INTRODUCTION
Hepatitis A virus (HAV), belonging to the Picornaviridae family, is one of the most common viral infections of the liver with a global distribution [Reference Koff1]. The modes of transmission are numerous, e.g. close individual contact or sexual contact [Reference Cotter2], ingestion of contaminated food or water [Reference Franco3], plus other means [Reference Koff1, Reference Almasio and Amoroso4]. This virus has a high prevalence in crowded regions with low socioeconomic conditions and poor hygiene [Reference Jacobsen and Koopman5, Reference Naoumov6]. Asymptomatic infection of this virus is seen during early childhood which results in long-lasting immunity [Reference Lednar7]. Severe disease and fulminant liver failure due to HAV may occur in adulthood or the elderly population and in patients with chronic liver disease (CLD) [Reference Vento8].
The Islamic Republic of Iran is an endemic area for HAV infection; however, its seroprevalence varies in different regions [Reference Mohebbi9]. During recent decades, due to improvements in sanitation and hygiene, the age at which infection by this virus occurs has shifted from childhood to adolescence or beyond [Reference Hendrickx10, Reference Lefilliatre and Villeneuve11]. Studies have shown that the overall case-fatality rate (CFR) in adults aged ⩾50 years is 1·8% [Reference Song12], whereas the CFR in all ages is 0·01–0·3% [Reference Fiore13], therefore the complications of this disease such as fulminant hepatitis and even death are more prevalent in the older population [Reference Saberifiroozi14]. More importantly, when patients with CLD are infected with HAV, fulminant liver failure worsens and mortality and morbidity rates increase [Reference Moon15, Reference Kim16]. There is increasing evidence that patients infected with HAV and who have CLD due to hepatitis C or B, have a more severe disease than those who do not have hepatitis C virus (HCV) or hepatitis B virus (HBV) infections [Reference Lefilliatre and Villeneuve11]. The CFR for acute hepatitis A was 5·6 times higher in HBsAg carriers than HBsAg-negative patients [Reference Yao, Hollinger, Lemon and Margolis17]. This can be seen in patients with chronic HCV and HBV who have also been infected with hepatitis A. Geographically, the prevalence rate of HAV antibodies in CLD patients has been studied in some regions of Iran [Reference Taghavi18–Reference Shavakhi20], but not in Jahrom. In this study we decided to first investigate the prevalence of HAV antibodies (anti-HAV) in patients infected with HCV or HBV and second, to determine factors that affect IgG anti-HAV seropositivity.
METHODS
Design and sample population
A descriptive cross-sectional study was designed, using patients with CLD (n = 159) who were registered at the Honary Medical Clinic Centre in Jahrom, a town in the south part of Iran, between September 2012 and February 2013. Patients provided signed informed consent prior to participation. Diagnosis of CLD patients was based on two positive results in the last 6 months which detected the presence of HBsAg, HCV antibody (anti-HCV), and HCV-RNA. The status of liver disease was classified into chronic hepatitis and liver cirrhosis (LC). The exclusion criteria consisted of patients with HIV infection, CLD caused by non-viral agents, and past history of HAV vaccination. The seroprevalence of IgG anti-HAV was evaluated. We then analysed our data according to sex, age, disease status, and compared it with the general healthy population and patients with CLD. Patients were classified into the following five age groups: (1) group A: 21–30 years; (2) group B: 31–40 years; (3) group C: 41–50 years; (4) group D: >50 years.
Data collection
Information was collected from each patient by questionnaire that included demographical data such as age, gender, marital status, socioeconomic characteristics including educational level, number of siblings, number of household members, salary, type of residence, income, and water supply.
Laboratory procedures
A 3-ml blood sample was taken from each patient. Sera were isolated, stored at −20°C, and used for assay. ELISA (a commercially available kit) was used for detection of antibodies against hepatitis A, anti-HAV IgG (Dia.Pro Diagnostic BioProbes Srl, Italy), HBsAg (DiaSorin, Spain), and anti-HCV (Dia.Pro Diagnostic Bioprobes Srl). The HCV RNA was amplified by RNA PCR using TaqMan technology according to Roche HCV RT-PCR assay (7500 Real-time PCR system; Applied Biosystems, USA).
