INTRODUCTION
Hepatitis E virus (HEV) is a small enterically transmitted RNA virus which causes an acute hepatic illness in humans. Hepatitis E manifests with symptoms of clinical hepatitis, including loss of appetite, malaise, dark urine, abdominal pain and jaundice. A blood test is required to confirm the infection. In Europe and North America HEV infections were previously believed to be associated with travel to endemic areas, but recently indigenous cases have been increasingly recognized in developed countries [Reference Teo1, Reference Dalton2]. Both imported and indigenous infections contribute to the burden of hepatitis E in England and Wales. The indigenous HEV genotype 3 found in human infections in Europe, including England and Wales, is similar to that found in pigs [Reference Banks3]. Contact with pigs and consumption of pig products have been reported as risk factors for some infections and outbreaks, however other food sources and risk factors, such as consumption of shellfish, wild boar and deer, have also been recognized [Reference Said4–Reference Wilhelm7]. Case-control studies have identified uncooked deer meat as a risk factor in Japan [Reference Tei8] and consumption of offal and wild boar in Germany [Reference Wichmann9]. However, the majority of hepatitis E infections are sporadic and the source is not identified.
Public Health England [PHE, formerly the Health Protection Agency (HPA)] has monitored the numbers of reference laboratory-confirmed cases of hepatitis E in England and Wales, since 2003 [10]. The national surveillance system collects data from the two reference laboratories in London and Birmingham. The purpose of the surveillance is to ascertain and investigate non-travel-associated cases of HEV and to identify potential risk factors in England and Wales. In 2005 there was a significant increase with 329 reference laboratory-confirmed cases of hepatitis E reported compared to 149 cases in the previous year; an increase of 55%. Enhanced surveillance during this period showed that the increase was mainly accounted for by non-travel-associated cases and that the majority of these were Caucasian males aged >55 years. In addition, an apparently British name was highly predictive for indigenously acquired infection [Reference Ijaz11, Reference Lewis12]. Despite detailed questioning of cases, specific risk factors could not be identified [Reference Lewis12]. Between 2006 and 2007 the number of reported cases declined (from 289 cases in 2006 to 162 cases in 2007) and numbers remained low in the intervening years, with an average of 175 cases, until 2010. Since 2010, there has been an increasing number of reports of HEV infection in England and Wales with 274 cases reported in 2010 (an increase of 36% from the previous year) and a further increase of 40% (456 cases) in 2011. Non-travel or indigenously acquired cases account for most of the increase and over 60% of these cases are males aged >55 years [13]. This 2011 increase in indigenous hepatitis E raised public health concerns and prompted epidemiological investigations to determine risk factors for the acquisition of infection in England and Wales. The investigation was approved by the HPA Hepatitis Programme Board and supported by the Hepatitis Regional Leads. Ethical approval was not required.
METHODS
Blood or serum samples received at the Virus Reference Department (VRD) Hepatitis E Reference Laboratory, Colindale, London were tested for HEV antibodies [immunoglobulin (Ig) G and IgM] using Fortress Diagnostics ELISAs (Fortress Diagnostics Ltd, Northern Ireland). Assays were run according to the manufacturer's instructions. Individuals were classified according to their serological results as recent acute hepatitis E infection (serologically confirmed by anti-HEV IgG and IgM) or no infection (serologically negative). IgM-positive samples were further tested for HEV RNA, and those that were RNA positive were genotyped as described previously [Reference Ijaz11].
Study population
Patient samples were submitted for hepatitis E testing due to evidence of liver disease, such as deranged liver function tests. Hepatitis E results were reported to the surveillance team on a weekly basis by the VRD reference laboratory. Patients were contacted by telephone within 2 weeks of the result report date. A member of the surveillance team explained the rationale for the investigation and obtained consent before the interview. Questionnaires were designed specifically for the investigation to examine a wide range of environmental, personal and food exposures using close-ended (yes/no/unknown) questions with further detailed information requested during interview if the interviewee answered ‘yes’. For hypothesis generation cases were recruited and interviewed during June and July 2011 (further details below). The hypothesis was tested in a case-control study with cases and controls recruited between August and November 2011. The process for recruiting to the case-control study is shown in Figure 1 and explained further in the Study design section.

Fig. 1. Recruitment to the case-control study, England and Wales, August–November 2011.
