Introduction
Infection with intestinal protozoa such as Giardia duodenalis (syn. Giardia lamblia, Giardia intestinalis) and Cryptosporidium spp. can cause recurrent and persistent diarrhoeal disease, leading to widespread morbidity globally [Reference Sow1, Reference Pires2]. In the USA, Cryptosporidium is a leading cause of waterborne outbreaks, with an estimated incidence of three reported cases per 100 000 persons per year [Reference Painter3]. In 2012, the rate of giardiasis was estimated to be six reported cases per 100 000 persons per year in the USA [Reference Painter4]. After accounting for underreporting, these two parasites combined are estimated to cause over 1 million total episodes of disease per year in the USA [Reference Scallan5]. In Alaska, annual incidence rates of Cryptosporidium tend to be low, but giardiasis incidence is routinely higher than the rest of the USA [6].
Both Giardia and Cryptosporidium are transmitted fecal-orally, either person-to-person, animal-to-person, or through contaminated food or water. Contamination can originate either from humans or from animals. In the USA, source water at 46% and 25% of 25 drinking water treatment facilities was found to be contaminated with Giardia cysts and Cryptosporidium oocysts, respectively, indicating the presence of the pathogens in the environment [Reference King7]. A recent meta-analysis showed that access to sanitation facilities and use of treated water decrease the risk of Giardia and Cryptosporidium infection [Reference Speich8]. In the USA, seropositivity to Cryptosporidium has also been associated with nonwhite race or ethnicity and low socio-economic status [Reference Frost9, Reference Becker, Oloya and Ezeamama10]. Some studies have identified risk factors that are specific to particular strains of Giardia (known as assemblages); human infection with assemblage A has been associated with contact with animals while assemblage B has been associated with human–human transmission [Reference Pijnacker11, Reference Minetti12].
Water insecurity is a pervasive concern among the geographically-isolated communities in rural Alaska [Reference Penn, Loring and Schnabel13]. In 2010, 22% of rural households did not have access to in-home water and sewer services [14]. Alaska is ranked last among all US states regarding complete plumbing, defined as hot and cold piped water, a bath- tub or shower and a flush toilet. Among the ten census areas in the USA ranked lowest in the proportion of homes served, seven are in Alaska [15]. Households that do not have piped or ‘running’ water may collect potable water from a treated community watering point or collect surface water, rain, snow, or ice to use as drinking and cooking water [Reference Penn, Loring and Schnabel13]. In this population, lack of access to piped water is associated with increased respiratory hospitalisations in children, skin infections, gastrointestinal infections, invasive pneumococcal disease and nasopharyngeal pneumococcal carriage [Reference Bruden16–Reference Thomas21]. Hunted mammals and other wildlife have been shown to carry Giardia and Cryptosporidium, which could lead to direct transmission to humans or contamination of water sources [Reference Hughes-Hanks22–Reference Siefker24]. However, associations between access to water and intestinal parasites have not yet been identified in Alaska.
In 2007 and 2008, we conducted a zoonotic pathogen serosurvey of four groups in Alaska: subsistence bird hunters and their families, sport hunters, wildlife biologists and non-bird hunters. In this analysis, we evaluated the prevalence of Giardia and Cryptosporidium antibodies, assessed correlates of exposure in each group and tested whether access to in-home running water was associated with the prevalence of these antibodies.