Statistical analysis
All data analyses were performed using SPSS statistical software v. 16.0.1 (SPSS Inc., USA). Continuous variables were expressed as mean and standard deviation for normally distributed data while categorical variables were expressed as frequency and percentage. A χ 2 test was used to determine whether significant differences exist between two categorical variables. For multivariate analyses, a stepwise multivariate logistic regression model was employed to assess the relative importance of variables showing a significant association (P < 0·05). Results of all multivariable analyses were reported as adjusted odds ratio, 95% confidence interval and exact P value.
RESULTS
Patients' demographics
The patients' values are shown in Table 1. The mean age (±s.d.) of patients was 41·03 ± 12·12 years (range 21–68 years). Males comprised the majority (74·8%) and most of the patients were aged >40 years (55·9%). This distribution reflects the current diversity in our population. Our data show that 35·8% of CLD patients were infected with HBV and 64·2% with HCV. Cirrhotic patients formed 10·6% of those with CLD. The overall prevalence of anti-HAV in CLD patients was 79·2%.
Table 1. Patients' characteristics

Prevalence of IgG anti-HAV based on the aetiology and status of liver disease
The prevalence of anti-HAV in patients with hepatitis C was 80·4% and in hepatitis B patients it was 78·9%. These values were not significantly different (P = 0·38). The prevalence of anti-HAV based on CLD status is shown in Figure 1. The anti-HAV seroprevalence in chronic hepatitis B patients was 77% and when this led to cirrhosis it became 100%. Similarly, in chronic hepatitis C patients this percentage was 78% which changed to 100%, respectively. Additionally, the anti-HAV prevalence was 77·2% in non-cirrhotic patients compared to 100% in cirrhotic patients.
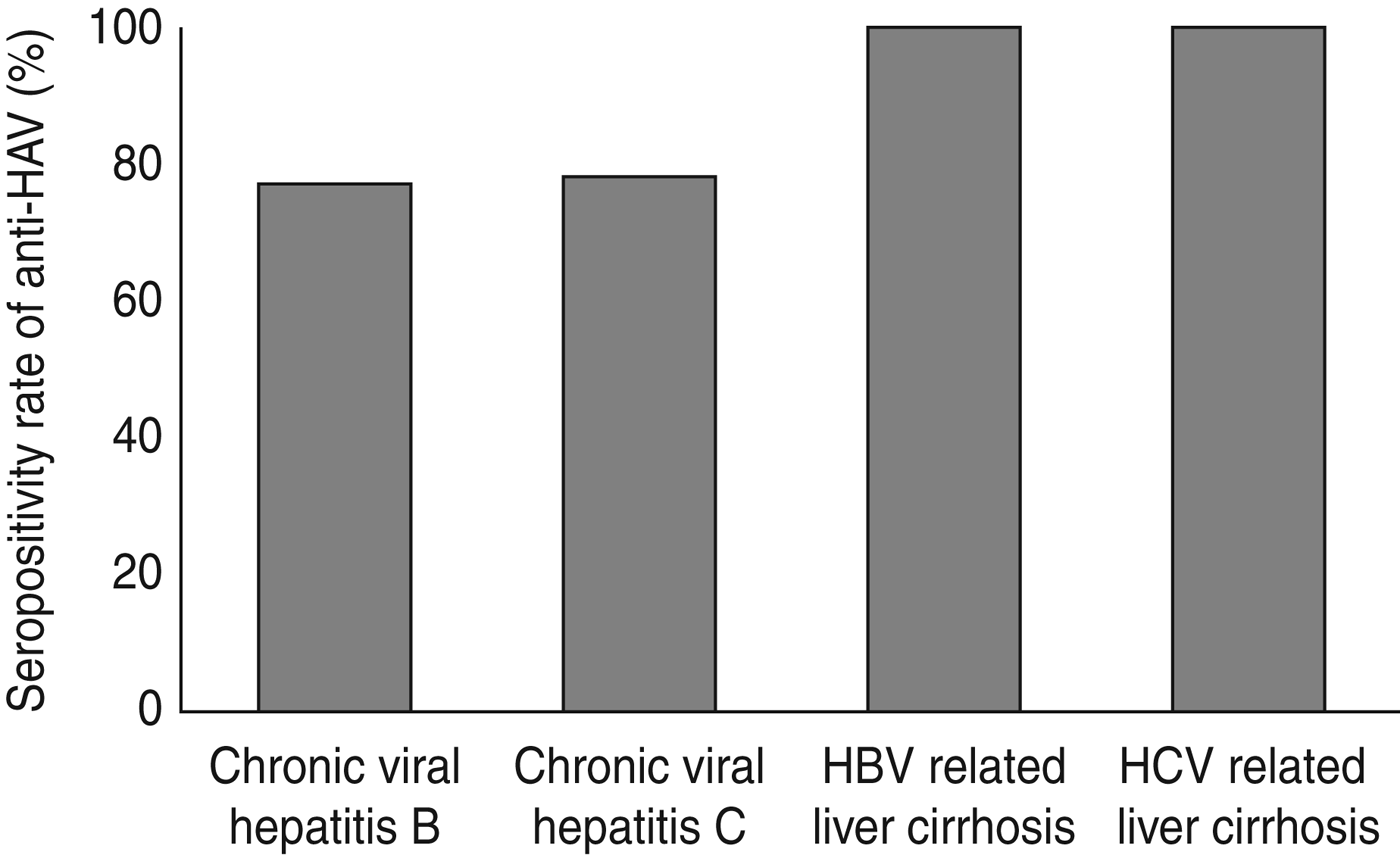
Fig. 1. The prevalence of IgG anti-HAV based on the aetiology and status of liver disease. HBV, Hepatitis B virus; HCV, hepatitis C virus, HAV, hepatitis A virus.
Prevalence of IgG anti-HAV according to age
The patients' ages (range 20–50 years) were divided into four groups (Table 2). The anti-HAV seroprevalence was 28·3% in group A, and >90% in the other groups. The prevalence of anti-HAV in CLD patients is shown in Figure 2. The seropositivity rate for anti-HAV increased gradually as age increased. The anti-HAV prevalence was significantly higher in patients aged >30 years compared to patients aged <30 years (P < 0·001). The prevalence of IgG anti-HAV according to age in the age- and gender-matched patients was 81·2%. There was no significant difference in anti-HAV seroprevalence between CLD patients and those from the Honary Medical Clinic Centre (P = 0·16).
