INTRODUCTION
One of the major concerns about the current global climate change is the increased frequency, intensity and duration of extreme rainfall events [1]. Heavy rainstorms and prolonged precipitations may cause pluvial flooding, increasing the transmission of several water-borne diseases [Reference Semenza and Menne2, Reference Cann3]. Over the last decades, there has been an increasing trend in heavy rainfall events during the summers in The Netherlands [Reference Jacobs, Heusinkveld and Holtslag4], causing recurrent pluvial flooding, especially in urban areas. Most urban drainage systems in The Netherlands can support ~20 mm of rainfall per hour. Heavier rainfall overwhelms this drainage capacity and causes street flooding in which 10–50 cm of pluvial floodwater accumulates temporarily on the surface [Reference ten Veldhuis5].
Microbial contamination of pluvial floodwater is notoriously high, especially during flooding of combined sewerage systems, when a mixture of sewage and rainwater containing different pathogens (mainly of faecal origin) flow onto the surface [Reference Fewtrell6]. Contact with floodwater may occur accidentally when passing through it (e.g. walking, cycling, etc.) or as a result of splash exposure, but it probably occurs more frequently or more substantially during post-flooding cleaning operations, or when improperly dealing with floodwater as if it were recreational (bathing) water. As there are pathogens in floodwater that can theoretically be transmitted to (and cause disease in) humans, people coming into contact with floodwater, especially children, may be at risk of acquiring a variety of clinically relevant infections via ingestion, inhalation and dermal contact with floodwater [Reference Jablecki7–Reference Schmid9]. Moreover, it has been shown that the risk for floodwater-borne gastrointestinal infections depends on the origin of the pluvial floodwater itself, i.e. combined sewers, drains, or surface runoff [Reference de Man8]. However, the estimated health risks need to be substantiated with empirical data on the extent to which people exposed to floodwater acquire clinically overt infections in order to provide decision makers with an evidence base to take appropriate actions.
The infection risks related to pluvial flooding are not confined to high-income countries like The Netherlands. Several parts of the developing world are struggling with increased (extreme) rainfall events and their impact on public health, as in those settings there are often multiple aggravating factors, such as sanitation deficit, widespread poverty and malnutrition, run-down infrastructures, poor access to healthcare, and endemicity of several water- and vector-borne diseases. As an example, in a particularly flood-prone developing country such as Bangladesh, pluvial flooding has been reported to be on the causal pathway with increased transmission of endemic infectious diseases like dengue, cholera, and typhoid fever [Reference Dewan10, Reference Hashizume11].
The main aim of this study was to quantify the household- and person-level occurrence of self-reported gastrointestinal, influenza-like illness (ILI) and dermatological complaints, as well as visits to the general practitioner (GP) following pluvial flooding in a high-income country like The Netherlands. To this purpose, a questionnaire-based cross-sectional survey was performed at seven flood locations in The Netherlands (Fig. 1), gathering data on the (type of) exposure to pluvial floodwater, symptoms experienced and GP visits during an observation period of 4 weeks following pluvial flooding. We then analysed these data together with other epidemiologically relevant information (e.g. age, underlying chronic conditions, etc.) to test whether and which type of exposure to (i.e. contact with) pluvial floodwater was associated with the occurrence of the self-reported health complaints. Therefore, this study will not only provide insights into the burden of post-flooding self-reported gastrointestinal, ILI, and dermatological complaints, but also into the associations between these complaints and (different types of) contact with pluvial floodwater, which is of public health significance given the increase in floodwater exposure and its potentially deleterious effects to human health throughout the world.
Fig. 1. Visualization of the seven flood locations (A–G) where the study was performed.
METHODS
Data collection
A retrospective cross-sectional survey of self-reported gastrointestinal, ILI, and dermatological complaints and GP visits following urban pluvial flooding was conducted after two extreme rainfall events (>30 mm rainfall/hour for >1 h) causing flooding on respectively 20 June and 23 July 2013 in seven municipalities in The Netherlands. Exactly 4 weeks after the flooding, 871 households received a self-administrable questionnaire by regular post. These households were known to have been surrounded by floodwater; flood locations were identified using topographic examination of mass media imagery, such as pictures/videos on websites and news reports of the flooded municipalities. Usually, flooding events in The Netherlands catch the media attention and are extensively documented and communicated in the press, TV and internet. This allowed us to clearly spot the flood areas, which were then manually traced back to addresses (using googlemaps.com) to send the questionnaires. To this purpose, several websites were searched (list available upon request to the authors), but the main source of information was the website of the Dutch fire brigades (www.112meldingen.nl).