Methods
Study design
This study is a secondary analysis of sera collected for an investigation of exposure to highly pathogenic avian influenza virus H5N1, as described by Reed et al. [Reference Reed25]. The overall study evaluated zoonotic pathogens among four different groups in Alaska who could be at high risk for avian influenza. The objectives of the current analysis were to evaluate the risk factors for IgG seropositivity to Giardia and Cryptosporidium in each group. Study participants were ⩾5 years of age and resided in urban or rural communities throughout Alaska. Groups included: (1) residents who likely had little to no exposure to wild birds (labelled hereafter as ‘non-exposed’), (2) wildlife biologists, (3) sport hunters and (4) subsistence bird hunters (labelled hereafter as ‘subsistence hunters’) and their family members. The non-exposed group comprised Alaskans who did not hunt wild birds and resided in Anchorage or Bethel (a rural hub community). These participants were recruited through flyers on health campuses and newspaper advertisements. Wildlife biologists must have been engaged in fieldwork for at least one field season in Alaska within the past 5 years. Biologists were recruited through flyers mailed to researcher offices, email lists, conference attendees and members of the Audubon Society. A sport hunter was defined as an individual who participated in the hunting of wild birds. These hunters were recruited in Anchorage and Dillingham (a rural hub community) through mailings to hunting license lists. A subsistence bird hunter was defined as an individual who participated in the hunting of birds and lived in a rural area of south-west Alaska. Hunters of either type must have hunted for at least 2 years in their lifetime and hunted at least one time in the 2 years prior to enrollment. Family members of subsistence hunters were also recruited. Subsistence hunters and their families were recruited through flyers posted in public areas in each community. Any study participants could have hunted animals other than birds; this information was not collected. Participants ⩾18 years of age provided written, informed consent. Participants <18 years old provided written, informed assent in addition to their parent or legal guardian's written consent. This study was approved by the regional Alaska Native Tribal Health Organizations, the Institutional Review Boards of the Alaska Area Native Health Service and the Centers for Disease Control and Prevention (CDC).
Questionnaire
Upon enrollment, the study team verbally administered a standard questionnaire to participants about location of residence, demographic information (age, gender, the highest level of formal education, occupation), household plumbing and water use practices. Water use questions included ‘Do you have running water in your home?’ and ‘Do you collect rainwater, ice or snow, to drink or cook within your home? If yes, do you treat the water? If yes, how is it treated?’ For community-level analyses, each community was assigned a proportion value based on the percentage of participants that reported water access or water collection.
Serological testing
The study team collected 10 ml of blood from adults and children. Participants who declined to submit a blood specimen were excluded from the analysis. The methods for Giardia and Cryptosporidium serological testing by multiplex bead assay (MBA) are described elsewhere [Reference Priest26, Reference Moss27]. Briefly, we used antigen-coupled SeroMap beads (Luminex Corporation, Austin TX) in the MBA to detect IgG antibodies to five of the Giardia variant-specific surface protein conserved structural regions (VSPs 1–5) representing both of the human-infective assemblages (A and B) [Reference Thomas21]. Samples were run in duplicate and data were reported as the average of the median fluorescence intensity minus the buffer-only background blank (MFI-bg). Giardia antibody response cutoffs were established previously using a set of sera from 65 US citizens with no history of foreign travel [Reference Moss27]. After removing the three highest responses for each antigen, the mean + 3SD cutoffs calculated for VSP1–5 were 209, 270, 262, 209 and 206 MFI-bg units, respectively. Participants were considered to have been exposed to Giardia if they had positive antibody responses to at least two of the recombinant VSP proteins. SeroMap beads coupled with recombinant Cryptosporidium parvum 17- and 27-kDa antigens were included in the assays to detect IgG antibodies to Cryptosporidium sp. [Reference Priest26]. Cutoffs of 933 and 1870 MFI-bg units for Cp17 and Cp23, respectively, were established using a panel of 42 human sera previously characterised using the ‘gold standard’ large format Western blot IgG assay [Reference Priest26, Reference Priest28]. Participants were considered to have been exposed to Cryptosporidium if they had positive antibody responses to both antigens. We also tested for IgG antibody responses to the carboxy-terminal, immunodominant peptide of the C. parvum 60S acidic ribosomal protein P2 (CpP2) [Reference Benitez29, Reference Priest30]. No CpP2 cutoff value was assigned. A SeroMap bead coupled with recombinant Schistosoma mansoni glutathione-S-transferase protein with no fusion partner was included in each well as a negative control.
Statistical analysis
Exposure groups were analysed separately. We described the risk factor distribution in each group using means and frequencies. We then described the prevalence of Giardia and Cryptosporidium antibody positivity by demographic factors within groups. For Giardia, statistical analyses were conducted using the continuous values for VSP3 (assemblage A) and VSP5 (assemblage B) and the binary values using the cut-offs for seropositivity, as described above. We used log-binomial general estimating equations clustered on the community of residence to assess risk factors for the serum MFI-bg for Giardia and Cryptosporidium among subsistence hunters using Stata14 (StataCorp LP, College Station, TX). We first evaluated univariable then multivariable models, controlling for variables chosen as potential determinants of exposure a priori. In these models, we used individual-level and community-level exposures to water access, as described above. Prevalence ratios (PR) are presented with corresponding 95% confidence interval (CI).
