INTRODUCTION
Cryptosporidiosis is a diarrhoeal disease caused by protozoa of the genus Cryptosporidium. Human infection is predominantly caused by the species C. hominis and C. parvum [Reference Chen1, Reference Sears and Kirkpatrick2]. Oocysts are passed in stool from infected humans and animals. The infective dose is very low: one study found the ID50 to be as low as nine oocysts for one C. parvum genotype [Reference Okhuysen3]. Faecal–oral transmission may be caused by direct contact with infected persons or animals, or indirectly by ingestion of contaminated drinking or recreational water or consumption of contaminated food. Illness is typically characterized by sudden onset of watery diarrhoea, often accompanied by abdominal cramping, low-grade fever, nausea, vomiting, dehydration and weight loss. Asymptomatic infections also occur. The incubation period ranges from 2 to 12 days (usually 7–10 days), and symptoms typically last for 1–2 weeks in otherwise healthy individuals. More severe and prolonged illness is seen in patients with HIV/AIDS, in otherwise immunocompromised patients and in young children in developing countries [Reference Chen1, Reference Sears and Kirkpatrick2, Reference Molbak4].
Although cryptosporidiosis is notifiable in several countries, the incidence in humans is probably underestimated. This is partly because testing for Cryptosporidium in many countries is not included in routine examination of human stool samples, and partly because of insufficient reporting systems. Nonetheless, cryptosporidiosis is recognized as one of the most common causes of waterborne disease in humans. The parasite is resistant to standard water disinfectants such as chlorine, and therefore waterborne outbreaks, often linked to public drinking-water supplies or recreational water use, are common worldwide [Reference Sears and Kirkpatrick2]. In both Europe and the United States an autumn pattern of seasonality has been described [5–Reference Yoder and Beach7]. Evidence from England and Wales further suggests that autumn cases are predominantly caused by C. hominis, while during the other seasons both C. hominis and C. parvum are prevalent [Reference Sopwith8]. Holiday travel and recreational water use during the summer months are major risk factors for C. hominis infection [Reference Khalakdina9–Reference Taylor11]. Nevertheless, widespread outbreaks associated with travel are rarely reported.
Although contaminated food items such as fresh fruit and vegetables are considered important vehicles, well-documented foodborne outbreaks of Cryptosporidium infections are few. In the United States two types of outbreaks have been described; outbreaks with apple cider as the vehicle, where contamination from manure to the apples appeared to be the cause [Reference Millard12, 13], and outbreaks from (solid) foods where the contamination appears to have resulted from infected food handlers [14–Reference Quiroz16].
We describe a large foodborne outbreak of cryptosporidiosis in Denmark, associated with eating from a salad buffet in a company canteen. The outbreak was recognized following an unusually high number of employees being absent with diarrhoea in the week 29 August to 2 September 2005. This was reported by the company management to the food safety authorities on Friday, 2 September 2005. An outbreak investigation was launched by the regional food safety authority in cooperation with the Danish Statens Serum Institut.
METHODS
Analytical studies
Two analytical studies were conducted. The first, designed as a case-control study, was launched on the day the outbreak was recognized (2 September). Questionnaires were given to about 50 employees known to have been ill and some 150 presumed-well employees at work by randomly handing out questionnaires to every third employee. Questions included usage of the company canteen from 22 August to 26 August and consumption of broad categories of items on the menu each day in that week. Data from the questionnaires were computerized using Epidata (www.epidata.dk).
The second study was an in-depth cohort study to identify a specific vehicle. This was undertaken from 26 to 30 September by email distribution of an electronic questionnaire to all employees in the company. This questionnaire contained a full list, obtained from the canteen management, of ingredients used in the salad bar between 22 and 26 August. Employees were asked to indicate which salad bar ingredients they had chosen (‘probably’, ‘perhaps’, or ‘most likely not’) during the week in question. Moreover, the questionnaire contained questions about canteen usage on 18 and 19 August in addition to 22–26 August, consumption of water with ice cubes and the occurrence of diarrhoea or foreign travel in the 3 weeks before 22 August. Questions about the items on the menu on 22 to 24 August were repeated in the cohort study questionnaire. Both questionnaires contained questions about symptoms (if any) and their duration and date of onset, medical attendance and stool sample submission, and respondents were asked whether any persons from their households had felt ill.