Hypothesis generation
Cases of recent acute hepatitis E defined as IgM and IgG positive, were identified from samples tested for anti-HEV at the VRD reference laboratory and reported during June and July 2011. Cases with no apparent travel outside the UK were contacted for an in-depth telephone interview using a trawling questionnaire. Cases were asked early in the interview if they had travelled outside the UK in the 9 weeks (the maximum incubation time for hepatitis E) prior to their illness. If they had travelled the interview was terminated and the case excluded from the investigation. Cases were asked to recall whether it was likely that in 9 nine weeks before their illness they had eaten a range of foods (including fish, shellfish, meat, meat products, offal, milk, dairy products, salad vegetables, fresh vegetables, fresh berries) at home or outside the home (e.g. restaurants) and whether the food was raw, undercooked or well cooked. Cases were asked about their place of residence, occupation, where they purchased their food, alcohol consumption, recreational exposures (e.g. gardening, fishing), animal exposures including pets, contacts with people who may have been ill with a similar illness, medical treatments before they became ill (e.g. dental treatment, transfusion), regular medication (e.g. statins, proton pump inhibitors), history of serious illness (e.g. liver disease) and travel history within the UK in the 9 weeks before they became ill. The hypothesis for testing was based on exposures that were reported by ⩾75% of the cases.
Study design
A case-control study was used to test the hypothesis generated. The cases previously interviewed for hypothesis generation were not included. Cases were defined as those individuals that had been diagnosed serologically with recent HEV infection (i.e. IgM and IgG positive) as reported by the VRD reference laboratory between August and November 2011 (see Fig. 1). From these, cases with a known recent travel history outside the UK were excluded. If no travel history was provided, the filter of an apparently British name (previously reported to be highly predictive for indigenously acquired infection [Reference Lewis12]) was used to presumptively identify possible indigenous cases. Travel history was confirmed at interview and if cases had travelled outside of the UK in the 9-week incubation period they were excluded from the investigation. Controls were defined as those who were tested for hepatitis E infection by the VRD reference laboratory and confirmed to be seronegative for anti-HEV over the same time period as the cases, and had no apparent travel history (Fig. 1). Three controls were recruited for each case, and were matched by age group (<45, 45–64, ⩾65 years) and gender. Where possible, controls were contemporaneous with and selected from the same geographical area as the cases. The geographical areas were based on HPA regions, i.e. East Midlands, East of England, London, North East, North West, South East, South West, West Midlands, Yorkshire & Humber, and Wales. Residential postcode was collected as part of the questionnaire in order to determine region for matching. When it was not possible to match a case with contemporaneous controls from the same region, an available control in an adjacent region was used.
Cases and controls were contacted within 2 weeks of result report date. Consent was obtained over the telephone prior to interview. The case-control questionnaire was developed from the trawling questionnaire used for hypothesis generation and covered: gender, age, ethnic group (i.e. white British or other), travel outside the UK, diet (including mussels/shellfish, chicken, pork, bacon, ham, cured pork, sausages, liver, pate, pork pie, game, offal), occupation, medical history (medication or serious illness) and contact with animals (contact with pets or farm animals). The case-control questionnaire incorporated all the exposures reported by ⩾75% of cases answering the hypothesis-generation questionnaire. The period covered was 9 weeks (the maximum incubation period for hepatitis E) before date of onset of illness, if known, or from the date the sample was taken. Where individuals were excluded because of travel outside the UK reported at interview, or consent refused, or they could not be contacted (Fig. 1), replacement cases or controls were recruited ensuring they continued to match appropriately by gender, age, time and region (as described above).
Statistical analyses
Risk factors and exposures for those with recent acute infection were compared with those for seronegative controls to identify the most likely cause of indigenous HEV infection during the study period. Univariate and multivariable logistic regression was performed, using logistic regression in Stata release 11·1 (StataCorp., USA).
Initially each of the exposures was analysed separately. Specific exposures with estimated odds ratios (OR) >1, a P value of <0·3, and with at least 30% of cases exposed were included in the multivariable model. In addition, as it has previously been suggested that non-travel-associated cases were more likely to live in coastal or estuarine areas [Reference Ijaz11], a univariate analysis was performed after stratifying the data into coastal and non-coastal, urban and rural, and by region based on the individual's postcode.
In the multivariable analysis, the factor that had an estimated OR < 1 or otherwise had the least significant association with HEV infection was removed from the model in a stepwise fashion until all the factors in the model exhibited some degree of association, i.e. P < 0·1 and estimated OR > 1. The variables, ‘alcohol consumption above recommended limits’ and ‘diet or medication for diabetes’, were included in the model and were not removed at any stage to ensure adjustment for any confounding by these two variables.
