was adjusted accordingly. An aliquot of the lysed product corresponding to the recommended volume was used for final DNA extraction.
PCR
For detection of Leptospira spp. a conventional PCR was used, based on the primers G1: 5′-CTG AAT CGC TGT ATA AAA GT-3′ and G2: 5′-GGA AAA CAA ATG GTC GGA AG-3′ as described previously [Reference Gravekamp22]. The primers amplify a 285 bp fragment of the SecY gene and have previously been shown to amplify DNA from at least six pathogenic Leptospira spp. namely L. interrogans, L. noguchii, L. santarosai, L. meyeri, L. weilii and L. borgpetersenii [Reference Gravekamp22]. PCR reactions were performed in total volumes of 100 μl: G1 2 μl (20 mm), G2 2 μl (20 mm), MgCl2 5 μl (50 mm), Platinum buffer 10 μl, dUTP mix 10 μl (25 μm dATP, 25 μm dCTP, 25 μm dGTP, 50 μm dUTP), Milli-Q water 61 μl, Platinum Taq 0·4 μl (5 U/μl) and template DNA 10 μl. The cycling reactions were performed on a GeneAmp PCR system 9600 (Applied Biosystems, USA) using a touch-down PCR profile consisting of an initial hot start at 94° C for 2 min followed by 10 cycles with denaturation at 95°C for 30 s, annealing at 60°C for 15 s with a 1°C decrement per cycle and elongation at 72°C for 30 s, followed by 40 cycles with an annealing temperature at 50°C and a final extension step for 5 min at 72°C. PCR products were visualized by ethidium bromide staining followed by electrophoresis on a 2% agarose gel (Nusieve GTG agarose, Seakem Lonza, Switzerland). Fragment sizes were estimated using a 100 bp ladder (New England BioLabs, USA).
MAT
Sera from a randomly chosen subsample of 17 rats from different locations were serologically tested by an accredited MAT as previously described [Reference Hartskeerl3]. Serum was tested against 16 serovars belonging to 11 pathogenic, one intermediate pathogenic and one saprophytic serogroup. The serogroups used were Patoc, Icterohaemorrhagiae (three different strains), Sejroe (two different strains), Poi, Canicola, Ballum, Bratislava, Pomona, Grippotyphosa, Saxkoebing, Bataviae, Hardjo and Hurstbridge. The end-point titre was determined as the highest serum dilution showing agglutination of at least 50% of the cells, i.e. a +2 reaction. A serum sample was considered positive at a titre ⩾1:100, following cut-off values that were standard for human serology in the same laboratory. Due to lack of serum, dilution was only performed until a positive end-point reaction at 1:100 for two rats and 1:450 for one rat.
STATISTICS
A logistic regression model was fitted to data using the proc logistic (SAS 9.1; SAS Institute, USA) to analyse the effect and possible interaction of the following parameters; location, body weight and sex on the outcome, presence or absence of Leptospira spp., respectively.
Only locations B2–B6 were included in the analysis because no rats with Leptospira spp. infection were seen in area B1. The best model was selected based on backwards elimination of effect parameters and parameters with significant (P<0·05) effect on the outcome were included in the final analysis.
In order to investigate the effect of age on the infection rate, we considered body weight to be a substitute parameter of the age of the rat. The predicted probability of infection as a function of weight was calculated based on the model above. The results are expressed both as a graph (Fig. 1) and fitted to a model were the relative change in infection rate according to weight intervals could be described by an odds ratio (OR).
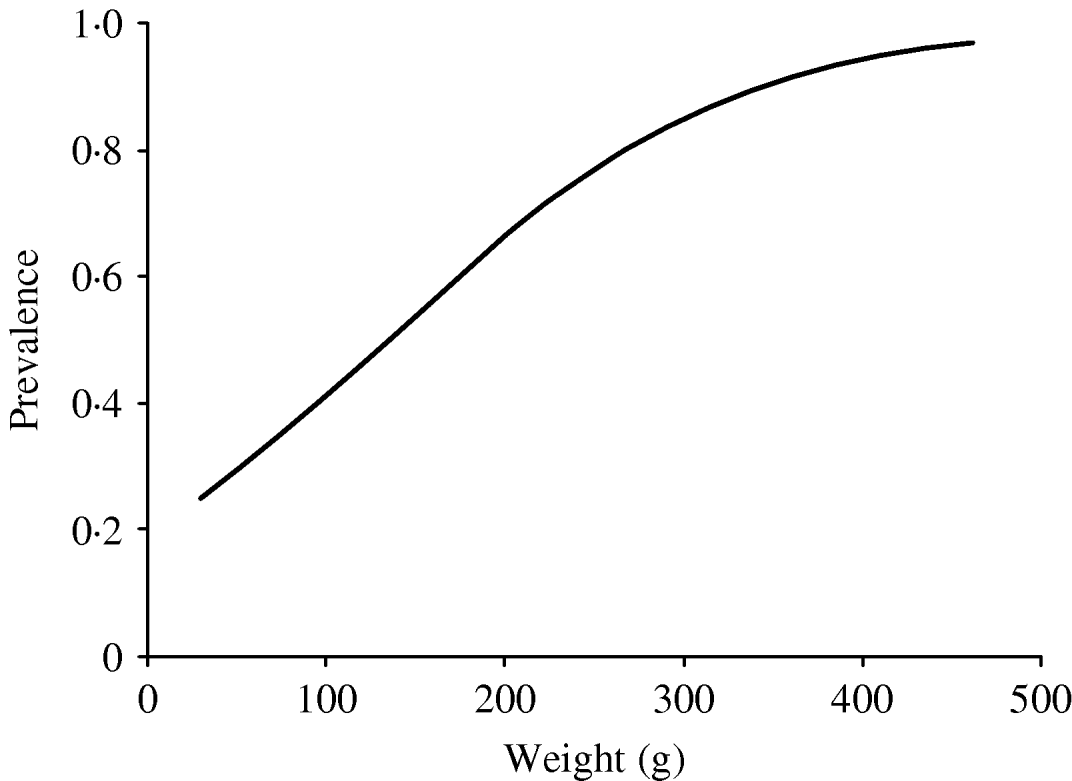
Fig. 1. Predicted prevalence of infected rats by weight, based on the body weight and infection status of the caught rats.