The same case definition was used in both studies; an employee was considered a case if he/she had diarrhoea (defined as three or more loose stools per day) between 21 August and 14 September. In the analysis of both studies the ‘do not recall’ answer was coded as ‘no’ and missing values were also coded as ‘no’; in the analysis of the cohort study the ‘probably’ category was coded as ‘yes’ and the two other categories as ‘no’. Data were analysed using the SAS version 9.1.3 software package (Cary, NC, USA). Univariate analyses were performed to calculate odds ratios (OR, case-control study) and relative risks (RR, cohort study) for the association between case status and the individual food exposures, using a 5% significance level. To perform multivariate analyses, logistic regression with the SAS Logistic procedure was used and the Wald χ2 statistic P values resulting from the model containing all the items from the salad bar were used to estimate the relative association with disease.
Microbiological and molecular analyses
Stool samples were examined for enteropathogenic bacteria, viruses and parasites using standard methods as previously described [Reference Olesen17]. Briefly, the analysis for Cryptosporidium was performed using Ziehl–Neelsen staining [Reference Henriksen and Pohlenz18] of faecal concentrates obtained by the formol ethyl-acetate concentration technique [Reference Young19]. Molecular typing of Cryptosporidium was performed as reported elsewhere [Reference Langkjaer20] using PCR and sequencing of the small subunit ribosomal RNA gene (18S rDNA locus, ~925 bp) [Reference Enemark21] and the HSP70 gene (325 bp) [Reference Morgan22].
Five samples, each of a minimum 1 litre, of water from ice-cube machines and drinking-water dispensers were tested for Cryptosporidium using membrane filtration and a modification of the EPA method 1622 [23]. Materials captured on the filters were eluted and concentrated, Cryptosporidium oocysts separated by immunomagnetic separation using the Dynabeads® anti-Cryptosporidium kit (Dynal A/S, Norway), fixed onto microscope slides and fluorescence-stained with the Merifluor Cryptosporidium/Giardia kit (Meridian Diagnostics Inc., Italy) and finally screened by fluorescent microscopy.
RESULTS
A total of 34 stool samples from 17 company employees were known to have been submitted for microbiological analyses. Most samples tested negative for enteropathogenic bacteria, although one patient was positive for Yersinia enterocolitica and another for enteroaggregative Escherichia coli. The patient samples were then sent for viral investigation and found negative for Norovirus and Sapovirus. What remained of the stool samples was then sent for parasitological analysis and samples from 13 of the 17 patients were found positive for Cryptosporidium. Subsequent molecular analysis identified all isolates as C. hominis, 100% and 99% identical to nucleotide sequences in GenBank (e.g. AF093489 and XM 661662) at the 18S rDNA and the HSP70 loci, respectively.
The outbreak affected employees in a company that occupied a single office building. There were about 600 employees; the company management could not state the exact number at the time the outbreak occurred. Of these most used the canteen on a regular basis. There were ill people from most of the different departments within the company. Apart from the canteen, the company did not have other locations or facilities, such as a day-care centre, gymnasium or swimming pool, where many of the employees could meet. No recent inter-departmental events had been held. The drinking (tap) water was also widely distributed within Copenhagen and there was no indication of a general water contamination. The investigation therefore quickly focused on the canteen in addition to dispensers of bottled water and ice-cube machines. Because the outbreak was not reported until the end of the week following the probable exposure, no food samples from the canteen were available for analysis. However, water from the ice-cube machines and from four different drinking-water dispensers was negative for Cryptosporidium.
A total of 118 employees took part in the initial case-control study; 56 were classified as cases and 57 as controls. The symptomatology was unclear in five persons who were therefore excluded from the analysis. Eating in the company canteen on Tuesday, 23 August was associated with disease (Table 1). Among the individual courses available from the canteen each day during the week, eating salad from the salad bar was consistently associated with illness, with especially strong associations on Tuesday 23 and Wednesday 24 August. Eating beef on Tuesday and salmon on Wednesday was also significant. Of the 13 laboratory-confirmed cases, 12 took part in the study. The numbers of these who reported eating in the canteen/eating salad from the salad buffet were 10/10, 12/12 and 10/10 for Monday, Tuesday and Wednesday, respectively.