RESULTS
Hypothesis generation
The hypothesis-generating study included 17 cases, 13 of whose samples were PCR positive with 11 confirmed as genotype 3. In the remaining two PCR-positive samples there was insufficient viral RNA to determine the genotype. All were white British and aged between 37 and 79 years (median 66 years). Twelve (71%) were male and five female. There was no association identified with area of residence, or with occupational, recreational or animal (including pet) exposures.
None of these cases were vegetarians and of the 29 food exposures included in the trawling questionnaire, six items were consumed by over 80% of those interviewed. These were chicken (88%), bacon (88%), ham (88%), sausages (82%), lettuce (82%) and other salad vegetables (82%). All these food items were included in the case-control study questionnaire. Since at least one pig-derived product was consumed by all 17 cases, the hypothesis was that hepatitis E infection is related to the consumption of pork products.
Case-control study
Twenty-five acute hepatitis E cases confirmed by the reference laboratory and 75 matched negative controls were interviewed. None of the cases had previously been interviewed for hypothesis generation. The exposure period for these 25 cases is likely to have been between June and October 2011 based on an incubation period for HEV of between 2 and 9 weeks.
HEV RNA was detected by PCR in the samples of 21 (84%) cases and of these ten were confirmed as genotype 3; in the remaining 11 cases the viral RNA was too low for successful amplification to determine genotype. Sequence and phylogenetic analysis across part of the ORF2 regions was possible on the ten genotype 3 samples. The data indicate that the viruses cluster closely with sequences from other known human cases and swine isolates from England and Wales.
Cases and controls were matched by gender and by age group (Table 1), as well as being matched to the closest geographical region. Patients were sampled from all regions; East Midlands (five cases, 11 controls), East of England (two cases, 17 controls), London (one case, 10 controls), North East (one control), North West (two controls), South East (four cases, seven controls), South West (nine cases, 18 controls), West Midlands (one case), Yorkshire & Humber (two cases, three controls), and Wales (one case, six controls).The majority of cases and controls lived away from the coast and in urban locations.
Table 1. Gender and age of cases and controls, England and Wales, 2011
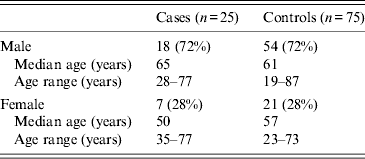
n values indicate number of subjects.
Eighteen (72%) cases currently consumed alcohol, although almost all (n = 23, 92%) stated that they had consumed alcohol in the past. Of the cases who currently consumed alcohol three (12%) drank above the recommended weekly limit (defined as a maximum weekly intake of 21 units for men and 14 units for women). Similarly, 54 (72%) of the controls currently consumed alcohol and 67 (89%) stated that they had consumed alcohol either in the past or present. However, only six (8%) of the controls who currently consumed alcohol drank above the recommended weekly limit. Around a third (n = 8, 32%) of cases and 40 (53%) controls stated that they had serious underlying illness, including diabetes which was adjusted for in the final model.
Statistical analyses
From the range of 19 food items, including chicken, shellfish, pork, sausages, liver, ham and pâté, in the case-control study questionnaire, only eight food exposures (Table 2) showed an association for acute HEV infections by univariate analysis. None of the cases or controls were vegetarian and there was no association with, for example, chicken [OR 1·00, 95% confidence interval (CI) 0·3-∞, P = 1·00], pork (OR 1·00, 95% CI 0·33–2·98, P = 1·00), pigs' liver (OR 1·60, 95% CI 0·46–5·55, P = 0·488) or shellfish (OR 1·12, 95% CI 0·45–2·74, P = 0·815).
Table 2. Univariate analysis of food exposures associated with the probability of having recent acute indigenous HEV infection, England and Wales, 2011
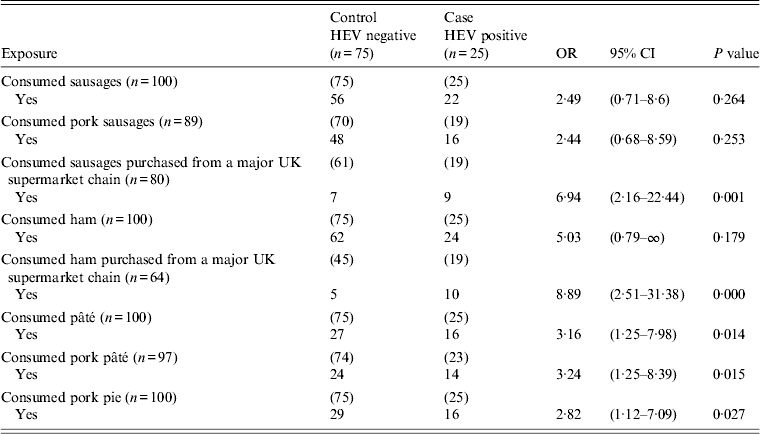
HEV, Hepatitis E virus; OR, odds ratio; CI, confidence interval; (n) values indicate number of responses received in each category.
