Worldwide, more than 240 million persons are living with hepatitis B virus (HBV) infection [1]. HBV is highly infectious and is transmitted by percutaneous and mucosal exposure with blood and other bodily fluids [1]. Given this large reservoir of infected persons, exposure of healthcare personnel (HCP) to this bloodborne pathogen is a well-recognized occupational risk [2, Reference Hadadi3]. In susceptible HCP, the risk of transmission from an HBV-contaminated needle without post-exposure prophylaxis can be as high as 30% [Reference Beltrami4]. Globally, an estimated 66 000 HBV infections occur annually in HCP worldwide due to occupational exposure [Reference Wicker5]. Because of the high risk of HBV infection in susceptible HCP, routine pre-exposure vaccination against hepatitis B has been recommended since 1982 by the United States Centers for Disease Control and Prevention (CDC) [6] and the World Health Organization (WHO) [7]. At the time of this study (September 2010), American Samoa was formulating a hepatitis B vaccination policy for HCP prior to patient contact. In this study, we examined demographic and risk information, and hepatitis B vaccination, testing, and serostatus (both self-reported and laboratory-confirmed) in hospital employees in American Samoa.
American Samoa is a territory of the USA located in the South Pacific Ocean. This study was conducted at the island's only hospital, the Lyndon B. Johnson Tropical Medical Center (LBJTMC), and was approved by the American Samoa Government Institutional Review Board. All employees of the LBJTMC were eligible to participate. After written informed consent, participants completed a questionnaire and reported demographic and risk information, hepatitis B vaccination status (including number of doses received), and hepatitis B testing status. Serum samples were obtained for testing of HBV markers, which were anonymously linked to the respondent's survey results by a unique identification number. Initially, serum samples were tested for antibody to hepatitis B surface antigen (anti-HBs) and antibody to hepatitis B core antigen (total anti-HBc). The specimens testing positive for total anti-HBc were subsequently tested for hepatitis B surface antigen (HBsAg). HBsAg-positive specimens were tested for IgM antibody to hepatitis B core antigen. Anti-HBs levels ⩾10 mIU/ml were considered protective or ‘positive’; anti-HBs levels <10 mIU/ml were considered not protective or ‘negative’. All testing was performed using VITROS Immunodiagnostic System (Ortho Clinical Diagnostics, USA) at the Hepatitis Reference Laboratory, CDC, in Atlanta, GA, USA.
Based on available literature [6] and job information obtained during the interview, hospital employees were divided into two groups (HCP and non-HCP) based on their potential for exposure to blood and blood products and to patients and/or to infectious materials, including bodily substances, contaminated medical supplies, equipment, and environmental surfaces. Occupations considered as HCP included physicians, nurses, laboratory technicians, medical technicians, housekeepers, and laundry workers. Non-HCP referred to all hospital employees whose job did not involve exposure to potentially infectious materials. Types of non-HCP occupations included educators, and maintenance, administrative, and dietary workers.
We classified participants according to standard hepatitis B serological interpretation profiles. Participants who tested negative for all HBV markers were considered susceptible to HBV infection. Participants who tested negative for HBsAg and positive for total anti-HBc and anti-HBs, and participants who tested negative for total anti-HBc and positive for anti-HBs were considered immune by a resolved infection and by hepatitis B vaccination, respectively. Participants who tested positive for total anti-HBc and negative for all remaining HBV markers were considered isolated core antibody positive. Participants who tested positive for HBsAg were considered HBV-infected.
We described demographic characteristics [sex, age, number of years in patient care services (HCP only)], risk information (history of needlestick injury, history of blood transfusion), and hepatitis B vaccination, testing, and serostatus. We compared the proportions of these categories by HCP status and HBV susceptibility status. Knowledge of hepatitis B vaccination, testing, and infection status was assessed by examining the proportion of participants who reported that they did not know their hepatitis B vaccination, testing, and infection status. For HCP participants, knowledge of HBV susceptibility status was assessed by comparing serologically confirmed HBV status with self-reported HBV status. Pearson's χ 2 test was used to assess the relationship between (1) HCP status and these characteristics and (2) HBV susceptibility status and these characteristics. P values <0·05 were considered statistically significant.
All analyses were performed using SAS software, version 9.2 (SAS Institute Inc., USA).
Of the 604 persons employed at LBJTMC, 231 (38·2%) completed the survey and provided serum for HBV testing (Table 1). The majority of respondents were female (75.1%), and the median age was 38·5 (range 19·0–70·0) years.