Table 1. Results of the case-control study in 56 cases and 57 controls, outbreak of C. hominis, Denmark, 2005
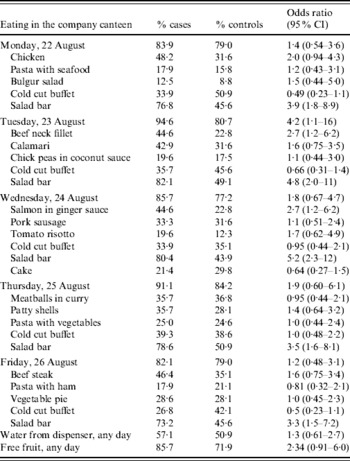
CI, Confidence interval.
Since the results of the first questionnaire study indicated that illness could be attributed to the salad bar, a second more detailed study of the individual ingredients in the salad bar was conducted, using an electronic questionnaire. The electronic link to the questionnaire was sent by email to all ~600 employees. In all 364 (~55%) responded. Nine respondents were on vacation or otherwise not present at the time of the outbreak; one person gave contradictory disease information. These 10 respondents were excluded, leaving 354 study participants of whom 79 met the case definition and were classified as cases. There was a clear association between illness and use of the canteen at any time within the defined period (RR 8·3, 95% CI 2·0–35). Fifty-one respondents, of whom two were cases, reported not having used the canteen at all. Furthermore, the risk increased with each additional day the respondents reported using the canteen (OR 1·3, 95% CI 1·2–1·5 per day). Illness was associated with eating in the company canteen on any of the seven individual weekdays, although the association was strongest for 22 and 23 August (Table 2). Multivariate analysis of canteen usage by weekday showed only 22 August (OR 2·9, 95% CI 1·2–7·1) and 23 August (OR 4·5, 95% CI 1·5–14) were associated with an elevated risk of disease. Although several menu items were associated with disease in the second study, the strongest association once more was with eating from the salad bar (Table 2).
Table 2. Results of the cohort study, 79 cases and 275 non-cases, outbreak of C. hominis, Denmark, 2005
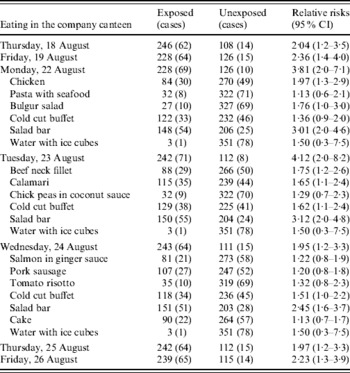
CI, Confidence intervals.
Concerning the individual salad bar items, the respondents were not asked to indicate what they had consumed on individual weekdays, but which items they were likely to have eaten during the week of 22–26 August when the salad bar contained the same ingredients. We therefore restricted the analysis of the salad bar ingredients to the respondents (75 cases and 210 non-cases) who indicated eating in the canteen on at least one of the days in that week. Although several salad bar items were moderately associated with illness in this analysis (Table 3), in a multivariate analysis only whole carrots were significant (OR 2·3, 95% CI 1·1–4·9). Stepwise elimination of the salad bar ingredients in the order of highest P value resulted in a set of three ingredients associated with disease: red peppers (OR 3·3, 95% CI 1·7–6·6), whole carrots (OR 2·1, 95% CI 1·1–4·0) and grated carrots (OR 2·1, 95% CI 1·2–3·9). Of the 75 cases, 26 reported most likely having eaten red peppers; 26 whole carrots and 46 grated carrots (52 cases most likely carrots, 20 both types, 26 only grated carrots and six only whole carrots). The whole peeled carrots were kept in a bowl of water at the salad buffet.
Table 3. Results from the cohort study concerning the 75 cases and 210 non-cases reporting eating in the company canteen 22–26 August; outbreak of C. hominis, Denmark, 2005
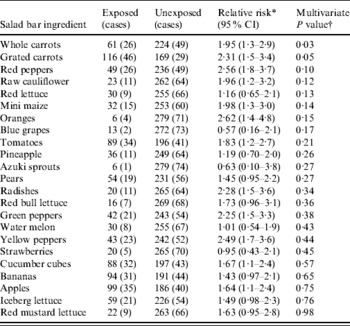
* Univariate analysis.