Five exposures, shown in Table 2, were significant (P < 0·1) and these were: consumption of sausages purchased from a major UK supermarket chain, consumption of ham purchased from the same supermarket, consumption of pâté, consumption of pork pâté, and consumption of pork pie.
After including these food exposures in a multivariable model adjusted for alcohol consumption and diabetes, consumption of pork pie and consumption of ham or sausages purchased from a major UK supermarket chain, were significantly associated with being a case (Table 3).
Table 3. Multivariable analysis model, adjusted for alcohol and diabetes, exposures associated with the probability of having recent acute indigenous HEV infection, England and Wales, 2011
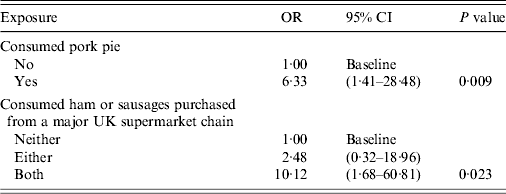
HEV, Hepatitis E virus; OR, odds ratio; CI, confidence interval.
Consumption of sausages from the supermarket was highly correlated with consumption of ham from the same supermarket with almost 90% of the study participants who answered these questions having purchased both of these products. Further analysis using two separate models for each of these variables showed that the consumption of sausages purchased from this major UK supermarket chain (OR 7·59, 95% CI 1·81–31·84, P = 0·004) and the consumption of ham purchased from the same supermarket (OR 10·98, 95% CI 1·84–65·35, P = 0·003) remained strongly associated with HEV infection.
There was no association with non-food exposures such as occupation or contact with animals. The analysis by region, coastal or non-coastal areas, and urban or rural areas did not show any pattern of association.
DISCUSSION
There has been a significant increase in the number of laboratory-confirmed hepatitis E cases identified in England and Wales since 2010. Indigenously acquired cases mainly account for this increase [13]. The hypothesis generated in this investigation was that HEV infection is related to the consumption of pork products. The case-control study to test this hypothesis included other previously implicated food exposures, such as shellfish, game and offal. Nevertheless, in the final multivariable model, only three processed pork products were implicated; these were sausages, ham and pork pie. Except for sausages, which require cooking, the other products are ‘ready-to-eat’ and do not require cooking or prior heating. In this context ham is pre-packed slices of ready-to-eat ham, which may be reconstituted, and is often used in sandwiches. Pork pie is a traditional British pie consisting of roughly chopped pork and pork jelly in a hot-water crust pastry which can be eaten cold [14]. HEV contamination has previously been found in raw products such as sausages and liver [Reference Berto15–Reference Feagins17]. However, the observation that two ‘ready-to-eat’ food items were also significantly associated with infection merits further careful consideration.
All cases and controls used for this study were identified through the reference laboratory where they were being tested for hepatitis E because they had biochemical evidence of deranged liver function or symptomatic liver disease. However, since this symptomatology applies to both cases and controls used this is unlikely to cause bias.
One study limitation was that although patients were contacted within 2 weeks of the result report to minimize recall bias, the long incubation period of up to 9 weeks for hepatitis E may lead to patients forgetting or incorrectly recalling an exposure. Nevertheless, questionnaires were completed comprehensively by telephone interview and we have no reason to suspect differential recall between cases or controls.
In this study, 14% of cases exceeded the recommended weekly alcohol limits, but this is less than the percentage in the general UK adult population with around a third of men and a fifth of women estimated to exceed the specified weekly intake of alcohol [18]. Factors such as excess alcohol consumption, some underlying medical conditions or medications could have effects on the liver and may pre-dispose HEV infection to cause clinically apparent illness by compromising hepatic function. However, these factors are unlikely to be causally linked to exposure. Alcohol and diabetes were adjusted for in the final model of this study to adjust for any confounding by these variables.