The questionnaire collected information for each household member (hereafter also referred to as an individual participant) about basic demographic characteristics, occurrence of gastrointestinal, ILI, and dermatological complaints, and visits to the GP during the 4 weeks before questionnaire completion, exposure to pluvial floodwater, history of chronic diseases, and medication use. Participants were also asked to report the type of exposure (i.e. contact) to floodwater as: (1) no contact with floodwater at all, (2) having been involved in post-flooding cleaning operations, (3) having cycled and/or walked through floodwater, (4) having played and/or been splashed with floodwater, (5) getting hands wet/dirty with floodwater. More than one of these types of contact with floodwater could be reported by each individual participant; those reporting one or more types of contact with floodwater were hereafter also generally referred to as being exposed to floodwater. Parents were asked to complete the questionnaire and give consent on behalf of their children. For logistical reasons, questions could be answered by/for up to four household members (as most Dutch households are composed of ⩽4 people according to Netherlands Statistics, http://www.cbs.nl), and they were left to decide on which of the household members to report information on. The questionnaire was developed by adapting validated questionnaires used in previous epidemiological studies in The Netherlands [Reference Doorduyn12–Reference Mughini-Gras14] along with some questions from a previous study focused specifically on quantification of exposure to pluvial flooding [Reference de Man8].
Definitions
Participants reporting to have experienced any of the following complaints in the 4 weeks before completing the questionnaire were defined as cases for the corresponding group of complaints (i.e. gastrointestinal, ILI, or dermatological complaints), and non-cases otherwise.
-
• Gastrointestinal complaints: diarrhoea, vomiting, nausea, abdominal pain, mucus in the stool, blood in the stool, or discoloration of the stool.
-
• ILI complaints: fever (>38 °C), headache, muscle pain, shortness of breath, sore throat, rhinitis (runny nose), ear pain, coughing, or sneezing.
-
• Dermatological complaints: eczema and eczema-like lesions, macule, bullae, blister-like lesions (e.g. papule, vesicles, pustules, etc.), urticaria or itching.
A participant with one or more of the above-mentioned complaints is also hereafter generically referred to as having health complaints.
Data analysis
We compared the cases with the non-cases for each group of complaints with regard to their (type of) contact with floodwater using logistic regression models incorporating two-way cluster-robust standard errors [Reference Cameron, Gelbach and Miller15, Reference Thompson16] to account for clustering of participants at the municipality (location) and household levels. Types of contact with floodwater reaching a significance level of α⩽0·20 for the association with the occurrence of each group of complaints in the univariable analyses were selected for inclusion in a multivariable logistic regression model that also included age (continuous variable), sex, medication use, and history of chronic complaints as covariates. A manual backward selection procedure was used to retain in the multivariable models only those variables significantly associated (α⩽0·05) with the outcome. However, variables producing a change of ⩾10% in the coefficients of the other covariates when removed from the models were retained regardless of their significance. Associations were expressed as odds ratios (ORs) providing 95% confidence intervals (CIs). Collinearities between variables were checked by observing their covariance matrix, and choosing between collinear variables was based on the improvement in model fit as revealed by Akaike's Information Criterion (AIC). All multivariable regression models showed an overall statistical significance of α < 0·05 (likelihood ratio χ 2 test,) and goodness-of-fit of α > 0·05 (Hosmer–Lemeshow test). A similar model-building procedure has been described in several sources [Reference Dohoo, Wayne and Stryhn17, Reference Hill and Lewicki18] and has been used in several previous studies, e.g. [Reference Doorduyn12, Reference Mughini-Gras19–Reference Clark22].
Because of the limited sample size, to support the inferences of regression models, bootstrapped ORs and bias-corrected bootstrap 95% CIs [Reference Vittinghoff and McCulloch23–Reference Nemes25] were also calculated (1000 replications) and reported along with the standard ones. The procedure consisted of drawing 1000 random samples with replacement from the observed data and using these samples to feed back the models. Bootstrap 95% CIs were calculated based on the model parameters at each replication; the bias statistic denoted how much each model parameter from the bootstrap distribution deviated from the parameter of the fitted models. Bias-corrected 95% CIs were then calculated so that the statistical significance of each parameter could be assessed in light of the fitted models applied to ‘different’ data, albeit drawn from the same population. This allowed us to examine the generalizability of the fitted models in order to cross-validate the inferences of the fitted models [Reference Efron and Tibshirani26]. For simplicity, only the results for the exposures of interest were presented. Statistical analyses were performed using Stata v. 13 (StataCorp., USA).