Results
We recruited 916 participants; 887 were tested for Giardia and Cryptosporidium antibodies, including 160 sport hunters, 454 subsistence hunters and their family members, 77 wildlife biologists and 196 non-exposed. The subsistence hunters and their families lived in six communities in rural southwest Alaska while the sport hunters, biologists and non-exposed groups lived in the Anchorage area, Fairbanks, Bethel and Juneau. The groups had significantly different demographic characteristics (Table 1). Sport hunters had the highest mean age (44 years) and the non-exposed group had the highest proportion of women (71%). Subsistence hunters and their families had the highest proportion of participants that did not have piped water in their homes. High proportions of biologists and subsistence hunters and family members reported collecting snow, rain, or ice for drinking or cooking. Among participants who reported treating any of their water, boiling was the most common method of water treatment (72%).
Table 1. Characteristics of sport hunters, subsistence bird hunters and families, wildlife biologists and non-exposed participants, Alaska 2007–2008
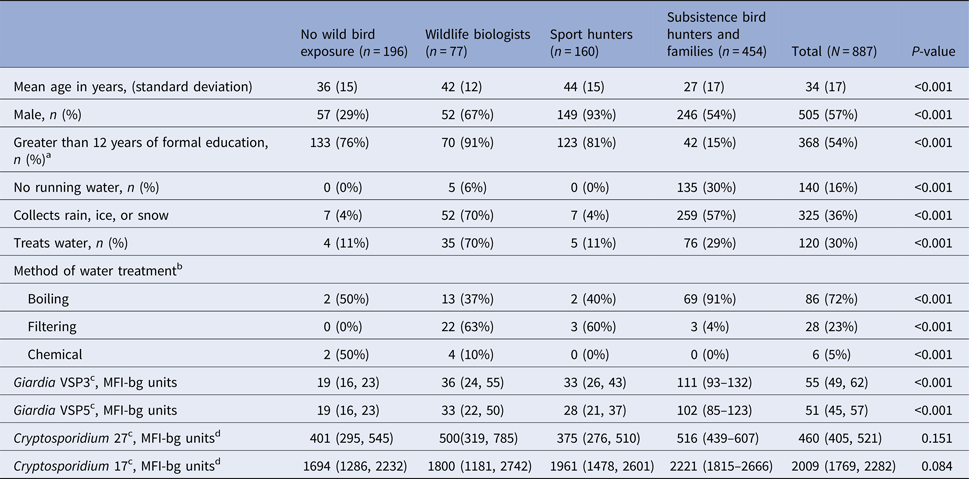
a Among participants ⩽18 years old.
b Among participants who reported treating water.
c Geometric mean concentration (95% CI).
d MFI-bg: median fluorescence intensity minus buffer-only background.
The geometric mean MFI-bg value of anti-VSP3 and VSP5 Giardia antibodies varied across groups (Table 1); subsistence hunters and their families had the highest geometric mean responses (geometric mean 111 (95% CI 93–132) for VSP3 and 102 (95% CI 85–123) for VSP5) . Cryptosporidium 17 and 27 antibody MFI-bg values did not differ across groups. Giardia values were positively correlated with each other and with both Cryptosporidium values (data not shown, P < 0.001 for all). Cryptosporidium CpP2 antibody response values were generally low (median <10 MFI-bg units, standard deviation = 43).
Similar to the MFI-bg continuous values, the prevalence of positive Giardia antibody responses was highest among subsistence hunters and their families (30% positivity), while the prevalence of positive Cryptosporidium antibody responses was similar across groups (Table 2). Giardia antibody prevalence tended to be higher among men while Cryptosporidium prevalence was higher among women in some groups. Prevalence of Cryptosporidium positivity was higher among older age groups. Neither Giardia nor Cryptosporidium antibody prevalence showed trends across formal education level.
