INTRODUCTION
Tularemia is a zoonotic disease caused by the bacterium Francisella tularensis, which can infect and cause disease in a large number of animal species, including humans. In Europe, the only subspecies causing disease is F. tularensis subsp. holarctica. The wildlife species most commonly associated with the disease historically in Sweden is the mountain hare (MH; Lepus timidus), a hare species that earlier studies have shown to die quickly from acute disseminated disease [Reference Mörner1]. During the last decade, tularemia has also been a cause of disease in the European brown hare (EBH; Lepus europaeus) in Sweden, with pathological changes similar to that found in the MH [Reference Hestvik2]. In central and western Europe, both acute and chronic forms of the disease have been described in the EBH [Reference Decors3, Reference Gyuranecz4].
In humans, the disease presentation differs according to the infection route. The ulceroglandular form arises when the infection route is via the skin from tick- or mosquito bites, or from direct contact with a diseased animal. When the source of infection is via ingestion of water or food, an oropharyngeal infection is contracted. Inhalation of infected dust, e.g. from dead infected small rodents in hay, results in pneumonia. According to the European Centre for Disease Prevention and Control (ECDC) [5], the number of reported confirmed human cases in 2014 increased by 70% compared with 2013, and Sweden accounted for most of the reported cases (150) in European Union/European Economic Area (EU/EEA) countries. During the outbreak year 2015, 859 cases were reported to the Public Health Agency of Sweden [6], and in 35 of the 434 confirmed cases (7%), animal contact was the cause. Hares are an often contract tularemia, and being a popular game species is a possible source of infection. Dressing of hares is a known source of human infection. The ECDC [5] identifies skinning as a risk factor. A feature not yet investigated is to what degree F. tularensis is present in the meat (musculature) of infected hares.
The aim of this study was to investigate if infected MH and EBH harbour bacteria in the muscle, thereby being a possible source of infection for humans consuming the meat.
METHODS
Material
Thirty-two EBH and 10 MH found dead or moribund necropsied during the period 2007–2015, and one hunted EBH were included in the study. From the latter, hare meat stored for consumption, was sent to the National Veterinary Institute (SVA) when the hunter dressing the hare contracted tularemia shortly after the hunting event. The EBHs originated from central and southern regions in Sweden, and the MHs from northern and central parts. The hares were diagnosed with tularemia in conjunction to the necropsy using indirect immunofluorescence (2007–2012), and real-time PCR or immunohistochemistry (IHC) (2012 onwards) on spleen, liver and/or lung. For the hunted EBH, a muscle sample was analysed. All 43 muscle samples were stored in a −20 °C freezer until the examination. When sampling the muscle tissue, great care was taken to avoid contamination of the samples. Since all hares had been diagnosed with tularemia, surface contamination during necropsy from infected organs was possible. Muscle surfaces were sterilised by flaming before the tissue was incised and the samples were taken from the inner part of the muscle with sterile instruments.
Real-time PCR for detection of F. tularensis subsp. holarctica
Material from the tissue samples was retrieved using sterile cotton swabs. The swabs were incubated in 570 µl G2 buffer and 30 µl proteinase K solution from the EZ1 DNA Tissue Kit (Qiagen, Hilden, Germany) at 56 °C for 15 min under continuous agitation, followed by 5 min incubation at 95 °C. DNA was extracted from 200 µl of the resulting lysate using the EZ1 DNA Tissue Kit and the EZ1 Advanced Instrument (Qiagen, Hilden, Germany). The DNA was eluted in 100 µl elution buffer and 2 µl was used as a template for each PCR reaction. A real-time PCR assay was used for the detection of F. tularensis subsp. holarctica DNA (Swedish Forum for Biopreparedness Diagnostics [7]). The PCR analysis was performed in a volume of 15 µl containing nuclease-free water, PerfeCTa qPCR Toughmix with low ROX reference dye (Quantabio, Beverly, MA, USA), 500 nM of each primer (forward primer 5′-CCATCAGCAGTAGTATAACCACCAA-3′ and reverse primer 5′-TGGCGCAGATATGACTAAAGTC-3′), 200 nM of each hydrolysis (probe 1 5′-FAM-TATTACTAGGAATGGCGCGC-MGB-3′, probe 2 5′-VIC-TACTAGGAATGGCGTGC-MGB-3′) and 2 µl template DNA. A 96-well format thermocycler, ABI 7500 Fast (Applied Biosystems, Foster City, CA, USA) was used for amplification and detection of the target sequence. Cycling conditions were as follows: 95 °C for 3 min followed by 45 cycles of 95 °C for 15 s and 60 °C for 30 s during which fluorescence intensity was measured. Samples with a CT-value ⩽38 for the FAM-probe were considered positive for F. tularensis subsp. holarctica.