RESULTS
A total of 149 households completed the questionnaire, corresponding to an overall response rate of 17% (range 5–28% between locations; Table 1). Seven (5%) households (corresponding to eight individual participants) were discarded because information about complaints and/or exposure was missing. The remaining 142 households reported data for 199 individual participants (64% women) with a median age of 52 years [25–75% percentile (P25–75): 20–64]. Compared to the Dutch general population in 2013 (16 778 025 inhabitants, according to official census data from Netherlands Statistics, www.cbs.nl), our sample contained relatively more women (64% vs. 50%) and was slightly older (average age 45 vs. 41 years). In total, 102 (72%) households reported data for one individual participant, 26 (18%) households for two individual participants, 911 (8%) households for three individual participants, and three (2%) households for four individual participants. The household attack rate varied between 0% and 45% per location, while the attack rate at the individual participant level varied from 0% to 50% per location (Table 1).
Table 1. Summary statistics per flood location and overall of the households invited and participants thereof along with the attack rates for the self-reported health complaints during the 4 weeks following pluvial flooding, The Netherlands, 2013
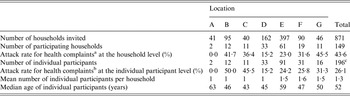
a Households with at least one participant reporting gastrointestinal, influenza-like illness or dermatological complaints.
b Participants reporting gastrointestinal, influenza-like illness or dermatological complaints.
c Three participants had missing information on location.
During the 4-week period after flooding, gastrointestinal complaints were reported by 25 (12%, 95% CI 8–18) individual participants (in 18 households), ILI complaints by 36 (18%, 95% CI 13–24) individual participants (in 27 households), and dermatological complaints by 19 (9%, 95% CI 5–14) individual participants (in 17 households) (Table 2). Overall, 52 (26%, 95% CI 20–32) individual participants reported health complaints (in 38 households), of whom 14 reported a combination of two of the three groups of complaints and seven a combination of all three groups of complaints. Nineteen (10%, 95% CI 5–14) individual participants (in 18 households) visited their GP because of the complaints (Table 2). The median duration of gastrointestinal complaints in individual participants was 3 days (P25–75: 2–10), that of ILI complaints was 14 days (P25–75: 8–20) and that of dermatological complaints was 17 days (P25–75: 5–28). Of individual participants, the most frequently reported gastrointestinal complaint was abdominal pain (8%, 95% CI 5–13), the most frequent ILI complaint was rhinitis (8%, 95% CI 5–13), and the most frequent dermatological complaint was itching (7%, 95% CI 4–12).
Table 2. Attack rates among participants exposed and not exposed to pluvial floodwater along with univariable and multivariable odds ratios for the association between exposure to pluvial floodwater and the occurrence of gastrointestinal, influenza-like illness, dermatological complaints, and visits to the general practitioner, The Netherlands, 2013
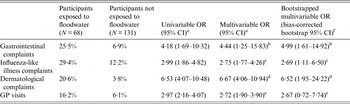
OR, Odds ratio; CI, confidence interval; GP, general practitioner.
Participants exposed (or not) to floodwater are people coming into contact (or not) with floodwater by having hands wet/dirty with floodwater, by cleaning up floodwater, by cycling or walking through floodwater, or by playing/being splashed with floodwater.
a Estimated by a logistic regression model with two-way cluster-robust standard errors [Reference Thompson16, Reference Harper21] to account for clustering at both the household and flood location levels.
b Adjusted for age and history of chronic gastrointestinal diseases (covariates).
c Adjusted for age and history of chronic respiratory diseases (covariates).
d Adjusted for age and history of chronic dermatological diseases (covariates).
e Adjusted for age and history of any chronic disease (covariates).
f ORs and bias-corrected bootstrap 95% CIs based upon 1000 replications.