Table 2. Giardia and Cryptosporidium IgG antibody seroprevalence by demographic factors, Alaska 2007–2008
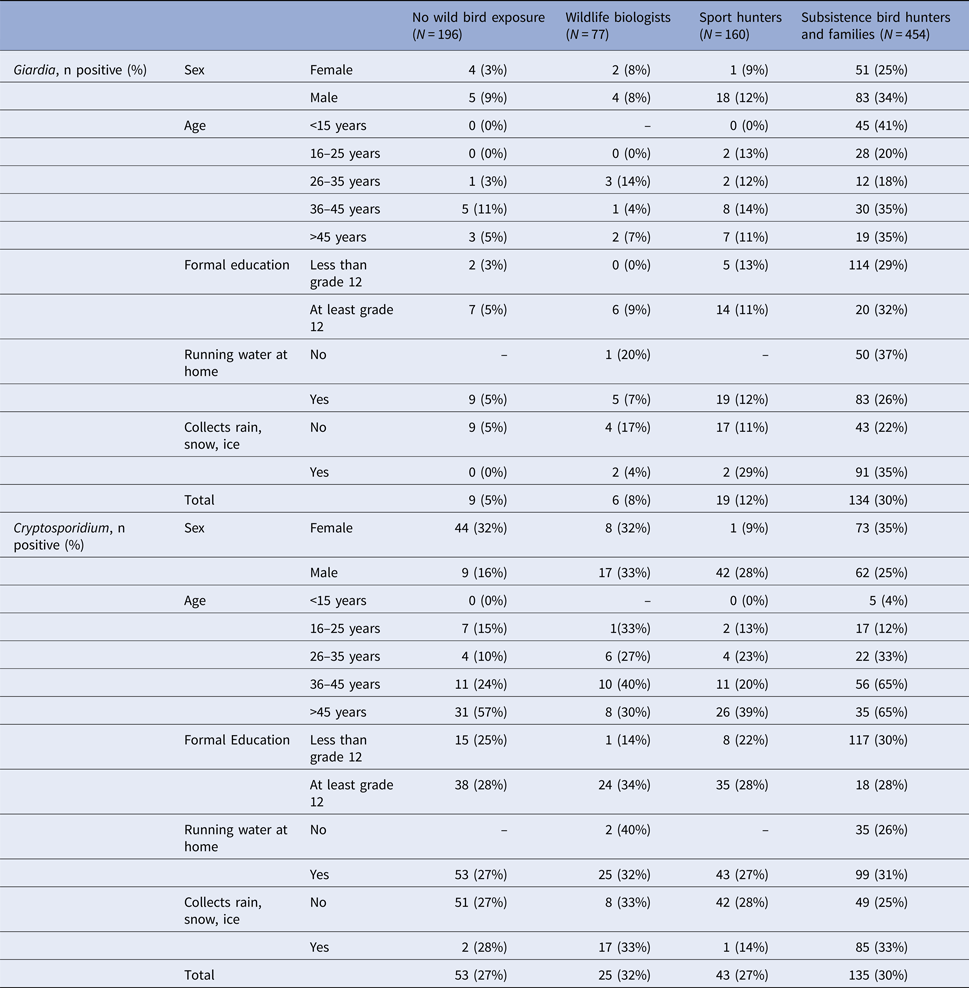
Over 90% of participants in the biologist, sport hunter and non-exposed groups reported access to piped water in their homes (Table 1). In contrast, only 70% of subsistence hunters and their families reported having piped water. Among subsistence hunters with no piped water, 37% had positive Giardia antibody responses, compared with 26% among participants who reported access to piped water at home (PR 1.41, 95% CI 1.05–1.88). Also among subsistence hunters and families, 35% of those who reported collecting rain, snow, or ice for water were positive for Giardia antibodies, compared with 22% of those who did not report collecting water (PR 1.6, 95% CI 1.15–2.15). However, these associations did not remain significant after accounting for clustering based on a community of residence (PR 1.12, 95% CI 0.82–1.54 and PR 1.05, 95% CI 0.73–1.52, respectively).