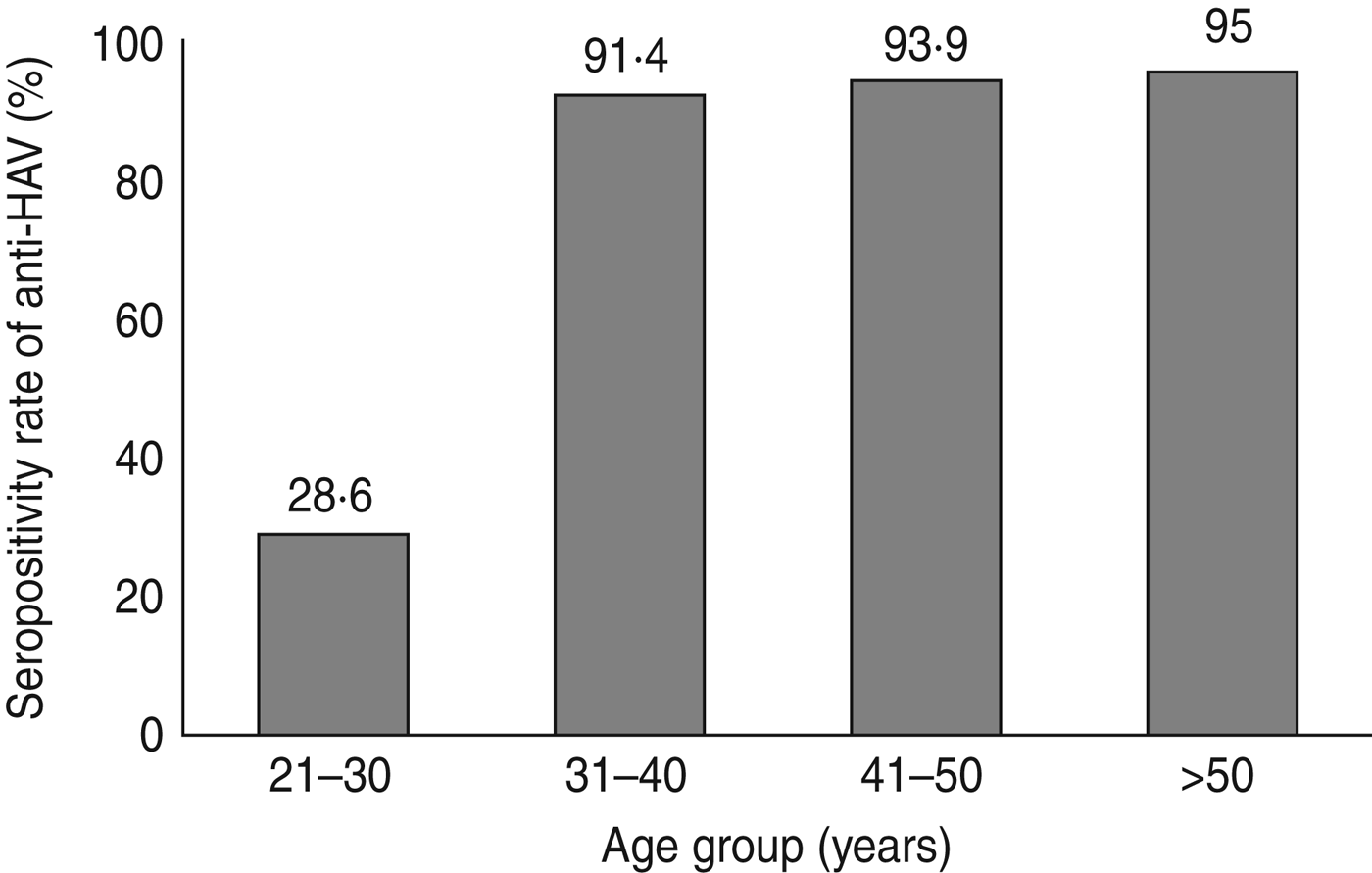
Fig. 2. Seropositivity rate of anti-hepatitis A IgG by age group.
Table 2. Factors affecting seropositivity for IgG anti-hepatitis A virus in the multivariate analysis
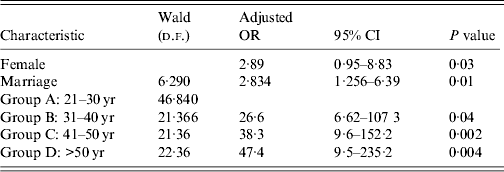
OR, Odds ratio; CI, confidence interval.
Factors affecting the seropositivity rate of anti-HAV
Based on sex, anti-HAV was more frequently detected in male (75·6%) than in female patients (90%, P = 0·038). Multivariate analysis of age group variables also showed a significant difference between patients aged <30 years and those who were older (P < 0·001). The general rate of anti-HAV was higher in married individuals than in unmarried patients (P = 0·012) (Table 2).
DISCUSSION
The epidemiological pattern of HAV infection is currently changing in many developing countries. In addition to data from Shanghai [Reference Yao, Hollinger, Lemon and Margolis17], epidemiological data from the Centers for Disease Control and Prevention (CDC) are often cited to quantify the risks of HAV super-infection in CLD patients with chronic HBV and HCV, and HAV infection superimposed on other forms of CLD [Reference Keeffe21, Reference Hadler, Hollinger, Lemon and Margolis22]. There are only limited published data about CLD patients with exposure to hepatitis A in Iran.