IHC for the detection of F. tularensis
F. tularensis spp. was detected with IHC performed on formalin-fixed tissues using a mouse primary monoclonal antibody FB11 (Meridian Life Science Inc., Nordic Biosite AB, Täby, Sweden) directed against F. tularensis lipopolysaccharide antigen. Sections were deparaffinised and incubated with H2O2 for 15 min to block endogenous peroxidase activity. To retrieve antigen, the sections were treated with proteinase K (Dako, Agilent, Glostrup, Denmark) for 6 min, followed by incubation with 2% bovine serum albumin (BSA) at room temperature for 20 min to block unspecific staining. The primary antibody was applied in 1 : 3500 dilution, and the sections were incubated at room temperature for 45 min. Antibody binding was detected by anti-mouse EnVision polymer detection (Dako, Agilent, Glostrup, Denmark) whereafter the sections were counterstained with Mayer's solution. A serial section incubated with 2% BSA was used as negative control, and a known positive tularemia sample from an EBH was used as positive control.
Histopathology
Pieces of muscle tissue were fixed in 10% neutral-buffered formalin, embedded in paraffin and routinely processed for histopathology. Sections were cut 4 µm thick and stained with haematoxylin and eosin (HE). Pieces of tissue from various internal organs sampled and treated likewise in conjunction with the necropsy of the 42 hares were also examined.
Bacteriology
Fourteen muscle samples were analysed by aerobic bacterial culture with the aim to investigate if the bacteria were able to grow after variable length of storage in a freezer at −20 °C. The samples had been stored for approximately 0·5 (one sample), 1 (two samples), 2 (two samples), 3 (two samples), 4 (three samples), 5 (two samples), 7 (one sample) or 8 years (one sample). The samples were cultured on T4 medium as described previously in harmonisation of methods at BSL-3 laboratories [8]. T4 medium contains 37 g/l brain–heart infusion (Oxoid, Hampshire, UK), 15 g/l Bacto-agar (Neogen, Auchincruive, UK), 10 g/l sodium chloride (Honeywell, Morris Plains, NJ, USA), 90 ml/l sheep blood (Håtunalab, Bro, Sweden), 1 g/l L-cysteine, 10 g/l glucose, 2·7 mg/l amphotericin B, 5 mg/l vancomycin, 2·5 mg/l trimethoprim and 2·8 mg/l cefsulodin (Sigma-Aldrich, St-Louis, MO, USA) making it selective for Francisella. The culture plates were incubated at 37 °C for 4 days and checked for growth daily.
RESULTS
The 42 necropsied hares had acute pathological changes with necroses and mild or no inflammation, in liver, spleen and bone marrow. Many hares also had lesions in other organs, such as lymph nodes, adrenal glands and lungs. Seven of the hares had no noticeable gross changes.
Thirty-nine of the 43 muscle samples were positive by real-time PCR, and in 13 of these, F. tularensis were detected by IHC. In one of the four real-time PCR-negative muscles, F. tularensis was detected by IHC which gives the overall result of 40 positive muscle samples by real-time PCR and/or IHC. Nine of the 43 samples were unsuitable for IHC due to post-mortal changes and the staining could not be evaluated with confidence. In five of these, routine histopathology on HE-stained sections could not be assessed either. Bacterial culture was positive in two of 14 samples, both stored for approximately 1 year. Real-time PCR was used to confirm that the bacteria found were F. tularensis subsp. holarctica.
By routine histopathology, the only signs of infection in the muscle tissue were mild infiltration mainly of macrophages and a few lymphocytes and plasma cells, mostly associated with the presence of F. tularensis shown by IHC. However, in two samples, small areas of necrosis were evident in the perimysium with mild infiltration of macrophages and lymphocytes (Fig. 1). Necrosis of myofibres could not be detected in any of the samples. The localisations of F. tularensis in muscle tissue observed by IHC are shown in Table 1.
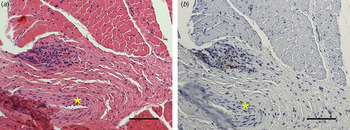
Fig. 1. (a) A necrosis in the perimysium with mild infiltration of macrophages and lymphocytes, adjacent to a medium-sized arteriol (*) (HE) is shown. (b) The IHC staining of F rancisella tularensis spp. in the necrosis is shown. Bars (a, b) 50 µm.