RESULTS
A total of 196 rats (92 males and 104 females) from the six different locations were tested for the presence of Leptospira spp. by PCR. The sex was determined for all 196 rats and body weight for 183 of the 196 rats.
A total of 104 rats (53%) were found to be infected. At one location none of the rats were infected with Leptospira spp., while the prevalence in the remaining five locations differed from 48% to 89% (Table 2). At some of the positive locations, Leptospira spp.-infected and non-infected rats were sampled in manholes which where located alongside each other. However, in some locations, we also sampled infected and non-infected rats from the same manhole.
Table 2. Number and percentage of infected rats at the different trapping sites
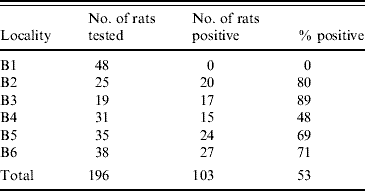
We found no significant difference in the prevalence between the two sexes but observed a significantly (P<0·05) higher prevalence in area B3 compared to the other four areas where infection with Leptospira occurred.
The number of individuals in each of the three categories, juvenile, sub-adult and adult was 28, 41 and 114, respectively. The three age groups were all represented in each location (Table 3). The mean body weight of all rats was 232 g with a range of 30–462 g.
Table 3. Number of juvenile, sub-adult and adult rats caught in the six localities (B1–B6)
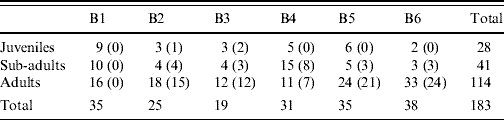
Values in parentheses are the number of infected rats.
In the five areas where infected rats were found, the percentage of infected juveniles was 16% (3/19). In the sub-adult group 21/31 (68%) were infected and in adults the numbers were 80/96 (83%).
A highly significant correlation (P<0·001) between body weight and probability of infection was found; this correlation was described by a model that gave an increase in OR of 1·33 (95% confidence interval 1·18–1·50) per 25 g increase in weight (Fig. 1).
MAT
Seventeen rats were tested by MAT of which 16 showed agglutinations at a titre between 100 and 1000; six were >100. The same 16 rats were positive by PCR and one remaining rat was negative. Of the six rats with a titre >1:100 the one having the highest titre was against Icterohaemorrhagiae. The remaining 10 rats with titre 1:100 most often showed agglutination towards the serogroups Pomona, Sejroe, and/or Icterohaemorrhagiae.
DISCUSSION
This survey shows that Leptospira spp. is highly prevalent in rats caught in sewers. In five of the six examined sewer locations, 48–89% of the examined rats were infected with Leptospira spp. The serovars belonging to the serogroup Icterohaemorrhagiae are considered among the most pathogenic to humans and are commonly found in rats [Reference Faine5, Reference Levett15, Reference Lindenbaum and Eylan16, Reference Thiermann23]. A subpopulation of 17 rats was tested by MAT and the highest titres were found against the serogroups Pomona, Sejroe and Icterohaemorrhagiae. Significant differences in the prevalence of Leptospira-infected rats were found between some of the locations (location B1 and B3).
Although Leptospira-infected rats seemed to be prevalent in the sewers, one location, B1, differed markedly from the others as none of the 48 rats captured here were infected. Since rats are known to be chronically infected with at least L. interrogans serovar Icterohaemorrhagiae [Reference Faine5, Reference Twigg6], they continue to shed the bacteria in urine. Thus, the non-occurrence of Leptospira in the B1 population indicates that the infection has been absent for a prolonged period of time, and seasonal variation is unlikely to play a major role in explaining its presence or absence. Therefore, the data suggest that the rats at B1 have lived as a population which has not been in contact with infected populations for quite some time. Leptospira has not been able to be maintained above a critical level in those rats.