Out of 68 (34%) individual participants exposed to floodwater, 16 (23%) reported gastrointestinal complaints, 20 (29%) ILI complaints, and 14 (20%) dermatological complaints. Overall, 30/68 (44%) individual participants exposed to floodwater reported health complaints, of whom 10 reported a combination of two groups of complaints and five of all three groups, and 11 (16%) visited their GP. Conversely, of the 131 (65%) individual participants unexposed to floodwater, nine (6%) reported gastrointestinal complaints, 16 (12%) ILI complaints, and five (3%) dermatological complaints (Table 2). Overall, 22/131 (16%) individual participants unexposed to floodwater reported health complaints and eight (6%) visited their GP. Multivariable logistic regression analyses revealed that exposure to floodwater was significantly associated with increased odds of reporting gastrointestinal (OR 4·44), ILI (OR 2·75) and dermatological (OR 6·67) complaints (Table 2). Individual participants exposed to floodwater were also significantly more likely to visit their GP (OR 2·72). Bootstrap analysis confirmed the significance of each of these associations except for the one with GP visits (Table 2).
Most of the 68 individual participants exposed to floodwater reported having had their hands wet/dirty with floodwater (84%), followed by those reporting being engaged in post-flooding cleaning operations outside the house (50%), those having walked/cycled through floodwater (28%) and those that had played and/or been splashed with floodwater (25%). None of the individual participants was exposed to floodwater within the house. Multivariable logistic regression analysis identified which types of exposures to floodwater were significantly associated with the occurrence of the different groups of complaints (Table 3). For gastrointestinal and dermatological complaints, as well as GP visits, having hands wet/dirty with floodwater was the only significant exposure (ORs 5·03, 8·47, 4·41, respectively). Having cleaned up floodwater (OR 4·28) and having walked/cycled through it (OR 3·60) were significant exposures for ILI complaints (Table 3). The duration of exposure was not significantly associated with the different groups of complaints (results not shown). The number of individual participants sharing more than one type of exposure to floodwater together with the ϕ coefficients and corresponding P values for the pairwise correlations between exposures are reported in Table 4.
Table 3. Odds ratios for the association between the type of exposure to pluvial floodwater and the onset of gastrointestinal, influenza-like illness, dermatological complaints, and visits to the general practitioner, The Netherlands, 2013
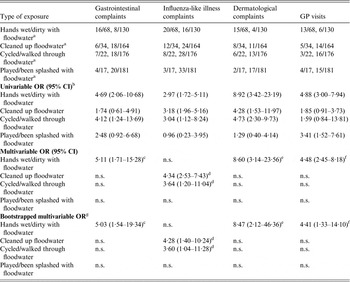
OR, Odds ratio; CI, confidence interval; GP, general practitioner, n.s., not significant (P > 0·05).
a Participants exposed to floodwater vs. participants not exposed to floodwater.
b Estimated by a logistic regression model with two-way cluster-robust standard errors [Reference Cameron, Gelbach and Miller15, Reference Thompson16] to account for clustering at both the household and flood location levels.
c Adjusted for age and history of chronic gastrointestinal diseases (covariates).
d Adjusted for age, history of chronic respiratory diseases and the other significant exposure to floodwater (covariates).
e Adjusted for age and history of chronic dermatological diseases (covariates).
f Adjusted for age, history of any chronic disease (covariates).
g ORs and bias-corrected bootstrap 95% CIs based upon 1000 replications.
Table 4. Number of individual participants sharing more than one type of exposure to floodwater (above the diagonal), and the corresponding ϕ coefficients and P values for the pairwise correlations between types of exposures to floodwater (below the diagonal), The Netherlands, 2013
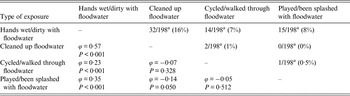
a For one participant information on the type of exposure to floodwater was missing.
DISCUSSION
This study provides evidence that exposure to urban pluvial floodwater is associated with increased risk of reporting gastrointestinal, ILI, and dermatological complaints, as well as of seeking medical attention, in a high-income country like The Netherlands. Gastrointestinal complaints were reported by 23% (16/68) of individual participants exposed to floodwater, which is of the same order of magnitude (4–33%) as the risk estimates for floodwater-borne gastrointestinal infections estimated by recent quantitative microbial risk assessments [Reference de Man8, Reference Sales-Ortells and Medema27]. This is striking, as typically a quantitative microbial risk assessment tends to overestimate disease incidence because it does not usually incorporate protective effects of immunity [Reference Swart28]. Moreover, our prevalence of 23% for gastrointestinal complaints among individual participants exposed to floodwater is significantly higher than that of 6% (93/1672) for syndromic gastroenteritis reported during summers from 2008 to 2013 for the general Dutch population by Friesema et al. in a repeated cross-sectional survey of self-reported infectious disease symptoms in The Netherlands [Reference Friesema, van Gageldonk-Lafeber and van Pelt29]. This difference is due in part to the use of a different case definition. Applying the case definition of Friesema et al. to our data (i.e. ⩾2 symptoms of vomiting, diarrhoea, stomach ache, nausea, fever, and blood in the stool) resulted in a gastroenteritis incidence of 12% (8/68) among individual participants exposed to floodwater, indicating that gastroenteritis indeed occurs more often in these participants.