Table 1. Characteristics of study participants employed at the Lyndon B. Johnson Tropical Medical Center by healthcare personnel status – American Samoa, September 2010
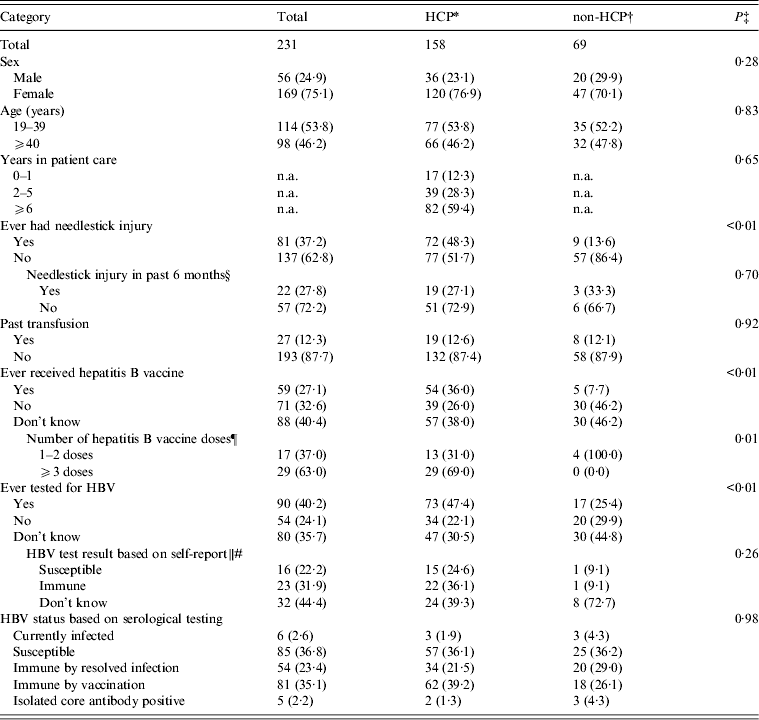
HCP, Healthcare personnel; non-HCP, non-healthcare personnel; HBV, hepatitis B virus; n.a., not applicable.
Data are presented as no. (%). The sum for each category may not add up to the overall column total because of excluded missing values. Serological testing revealed no acute HBV infections. HCP status on four hospital employee participants could not be determined due to insufficient job information.
* Occupation listed: 75 nurses/nurse assistants, 17 housekeepers, 15 laboratory technicians, 13 physicians, 11 medical, dental, respiratory, and operating-room technicians/assistants, 11 phlebotomists, nine laundry workers, one dentist, one respiratory therapist, and one mortician. Department listed with no occupation mentioned: two in emergency room, one in respiratory therapy, and one in radiology.
† Occupation listed: 36 administrators and assistants, 13 maintenance technicians, nine dietary/food workers, four security agents, three educators, two communication specialists, one pharmacy technician, and one social worker.
‡ For statistical testing of significant differences between HCP participants and non-HCP participants using Pearson's χ 2 test, categories with greater than two levels were collapsed into two levels. These were: years in patient care = 0–5 years and ⩾6 years; ever received HB vaccine and ever tested for HBV = yes and no/don't know; HBV test result based on self-report = susceptible and all others; and HBV status based on serological testing = susceptible and all others.
§ Denominator included only study participants who responded ‘Yes’ to the question ‘Have you ever had a needlestick injury?’
¶ Denominator included only study participants who responded ‘Yes’ to the question ‘Have you ever received the hepatitis B vaccine?’
∥ Denominator included only study participants who responded ‘Yes’ to the question ‘Have you ever been tested for hepatitis B?’
# One non-HCP participant self-reported chronic HBV infection.
Of the 231 participants, 158 (68·4%) were HCP. Of these, 76·9% were female and 53·8% were aged <40 years (Table 1). The majority (59·4%) of HCP participants reported ⩾6 years in patient care services. HCP participants more frequently reported history of needlestick injury (48·3% vs. 13·5%), hepatitis B vaccination (36·0% vs. 7·7%), receiving ⩾3 doses of the hepatitis B vaccine (69·0% vs. 0·0%), and prior HBV testing (47·4% vs. 25·4%) than non-HCP participants (P⩽0·01 for all comparisons). In the 6 months preceding the study, 19/70 (27·1%) HCP participants reported needlestick injury compared to 3/9 (33·3%) non-HCP participants (P = 0·70). Of the 61 HCP participants who reported ever being tested for HBV and reported a test result, 22 (36·1%) reported their status as immune, 15 (24·6%) reported their status as susceptible, and 24 (39·3%) did not know their test result.