† Wald χ2 statistics.
Based on the results from both analytical studies 99 persons meeting the case definition were identified. Figure 1 shows the dates of onset of symptoms, Monday, 29 August was the date when most patients reported falling ill. The curve indicates a point-source outbreak and is compatible with the analytical findings that the exposure took place at the beginning of the week preceding the peak in cases. The second questionnaire to the employees contained several additional questions. Among those reporting diarrhoea, 81% also reported abdominal pain, 37% fever and 19% vomiting; a quarter of the cases reported having seen a doctor and 15% reported that close friends or family had developed diarrhoea following the illness in the cases. Of 31 cases who answered a question about the duration of illness, the median duration was 9 days (range 2–31 days). In the 3-week period prior to the beginning of the outbreak, 6·7% reported having had diarrhoea and 12·5% having travelled outside Denmark.
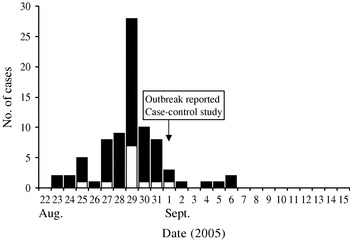
Fig. 1. Outbreak of C. hominis infection, Denmark, 2005: symptom onset dates for 81 cases (![]() ), including 12 confirmed cases (□).
), including 12 confirmed cases (□).
DISCUSSION
Eating in the company canteen was identified as the probable source of the outbreak. Most patients reported falling ill on Monday, 29 August, and results from the two analytical epidemiological studies indicated that infection took place on one or more days at the beginning of the previous week. Tuesday, 23 August was clearly associated with disease in both studies; the second study also indicated that infection may have occurred the day before. The epidemic curve indicated a point-source outbreak. The probable median incubation period was 6 days with onset of illness for most cases between 4 and 8 days.
Furthermore, the analytical studies indicated that the salad bar was the most likely source of infection, and eating from the salad bar most strongly associated with illness. Due to the design of the second questionnaire study, it was not possible directly to pinpoint any salad bar item as the source of the outbreak. However, among the salad bar ingredients, three items – red peppers, whole carrots and grated carrots – appeared to be associated with disease. On balance, the whole, peeled carrots would seem the most probable vehicle as they were kept in a large bowl of water placed in the salad buffet. Oocysts of C. hominis survive well in water and the spread in the water combined with the low infectious dose of the organism can make a bowl of water an efficient vehicle for spread of the infection. Inquiries to the canteen staff revealed that 200 whole carrots were sold daily. Moreover, the water bowl was refilled with carrots twice without changing the water, and the water was not necessarily changed from one day to the next. Many costumers complained that the utensils for taking the carrots were missing, forcing costumers to dip their hands into the water. Thus, if any of the customers were carriers of the parasite, they could have contaminated the carrots with their hands. If so, the association with grated carrots is less easy to explain. One possibility is reuse of whole carrots to make grated carrots the following day, assuming exposure on two successive days (e.g. 22 and 23 August). Another possible explanation is recall bias; it may have been difficult to remember which type of carrot was consumed. Ingredients in the salad bar may also have become contaminated as a result of canteen staff excreting the parasite. Based on interviews with staff this was considered less likely, primarily because none of the staff had shown symptoms of diarrhoea before the outbreak. However, none of the staff was tested for carriage of Cryptosporidium; Danish regulations do not allow for compulsory testing in such situations.