Table 1. Localisation of Francisella tularensis spp. in 14 muscles from hares sampled in Sweden 2007–2015

Common for all the muscle samples positive at IHC was that the number of bacteria was low to moderate. The most common location was in the connective tissue of the perimysium surrounding medium-sized arteries. Here, F. tularensis was commonly intracytoplasmic in macrophages, but could also be seen in the cytoplasm of fibroblasts and lymphocytes, or extracellularly (Fig. 2). In the endomysium, the number of bacteria was low, and those found were mostly intracytoplasmic in spindle cells, presumably fibroblasts (Fig. 3). Occasionally bacteria were also seen extracellularly. In three of the samples, F. tularensis was detected intravascularly, both in the cytoplasm of macrophages and extracellularly (Fig. 4).
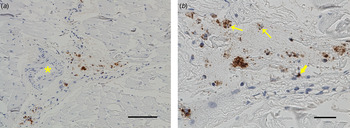
Fig. 2. F rancisella tularensis spp. was most commonly located in the connective tissue of the perimysium surrounding medium-sized arteries (*) (a). The bacteria were often intracytoplasmic in macrophages (arrows), but also in the cytoplasm of fibroblasts (block arrow) and extracellularly (b). Bar (a) 50 µm, bar (b) 20 µm.
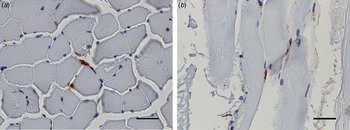
Fig. 3. In the endomysium, the number of bacteria was low, and they were commonly located in the cytoplasm of spindle cells, presumably fibroblasts. Bars (a, b) 20 µm.
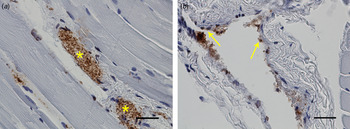
Fig. 4. In three of the samples Francisella tularensis spp. was detected intravascularly (*) (a). Bacteria could also be seen in the cytoplasm of endothelial cells (arrows) (b). Bars (a, b) 20 µm.
DISCUSSION
F. tularensis was detected by real-time PCR and/or IHC in muscle samples examined from hares with tularemia. The meat may contain F. tularensis bacteria, either due to transportation via the blood stream during the disease process, or due to post-mortal contamination from infected internal organs. In our study, in the 14 muscle samples where the bacteria could be visualised with IHC, F. tularensis were often seen in the cytoplasm of various cell types in the inner parts of the muscles, representing true infection. Common gross changes in MH and EBH that die of acute tularemia with sepsis are: enlarged liver and spleen, and necroses in liver, spleen and bone marrow. However, necropsies performed at SVA have shown that gross lesions are frequently absent in F. tularensis-infected hares, while microscopic changes are detected [Reference Hestvik2]. Seven of the hares from the present study had no evident gross changes, making it difficult for the hunter to realise that the hares were infected. Infection of hunters acquired when dressing infected hares has been described [Reference Wahab and Bjuggren Zelano9, Reference Hofstetter10].
The risk of contracting infection can be diminished by cautious dressing and by cooking the meat thoroughly. In Sweden, there are no reports of people contracting tularemia after consumption of hare meat, but there are reports from other countries. In a French study including 101 diseased humans, the source of infection was reported as hare meat in nine cases and roe deer meat in one case [Reference Maurin11]. In Serbia, two cases have been reported to be caused by undercooked rabbit meat [Reference Djordjevic-Spasic12]. In the Netherlands seven persons in the same family, and in France three in the same family contracted disease after consumption of infected hare meat [Reference Benlyazid13, Reference Warris-Versteegen and van Vliet14]. Freeze storage affects the survival of F. tularensis and attempts to culture the bacteria were conducted for 14 muscle samples with variable length of freezing time. Two of the samples, stored <1 year, cultured positive, while the other samples stored 6 months to 8 years were negative. From this could be inferred that the presence of viable bacteria decreases with increased length of freezing time, but one should also take into account that F. tularensis is a bacterium difficult to culture. In a Russian study, sheep were experimentally infected with F. tularensis and the meat still contained viable bacteria after storage at −20 °C for 60–75 days [Reference Airapetyan, Khachatryan and Pogosyan15]. Since hunters in Sweden are allowed to sell small amounts of wildlife or meat from wildlife to the public shops and restaurants without preceding professional meat inspection, there is a risk that others than the hunters themselves might get infected.
Hares infected with tularemia do not always have readily detectable gross changes, and this study reveals that F. tularensis often is found in the muscle of infected hares as a part of the systemic infection. This, together with the finding that F. tularensis is still viable in meat after freeze storage for at least up to a year, indicates that people consuming undercooked hare meat are at risk of contracting tularemia.
ACKNOWLEDGEMENTS
The authors would like to thank Eva Westergren for conducting the histopathology and immunohistochemistry.
The study was funded by Stiftelsen Ivar och Elsa Sandbergs stipendiefond.
DECLARATION OF INTEREST
The authors declared no conflicts of interest.