Nine (36%) of the 25 individual participants with gastrointestinal complaints visited their GP. This is higher than the proportion of GP visits in the study of Doorduyn et al. [Reference Doorduyn, Van Pelt and Havelaar13], who estimated that 8% of the cases with infectious intestinal disease (IID) in The Netherlands visited a physician. Applying the case definition of Doorduyn et al. [Reference Doorduyn, Van Pelt and Havelaar13] to our data (i.e. vomiting and/or diarrhoea in absence of other conditions that would have caused these symptoms) resulted in an estimated 33% (4/12) of IID cases visiting their GP in our study, which is still considerably higher than the 8% reported by Doorduyn et al. [Reference Doorduyn, Van Pelt and Havelaar13]. According to Doorduyn et al. [Reference Doorduyn, Van Pelt and Havelaar13], the strongest predictor for contacting a physician was the duration of symptoms: cases suffering from IID for ⩾3 days were more likely to contact a physician than those with shorter episodes of IID. This is consistent with our data, as the mean duration of symptoms in the 12 above-defined IID cases in our study was 4 days (range 1–14). Furthermore, the mean duration of ILI and dermatological complaints was 14 and 17 days, respectively, which may explain why 19 (37%) of the 52 participants with health complaints (i.e. one or more gastrointestinal, ILI, or dermatological complaints) visited the GP. An alternative explanation is that people were more likely to seek healthcare services because they were scared or assumed that their symptoms were due to the flooding event.
Contact with floodwater was associated with increased occurrence of gastrointestinal, ILI, and dermatological complaints, as well as visits to the GP. The effect size of the association between exposure to floodwater and the occurrence of dermatological complaints was somewhat more pronounced than the effect sizes for gastrointestinal or ILI complaints, having an OR of 6·7 that appeared to be of the same order of magnitude of the relative risk of 6·5 for itching in households exposed to contaminated drinking water found in a previous cohort study in The Netherlands [Reference Fernandes30]. Such dermatological complaints are probably due to dermal contact of contaminated water during showers or baths in individuals with increased skin sensitivity [Reference Fernandes30]. This is also substantiated by our study, as having hands wet/dirty with floodwater was associated with the development of either gastrointestinal or dermatological complaints, whereas ILI complaints were associated with being engaged in post-flooding cleaning operations and having walked/cycled through floodwater. Assuming a causal relation, these associations may reflect, to some extent, the primary transmission routes. While inhalation of aerosolized water particles is likely to occur more substantially when people have to dispose of or move through floodwater, dermal and hand-to-mouth contact, including accidental ingestion of water droplets, do occur when hands are wet/dirty with floodwater, potentially leading to transmission of several pathogens typical of flood-ravaged settings in developed countries [Reference Jablecki7, Reference Schmid31]. If this holds true, our findings would indicate that floodwater-related health risks may be reduced, to a certain extent, by hand washing and possibly also by wearing protective clothing, such as gloves and masks [Reference Seto32]. These preventive measures may therefore be recommendable by local authorities to reduce the risk of developing gastrointestinal, ILI, and dermatological complaints in case of flooding.