The prevalence of natural protection against HAV in CLD patients was 79·2% as shown in Table 1. This percentage (79·2%) was lower than the percentage (90%) found in other studies from Iran and Italy [Reference Saberifiroozi23, Reference Sagnelli24]. This is probably due to differences in cultural and socioeconomic standards. Patients aged >30 years had higher prevalence (90%) than younger patients. This suggests that most of these older patients were exposed to HAV when they were young and acquired immunity spontaneously against hepatitis A, whereas for patients aged <30 years, the positive rate for anti-HAV was found to be lower than 30% suggesting that most of these younger patients lacked immunity and had not been exposed to HAV. Thus individuals are more susceptible to HAV infection when younger whether they are in good health or CLD patients, as has been shown in Asian populations [Reference Joshi25, Reference Cho26]. Moreover, in regions where hepatitis A vaccination is not routine, HAV seroprevalence usually increases as people age. Studies have shown there is a pattern in the shifting of age at which people become infected from young to old in developing countries [Reference Saberifiroozi14, Reference Movahedi27]. Therefore, less infection in younger people causes less seroprevalence in this group. Another reason for low HAV seroprevalence in young people is improvements in sanitary conditions, hygiene practices, and socioeconomic conditions [Reference Shavakhi20, Reference Cho26, Reference Zhu28]. Therefore, appropriate vaccination programmes for young people should be implemented, as in other countries. Vaccination against HAV is important. If young people are not vaccinated they will become more sensitive to infection as they age. They will become even more sensitive to HAV and death may occur if they have CLD and become infected with HAV [Reference Moghani, Manzouri and Alavian29–Reference Lee31].
In the current study, the prevalence of IgG anti-HAV in the general healthy population aged between 21 and 30 years, and ⩾31 years was found to be similar to that of CLD patients, which parallels that of developing and developed countries [Reference Cho26, Reference Deoliveira, Comácio and Gonçalves32, Reference Saab33]. In this study there was no significant difference in the anti-HAV rate between the two groups of CLD patients and the general population (79·2% vs. 81·2%, respectively, P = 0·16) regardless of age. We expected the seroprevalence of anti-HAV to be higher in CLD patients than healthy individuals, considering the relatively low socioeconomic status of CLD patients, but this was not the case.
As suggested by others, we believe that a universal vaccination is better for older people in the general population [Reference Lee31, Reference Klevens34]. This may appear costly but we believe it will be beneficial. This result is also consistent with the results of the multivariable analysis in our study. The seropositivity for HBV and HCV infection in CLD patients was 78·9% and 80·4% (P = 0·38), respectively. This lack of statistical difference may indicate that the immune system response against HAV infection is not altered in this study and also that there is a significant relationship between anti-HAV seropositivity and CLD aetiology, as demonstrated by other studies [Reference Somi19, Reference Joshi25, Reference Ahmad35, Reference Ahn36].
As shown in the multivariable analysis and Figure 1, patient's age was the most important factor for determining the anti-HAV seropositivity rate. The seropositivity of anti-HAV was more frequently detected in patients with LC than in those with CLD (100% vs. 70·4%); because LC patients were older than CLD patients we believe age is a contributing factor, in accord with other studies [Reference Cho26, Reference Saab33]. The higher seropositivity of anti-HAV in older people may be explained by the fact that they lacked good hygiene practice in the past and were probably exposed to HAV leading to production of anti-HAV. This view is in line with other studies [Reference Cho26, Reference Acharya37].
Gender in our study is another factor influencing HAV seropositivity as shown previously [Reference Cho26]. This data might be explained by the fact that females in Jahrom have a larger number of social and household tasks and therefore probably have more contact with sources of HAV.
Married people had a higher seropositivity of anti-HAV probably because of greater contact between individuals. This might also be due to the number of family member living in crowded accommodation.
The lower seroprevalence of HAV in this study (79·2%) than other studies in Iran (<90%) may be due to the religious culture in Jahrom. Therefore, due to frequent washing of hands, the chance of exposure to sources of HAV infection is decreased. In conclusion the anti-HAV seroprevalence in young CLD patients was lower than in older patients and there was no relationship between this HAV seropositivity and the presence of HBV and HCV in CLD patients. Finally seropositivity was higher in LC patients than non-cirrhotic individuals and vaccination is best administered at a younger age.
DECLARATION OF INTEREST
None.
ACKNOWLEDGEMENTS
The authors acknowledge the cooperation of the staff of the Department of Microbiology, Jahrom University.