Three HCP participants and three non-HCP participants had chronic HBV infection; there were no acute infections (Table 1). Overall, 57 (36·1%) HCP participants and 25 (36·2%) non-HCP participants were susceptible to HBV; and 96 (60·8%) HCP participants and 38 (55·1%) non-HCP participants had evidence of immunity to hepatitis B (P > 0·05).
Of 57 HCP participants who were serologically confirmed to be susceptible to HBV, 73·7% were female, 54·7% were aged <40 years, 49·0% had ⩾6 years in patient care, 52·8% had no history of a needlestick injury, and 90·9% had no history of a past blood transfusion (Table 2). Assessment of hepatitis B status revealed that 62·9% either had not or did not know if they have received hepatitis B vaccination, and 56·2% either had not or did not know if they were ever tested for HBV. Of 25 susceptible HCP participants who were ever tested for HBV, 10 correctly classified their status. Susceptible HCP participants did not differ from non-susceptible HCP participants by sex, age, years in patient care services, history of needlestick injury, needlestick injury within the last 6 months, past transfusion, receipt of hepatitis B vaccine, and ever tested for HBV (P > 0·05). There was no difference in susceptibility in HCP who had direct patient contact (e.g. physicians, nurses) and HCP who had indirect patient contact (i.e. housekeepers and laundry workers) (35·6% vs. 38·5%, respectively; P = 0·78) (data not shown).
Table 2. Characteristics of healthcare personnel employed at the Lyndon B. Johnson Tropical Medical Center by laboratory-confirmed HBV susceptibility status – American Samoa, September 2010
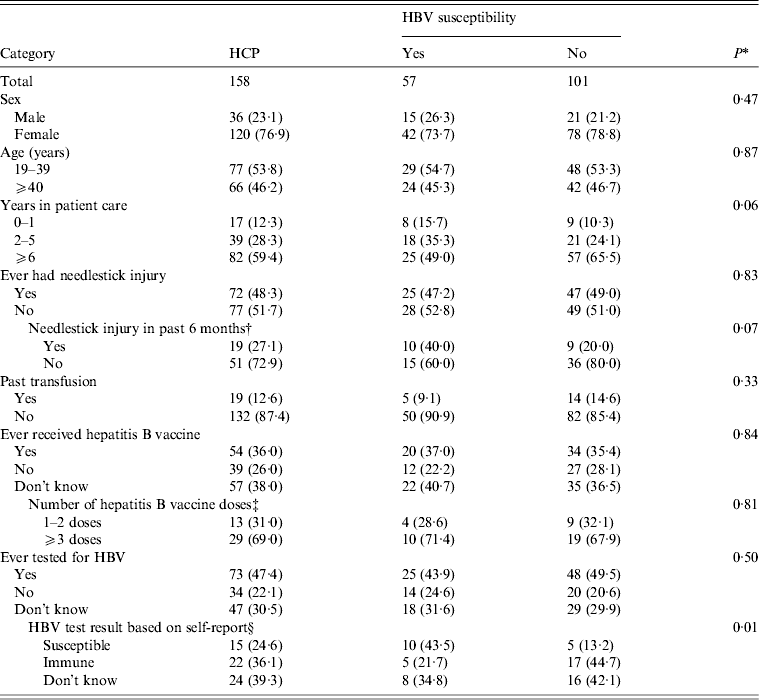
HCP, Healthcare personnel; HBV, hepatitis B virus.
Data are presented as no. (%). The sum for each category may not add up to the overall column total because of excluded missing values.
* For statistical testing of significant differences between susceptible HCP participants and non-susceptible HCP participants using Pearson's χ 2 test, categories with greater than two levels were collapsed into two levels. These were: years in patient care = 0–5 years and ⩾6 years; ever received HB vaccine and ever tested for HBV = yes and no/don't know; and HBV test result based on self-report = susceptible and all others.
† Denominator included only study participants who responded ‘Yes’ to the question ‘Have you ever had a needlestick injury?’
‡ Denominator included only study participants who responded ‘Yes’ to the question ‘Have you ever received the hepatitis B vaccine?’
§ Denominator included only study participants who responded ‘Yes’ to the question ‘Have you ever been tested for hepatitis B?’