In comparison with previous surveys the prevalence found in the five infected sewer locations of this study was high. Sunbul et al. [Reference Sunbul14] have reported a Leptospira spp. infection rate of 27% by PCR in Turkish rats caught along the seashore. Studies where MAT has been used have similarly shown infection rates of 21% and 41% in Israeli and Thai rats, respectively [Reference Doungchawee12, Reference Lindenbaum and Eylan16]. In a survey of urban settings in Brazil [Reference Faria11], a higher prevalence was found in rats (R. norvegicus). They found that 80% were positive by culture and/or PCR/MAT.
One reason for the high infection rate in the rats caught in sewers could be an accidental sampling of individuals belonging to the same deme, assuming that there is a higher transmission rate within a deme than between demes. The sampling would then not be representative for the population within each location as a whole. We do not yet know if sewer rat populations are structured into family groups as are rat populations living outside the sewers [Reference Klemann and Pelz24]. However, unpublished results from an ongoing study on the biology of rats in sewers indicate that most of these rats may be limited in their movements and thus may form smaller family units. With this knowledge, our data do not suggest a gathering of infected rats in particular ‘hotspots’, rather, infected and non-infected rats were dispersed more or less evenly within the various sampling locations and thus probably representative of numerous family units.
We found a rise in the prevalence of infected rats with increasing age, with only 16% of juvenile rats and 83% of the adult rats being infected with Leptospira spp. This is in accord with the findings of other authors [Reference Levett15, Reference Lindenbaum and Eylan16, Reference Easterbrook20, Reference Thiermann23]. The low infection rate in juvenile rats indicates that transplacental transmission of Leptospira is of minor importance, if at all, in sewer rats. It is probable that the increase in infection rate with age is explained by a longer period of exposure to the environment, increased social behaviour in adults and the fact that infection with at least L. interrogans serovar Icterohaemorrhagiae in rats is found to be chronic [Reference Faine5, Reference Twigg6].
The present study raises many questions regarding environmental and behavioural factors influencing the transmission of Leptospira infection in rats. While the study was not designed to address this issue, and thus definitive conclusions on this topic cannot be drawn, there are some interesting indications. Leptospira spp. are known to survive in wet surroundings and despite the presence of detergents, etc. in wastewater the sewers could provide a habitat conducive for the transmission of Leptospira. Such a mechanism, where both sexes are equally exposed to possible infection, would be consistent with the fact that we did not find any differences in prevalence between males and females. Other studies have examined sexual differences. Nutall et al. [Reference Nuttall25] found no sexual difference in infection rates, whereas Easterbrook et al. [Reference Easterbrook20] suggested that female rats should be more prone to infection, based on the findings in urban rats of Baltimore, USA. Moreover, living in a sewer system limits movements which can cause more frequent interactions between the rats. This, and the constant presence of contact with water which facilitates survival of excreted Leptospira, can be the cause of the observed high prevalence compared to studies on rats living on the surface.
CONCLUSION
In conclusion, the present study reports a high overall prevalence (53%) of Leptospira spp. in rats caught in sewers, ranging from 48–89% in five of the six locations studied, while in one location no Leptospira infection was observed. We found a strong correlation between prevalence and body weight and observed a rapid increase in the prevalence post-infancy. No gender difference was found.
High prevalence of Leptospira infection in rats caught in sewers should be a great cause of concern for health authorities, and also in cities with temperate climate. Since most surface rats in urban areas, with which humans may have contact, originate from the sewer population, there is a considerable and probably underestimated risk for transmission of this zooanthroponosis. Ageing sewer systems and growing problems with the control of rat populations, e.g. due to resistance to rodenticides, may aggravate this problem.
ACKNOWLEDGEMENTS
This project is part of an ongoing project concerning the biology of sewer rats. The sewer rat project is funded by the Environmental Protection Agency, the ‘Kommunernes Momsfond’ and the Danish Pest Infestation Laboratory (DPIL), University of Aarhus, Denmark. The molecular work and identification of Leptospira spp. was funded by DPIL and Statens Serum Institut (SSI), Denmark. The authors thank staff members Folmer Jensen, Iver Skadborg and Sarah Adams at DPIL as well as Berit Jensen and PCR laboratory technicians at SSI for providing technical assistance in animal handling and testing. Additional thanks to Claus Bo Svendsen, SSI and Steen Ethelberg, SSI for support with statistical analysis, and to supervisors Jes Søe Pedersen, Copenhagen University and Karen A. Krogfelt, SSI, for helpful discussions during the process.
DECLARATION OF INTEREST
None.