In this outbreak investigation, microbiological evidence from food was lacking and the conclusions reached were supported solely by the results of the epidemiological studies. Two different analytical studies were performed. The first was conducted quickly following the recognition of the outbreak. The aim was to confirm that the outbreak was foodborne and if possible identify a category of food as the most likely source. Guided by the results of the first study, the second was conducted with the aim of corroborating the results of the first study and identifying the source of the outbreak in more detail. Because the outbreak occurred in an office environment, an electronic questionnaire was utilized for this (larger) study. Electronic data collection provided a number of advantages, including the ability to address all employees easily and efficiently, enabling rapid data collection and omission of the data-entering step before analysis. As the second study was carried out 5 weeks after exposure, it was not considered likely that the respondents would be able to remember specifically which food items they had chosen from the salad bar on individual weekdays. We therefore asked the employees to indicate whether they, had eaten (‘probably’, ‘perhaps’, or ‘most likely not') any of the individual food items. The answers thus reflect the most likely consumption (i.e. food preferences) rather than actual exposures. This was an obvious limitation to the study, but still did not compromise it, since the effect of this potential recall bias problem would be a non-differential misclassification of the answers, because there was no hypothesis concerning the carrots as the vehicle prior to the study. We therefore believe that the results of the second study are valid. Another potential bias relates to the response rate which in both studies was only around 55–60%. Theoretically, if ill persons who did not often use the canteen were less likely to respond than those who regularly used the canteen, the results might have been distorted, but it was not possible to examine if there were systematic differences between respondents and non-respondents.
The outbreak took place in a fairly large Danish branch of an international company. Many of the employees frequently travelled abroad and the company received many guests, including persons who had recently travelled abroad. According to the company management, an average of 100 persons who were not normally working in the building would eat in the company canteen every day. Results from the second questionnaire study showed that several persons had travelled outside Denmark in the weeks preceding the outbreak. Moreover, several employees had had diarrhoea in that period. It is thus a possibility that the outbreak strain was imported by one of the employees or guests.
Cryptosporidium outbreaks appear to be rare in Denmark. Only one outbreak has been described previously. This took place in 1990 in a hospital ward with AIDS patients and was caused by contaminated ice cubes from an ice-cube machine. The ice cubes were most likely contaminated by an infected AIDS patient [Reference Ravn24]. In Denmark, Cryptosporidium infections are not notifiable, neither clinically nor by laboratory and an accurate incidence of the infection is not available [Reference Enemark25]. Recent studies have demonstrated the infection to be very common in Danish farm animals with herd prevalences as high as 100%, 96% and 84% in pig weaners, young and older calves, respectively [Reference Maddox-Hyttel26]. Many of these infections are caused by potentially zoonotic species/types [Reference Langkjaer20]. Nevertheless, the available evidence suggests that infections in humans are rare. Thus in 2005, only 82 episodes were diagnosed at the Laboratory of Parasitology at Statens Serum Institut [27]. Although insufficient diagnosis may play a role, the lack of reported outbreaks also seem to indicate that the organism does not occur commonly in Denmark. This is in contrast to some other industrialized countries where cryptosporidiosis is found to contribute more heavily to the burden of infectious gastrointestinal illness, such as in the United States [Reference Mead28] and the United Kingdom [Reference Lake29]. The fact that drinking water in Denmark is almost exclusively derived from ground water, as opposed to surface water, may be part of the explanation for the apparent scarcity of the infection in Denmark.
The outbreak reported in the present study was large. A total of 99 individuals reported onset of illness. Because cryptosporidiosis patients typically experience symptoms for more than 1 week, canteen outbreaks such as the present one may pose considerable costs to the workplace, in addition to human discomfort. It is possible that the case definition we used would have included a few patients that were ill due to other causes. However, only about two thirds of the individuals who probably consumed the canteen food during the relevant time period participated in the studies, and so the actual number of infected patients is likely to have been higher than 99. The fact that four out of 17 stool samples were not found positive for Cryptosporidium was primarily a result of insufficient material to examine and did not indicate that these patients were ill due to other causes.
As shown here, C. hominis has the ability to cause foodborne outbreaks given the right circumstances. This outbreak also shows that salad buffets may pose a particular risk as a cause of foodborne outbreaks. Good hygienic measures in the kitchen will help limit the risk of food handlers contaminating buffets, but the risk remains that a customer will contaminate a buffet, making others ill. Care should be taken to avoid direct contact between the hands of customers and the food. Utensils should always be available and fresh salad bar ingredients should not be served immersed in water.
ACKNOWLEDGEMENTS
We thank Lene Nørby Nielsen and Mette Bomhold Christensen for help with entering questionnaire data.
DECLARATION OF INTEREST
None.