This study has some limitations. Addresses to which the questionnaires were sent were selected based on mass media imagery and reports of flood locations. The whole street of an identified flooded household was asked to complete the questionnaire, meaning that there might have been locations in which the flood was relatively lighter than the actual length of the street so that some questionnaires were sent to households that did not actually experience the flooding, at least not to the same extent as other households. This means that within the exposed group, there might have been different magnitudes of exposure that could not be studied here. Although questions were posed neutrally (i.e. they did not emphasize the association between health complaints and flooding), people who did not experience the flooding might have somehow been less motivated to participate in the study, which may have led to the relatively low response rate (17%) observed here. Moreover, exposed people who also suffered from health complaints might have been more motivated to participate than those who did not, especially since we left the decision to the household on whom to enrol in the study, leading to a possible overestimation of the occurrence of complaints. Because we had no information about the characteristics of the non-respondents, we could not check the extent to which our sample was representative of the population from which it was drawn. Moreover, the limited sample size did not allow us to use a stricter, symptom-based syndromic case definition using a combination of specific symptoms, e.g. [Reference Majowicz33], which would have undoubtedly been best. Indeed if we would have done so, we would have had less than a dozen cases per syndrome, which would have prevented any analysis of the data to pursue the objectives of this study. It follows, therefore, that our results can only refer to complaints as a whole and not to specific illnesses or syndromes. Another limitation of this study is that we did not ask for the first date of complaints and we could therefore not differentiate between primary and secondary cases, which might have occurred, especially between household members [Reference Heijne34]. While recall bias may have underestimated the occurrence of complaints because people might have forgotten (mild) illness episodes, ‘telescoping’, i.e. when people remember illness episodes as being more recent than they actually were, might also have occurred, leading to an overestimation of self-reported complaints [Reference Wheeler35]. Last, some reporting bias might have occurred, as the questionnaire did not clearly define the frequency or severity of complaints to be reported, possibly leading to discrepancies between the perceived and actual health status of a participant.
Because of the retrospective nature of this study design, it was not possible to assess the levels of microbial contamination in the floodwater. However, intensive sampling of pluvial floodwater in The Netherlands [Reference de Man8] has shown that such water is always contaminated with faecal matter. This has been demonstrated by the presence of E. coli and intestinal enterococci, together with the occurrence of frequently encountered enteropathogens, such as enterovirus, norovirus, Campylobacter, Giardia, and Cryptosporidium. Moreover, viable Legionella bacteria have been isolated from pluvial floods in The Netherlands [Reference Schalk36]. The prevalence of these pathogens in floodwater is expected to vary according to the local environment [Reference Smith, Kay and Fewtrell37, 38] and the origin of the floodwater [Reference de Man8], which may explain the different attack rates of gastrointestinal, ILI, and dermatological complaints we observed between locations.
In the field of (life-threatening) waterborne diseases such as cholera and typhoid fever, the association with extreme rainfall events is well-known [Reference Dewan10, Reference Hashizume39], although in most countries where these diseases are (or threaten to be) endemic the magnitude of pluvial flooding events, infrastructural failure and poverty-related factors lurking in the background usually exceed the ones relative to this study. Thus far, knowledge of the infectious disease risks related to pluvial flooding in high-income countries has been somewhat elusive in contrast to the developing world. The Netherlands is highly populated (~400 people/km2), and while this does not allow floods to easily pass unnoticed, it is also true that floodwater do not usually persist for protracted periods, as it is often drained off in a few hours or days. Therefore, significant transmission of diseases whose presence and/or increase can be associated with prolonged rainfall or flooding events that provide new breeding grounds, i.e. wet areas and stagnant waters, for arthropod vectors such as mosquitoes transmitting malaria, dengue and West Nile fever in different parts of the world [Reference McMichael40], are unlikely to pose a threat in The Netherlands and neighbouring countries. Yet, other diseases like leptospirosis might well feature [Reference Goris41] if floods were to increase exposure to leptospires and/or boost the fitness of rodent vector populations. In this regard, our study provides essential information to tackle the increase in pluvial flooding events in a developed country by highlighting and characterizing the associated risks for public health. People should be made aware that in developed countries pluvial floodwater may contain pathogens and that good hygiene practices should be enforced to prevent transmission [Reference de Man8]. This becomes even more crucial since recent studies have estimated that around 2 billion additional extreme rainfall events annually are to be expected due to climate change in the years to come [Reference Watts42].
In conclusion, besides providing evidence that self-reported gastrointestinal, ILI, and dermatological complaints, as well as GP visits, occur significantly more often in people exposed to floodwater than in those not exposed to floodwater, we also found that different types of exposure to pluvial floodwater were associated with the occurrence of different complaints, a possible reflection of the primary transmission routes of the pathogens in question. This study also points out that extreme rainfall events, which may become more frequent in the future because of climate change, may in turn increase the occurrence of floodwater-associated diseases in a high-income country like The Netherlands. Therefore, further research is warranted to determine the disease burden of pluvial flooding and the impact of different intervention measures, including raising awareness among stakeholders. This is required for policy-making, development of emergency response plans, and preparedness of public health services to mitigate the effects of flooding.
ACKNOWLEDGEMENTS
The authors thank Marie Jose Strik for assistance in sending surveys.
DECLARATION OF INTEREST
None.