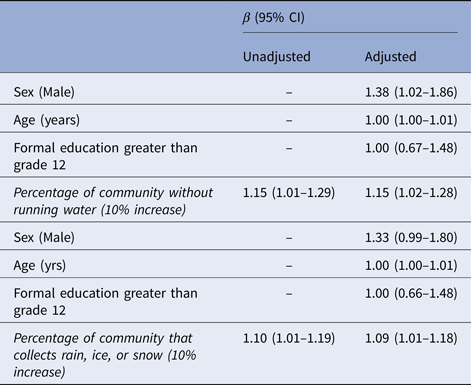
The proportion of participants who reported either not having access to in-home running water or collecting rain, ice, or snow was associated with Giardia antibody prevalence at the community-level (Table 3) but was not associated with Cryptosporidium prevalence (data not shown). As the percentage of participants who reported either not having access to in-home running water or collecting rain, ice, or snow in a community increased, the prevalence of participants with Giardia antibody responses also increased. On average, an additional 10% of a community population that did not have access to running water was associated with a 15% increase in the prevalence of Giardia antibodies among participants, after adjusting for an individual's age, sex and formal education level (Table 3). Similarly, on average each additional 10% of a community population that collected rain, ice, or snow was associated with a 9% increase in the prevalence of Giardia antibodies among participants, adjusting for an individual's age, sex and formal education level. Communities with higher proportions of participants that reported no access to running water had higher proportions of participants also reporting collecting rain, ice, or snow (data not shown; P < 0.001).
Table 3. Giardia seroprevalence ratios among subsistence bird hunters and their families in rural Alaska (n = 454)
Discussion
We assessed the seroprevalence of Giardia and Cryptosporidium in sport hunters, biologists, subsistence hunters and participants who were not exposed to wild birds in Alaska. Almost one-third of participants in each group had evidence of current or recent exposure to Cryptosporidium. We found evidence of previous Giardia exposure in 30% of subsistence hunters and their families, compared with 5–12% of the other groups. Among subsistence hunters and their families, Giardia antibody prevalence was associated with the percentage of their community of residence that had access to piped water and the percentage of the community that reported collecting rain, ice, or snow as drinking or cooking water.
Prevalence studies frequently focus on acute infections and parasites are usually detected using stool microscopy or fecal antigen tests. Prevalence studies based on stool microscopy or fecal antigen tests have not been conducted in Alaska. However, 101 cases of Giardia infection and 12 cases of Cryptosporidium infection were reported in 2011 [31]. Our serosurvey suggests that cases might be underreported, especially given the high prevalence of Cryptosporidium antibody positivity but low numbers of reported cases.
Seroprevalence studies of Giardia and Cryptosporidium are less common and it is often difficult to compare results as the antigens used in the serologic tests vary between surveys and the methods for determining positive cut-off values are not universally standardised. Other studies have found varying seroprevalences of Giardia and Cryptosporidium. In the Caribbean, 41% of pregnant women were found to be seropositive for Giardia [Reference Guo32]. A national survey in Mexico showed a Giardia seroprevalence of 55% [Reference Cedillo-Rivera33]. The National Health and Nutrition Evaluation Survey (NHANES) in the USA found Cryptosporidium seropositivity to be 21% (CI = 18.5–23.9%) for individuals between 6 and 49 years of age in 2000, using the same antigens as the current study [Reference Becker, Oloya and Ezeamama10]. The seroprevalence of Giardia and Cryptosporidium that we identified in Alaska falls within a similar range to these prior estimates, despite the lack of standardisation.
Although the seroprevalence of Cryptosporidium-specific antibodies was moderately high in this study, very few participants demonstrated antibody responses to the Cryptosporidium antigen P2. Previous work suggests that this antibody is a proxy for acquired immunity to Cryptosporidium and may be present in areas of high disease burden [Reference Priest30]. Cryptosporidium seroprevalence did not vary between groups and was not associated with in-home water access. This may be a result of Cryptosporidium’s higher resistance to disinfection [Reference Betancourt and Rose34]. Other factors that might influence Cryptosporidium exposure aside from geography and water might include international travel, contact with animals, or contact with persons with diarrhea [Reference Roy35, Reference Khalakdina36].