This is the first study to examine hepatitis B vaccination, testing, and infection status in HCP in American Samoa – a US territory that had no formal hepatitis B vaccination policy for HCP. Research from the 1980s established an intermediately high HBV prevalence (5–8%) in the territory [Reference Mahoney8]. However, we expect the overall prevalence to have decreased since then because of the island-wide hepatitis B vaccination policy implemented in 1986 for infants and children. A recent study conducted in American Samoan college students found that of those who had completed the primary hepatitis B vaccination series during infancy, nearly 90% had an anti-HBs level below the protection threshold around 20 years later [Reference Spradling9]. Of these, 50% with a baseline anti-HBs level of 0 IU/l and 83% with levels between 1 and 9 IU/l achieved an amnestic response with a challenge dose [Reference Spradling9]. In the healthcare setting, findings revealed several major programmatic gaps resulting in opportunities for acquiring HBV infection. Because the majority of HCP are older and less likely to be vaccinated as part of the island-wide hepatitis B vaccination policy, it is important that all new and existing susceptible HCP are protected from hepatitis B through implementation of a hepatitis B vaccination policy for HCP.
We found that almost half of HCP participants reported sustaining a needlestick injury, and a report of this type of injury occurred nearly four times more frequently in HCP participants than non-HCP participants. This finding indicates that the implementation of universal precaution measures, such as sharps training, would be extremely beneficial to all HCP employees. An example of such precautionary measure is the use of auto-disabled syringes, which could prevent both needlestick injury and syringe re-use. An unexpected finding, however, was that in the 6 months preceding this study, HCP participants were less likely to have a needlestick injury than non-HCP participants. Although the difference was not statistically significant, the difference may have existed because we considered housekeepers and laundry workers as HCP. These two occupational groups have a high chance of blood exposure but low chance of needlestick injury, and together, accounted for 16·5% of HCP. In addition, it is possible that some non-HCP could have been working as HCP in the 6 months prior to the study. For example, an administrative clerk could have been working as a nurse within 6 months of this study. However, we did not systematically collect information regarding changing duties and responsibilities while employed by the hospital.
For HCP participants, we also found that the prevalence of resolved HBV infection was 11 times higher than the prevalence of chronic HBV infection. Because chronic infection develops in up to 90% of persons infected as infants compared to 25–30% of persons between ages 1–5 years and about 10% of persons infected at age >5 years [Reference McMahon10], our data suggest that HBV infection occurred more during adulthood rather than during birth or childhood. Of concern, all of the chronically infected hospital employee participants and the majority of HCP participants were either never tested for HBV, did not know if they were ever tested for HBV, or if tested, were unaware of their HBV status. While CDC recommends that HBV infection alone should not disqualify infected persons from the practice or study of surgery, dentistry, medicine, or allied health fields, the HBV DNA serum level of HBsAg-positive HCP should be monitored to assess safe levels of HBV DNA (<1000 IU/ml) for those performing exposure-prone invasive procedures.
This study is not without limitations. First, participation was voluntary in an effort to reach and offer hepatitis B testing to as many hospital employees as possible. Still, we were able to recruit 38·2% of all hospital employees in American Samoa. Second, we were unable to compare characteristics between participants and non-participants in order to assess the representativeness of our sample. Therefore, results may not be generalizable to all LBJTMC employees. Finally, the self-reported responses from the survey portion of our study may be subject to recall or social desirability bias. Despite these limitations, this study is still the first to assess hepatitis B vaccination coverage and testing, and to examine the prevalence of serologically confirmed HBV infection, susceptibility, and immunity in HCP in American Samoa.
In conclusion, the adoption of a hepatitis B vaccination policy for HCP by America Samoa, as currently recommended by the CDC and the WHO is crucial. Our study suggests that HCP in American Samoa would benefit from the implementation of a comprehensive hepatitis B vaccination programme that includes post-vaccination testing to confirm that a protective response has occurred.
ACKNOWLEDGEMENTS
The authors thank Natalia Khudyakova of the Assay Development and Diagnostic Reference Laboratory, Division of Viral Hepatitis, Centers for Disease Control and Prevention, and the staff of the Immunization Program of the American Samoa Department of Health for their generous assistance during this project.
Kathleen Ly was supported in part by an appointment to the Research Participation Program at the U.S. Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an inter-agency agreement between the U.S. Department of Energy and the Centers for Disease Control and Prevention.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.