Once again we report the dominance of older males in indigenous hepatitis E cases. The excess in middle-aged and elderly men has been previously reported both in England and Wales [Reference Said4, Reference Ijaz11, Reference Lewis12] and in other European countries [Reference Buti19–Reference Mansuy21]. Although the excess remains unexplained, two possible mechanisms could account for this persisting observation. The first is that this truly reflects a higher rate of infection in males and the observed excess could be a genuine difference in exposure [Reference Said4]. With overall pork consumption, including ham and sausages, increasing in England [22], one possible explanation is that men tend to eat larger quantities of meat, including processed pork products. The National Diet and Nutrition Survey, which is a survey of food consumption in private households in the UK, showed the total daily meat consumption for women was 88 g compared to 135 g for men [23]. The breakdown was similar for individual pork products, where, for example, sausage consumption was 27 g/day for women and 35 g/day for men. However, a 20–30% increase in consumption between women and men may be too fine a margin to generate the observed male dominance. The alternative mechanism may be gender-driven differences in clinical susceptibility for HEV genotype 3 to cause illness, whereby females may be less likely to exhibit acute clinical disease if infected. This explanation is supported by the age prevalence of anti-HEV in the UK showing little male/female bias [Reference Ijaz24] and liver diseases as a whole exhibiting reduced female clinical expression [Reference Baig25, Reference Nasta26].
In this study, HEV genotype 3 was identified in cases where the serum viral load was sufficient for amplification. These findings are consistent with a zoonotic route of transmission which is supported by evidence of a similar genotype 3 virus circulating in pigs in the UK in 2000 [Reference Banks3]. Although more recent molecular epidemiological data is required for HEV circulating in pigs to compare with HEV causing disease in humans, phylogenetic analysis of genotype 3 viruses from this study indicate that they cluster closely with sequences from swine isolates from England and Wales.
A 2008 study demonstrated anti-HEV prevalence in pigs in the UK of 21·5% with about 8% of ‘finishers’ (22- to 44-week-old pigs) infected and shedding virus in faeces at the time of slaughter [Reference McCreary27]. HEV shed in faeces could be a source of contamination at point of slaughter. Meat and pig derivatives from these almost certainly viraemic animals are likely to enter the pork production chain and contaminate pork products. In addition, there could be cross-contamination through butchery and subsequent display or point of sale. A study from The Netherlands found 6·5% of pig livers were HEV RNA positive at point of sale in butchers' shops [Reference Bouwknegt16]. Potentially, HEV could be widespread throughout the food chain and the association of a major UK supermarket chain with infection is probably a feature of a contaminated batch. Processed pork products in particular are likely to contain meat and pig derivatives from multiple pigs and therefore a single highly viraemic animal has the potential to contaminate many different products or processed foods made from the same starting batch of meat. In addition, a recent study has shown 10% of pork sausages sampled at point of sale from UK retailers contained detectable HEV RNA [Reference Berto15], supporting pork sausages as a potential vehicle for HEV transmission.
As well as the finding that indigenous HEV infection was associated with the consumption of sausages from a major UK supermarket chain, our study also identified the ready-to-eat products pork pie and ham, as risk factors for the acquisition of HEV. These products can also contain meat and pig derivatives from multiple pig sources. In the UK the minimum industry standards for cooked pork products, such as pork pie, is 70°C for 2 min. However inactivation studies suggest that this may be insufficient to destroy infectious HEV, with recent studies showing that heating food to an internal temperature of 71°C for 20 min is necessary to inactivate HEV [Reference Feagins17, Reference Barnaud28]. This is unlikely to be achieved during the preparation of ready-to-eat pork products or during the cooking of sausages by consumers. These products will also have a high content of cellular and tissue proteins as well as fat and may be a matrix which can stabilize the virion and protect it against heat degradation.
Our study found that HEV infection, in cases with no recent travel history outside the UK, was associated with the consumption of sausages purchased from a major UK supermarket chain or ham purchased from the same supermarket or consumption of pork pie. Although contamination of sausages with HEV has been described previously, this study also raises public health concerns about other processed pork products as a potential source of HEV infection. As the evidence suggests that current recommended food processes, particularly cooking temperature and times used for ‘ready-to-eat’ processed pork products may be insufficient to inactivate the virus, the minimum industry standards should be reviewed. Further studies are necessary to ascertain the prevalence of HEV in the food chain and provide advice for safe preparation of these products.
ACKNOWLEDGEMENTS
We are grateful to the HPA Regional Hepatitis Leads for their support and colleagues nationwide in the Health Protection Teams and Public Health Wales, for assistance in contacting and interviewing cases and controls.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.