Untreated water consumption has often been associated with exposure to Giardia and Cryptosporidium [Reference Speich8, Reference Farkas37, Reference Isaac-Renton38]. In our study, participants without piped water in their homes had higher unadjusted seroprevalence of Giardia. However, the individual-level effect disappeared when we took into consideration the high correlation of Giardia results within communities. This suggests that the water access within participants’ homes was not truly associated with their likelihood of exposure to Giardia after accounting for their community of residence. In contrast, the water access level of the community in which each participant lived remained statistically significantly associated with Giardia exposure, even after adjustment for potential confounders and accounting for community-level clusters. A causal interpretation could be that the community-level access to water was related to the level of circulating Giardia, which affected the potential for individual exposure. We were not able to control all potential community-level confounding, though, and therefore, it is possible that this effect is non-causal. It may be that communities with lower proportions of water access are different from other communities based on other factors that are associated with Giardia, such as animal or social contact patterns, cooking practices, or socio-economic status.
Other possible routes of transmission for Giardia and Cryptosporidium exist in Alaska. For example, companion and hunted animals have been shown to carry Giardia and Cryptosporidium, which may lead to transmission [Reference Hughes-Hanks22, Reference Lebbad39, Reference Bryan40]. In Alaska, both Giardia sp. and Cryptosporidium sp. have been identified in marine mammals, which are commonly hunted by subsistence hunters [Reference Hughes-Hanks22]. These animals might also serve as reservoirs that can contaminate the environment. Giardia transmission has also been linked to daycare centers in Alaska [41]. Despite these other transmission routes, improved access to in-home running water in Alaska could potentially decrease the likelihood of exposure to these parasites [Reference Speich8, Reference Thomas18, Reference Nasser42].
This study provides a unique insight into the burden of diarrhoeal parasites in Alaska. However, there are some limitations to the interpretation of these data. First, the data presented here were not collected with the intent to evaluate water access as a risk factor for Giardia or Cryptosporidium exposure. As previously described, the original study was to evaluate exposure to wild birds [Reference Reed25]. For this reason, not all of the water-, sanitation- and hygiene-related variables, which are required to fully assess the relationship between water access and seropositivity for Giardia and Cryptosporidium, were available for analysis. As a result, we are not able to adjust for all potential confounders in the community-level comparison of Giardia prevalence and water access, which may lead to confounding in the association. Also, the sample frame for a study intended to understand risk factors for Giardia and Cryptosporidium exposure would likely be different than the current study. For example, a better design would have more deliberate inclusion of participants from communities with and without in-home water and sanitation and from communities from environments with different likelihoods of exposures to vectors, such as river vs. coastal locations. Second, because seroprevalence studies for enteric parasitic diseases are not yet common, no calibrated positive serum standards are available and methods for data interpretation and cutoff identification are not globally standardised. Additionally, the study was conducted as a cross-section, so we cannot draw temporal associations. However, given waterborne transmission patterns of these parasites, the associations benefit from biologic plausibility. Finally, while the seroprevalence of these pathogens does indicate historic exposure, it does not equate to current infection status. In this study, we did not evaluate infection status in the positive participants.
Giardia and Cryptosporidium are important contributors to the global burden of diarrhoeal disease. This analysis reflects the first seroprevalence survey of these parasites to be conducted in Alaska. The seroprevalence estimates in these demographic groups can provide a baseline for comparison in the rapidly changing Arctic environment. Although further study is needed, we showed a large disparity in Giardia seroprevalence between subsistence bird hunters and their families compared with other residents of Alaska. In particular, subsistence hunters and their families who live in communities with low levels of in-home running water access had a higher prevalence of Giardia seropositivity. Improved access to potable, in-home running water in these communities could help alleviate this disparity.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Acknowledgments
We would like to acknowledge Kathy Byrd, Helen Peters, Kim Boyd Hummel, and Justin Ortiz for their help recruiting study participants. We would also like to thank Stephen Bentley for data management.
Conflict of Interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. Use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the US Department of Health and Human Services.





