INTRODUCTION
In Latin America visceral leishmaniasis (VL) or kala-azar is caused mainly by Leishmania chagasi. In Brazil VL is transmitted by Lutzomyia longipalpis sandfly species and is considered the most severe form of the disease. Dogs are accepted as the most important source of infection for the vector [Reference Sherlock1, Reference Ikeda-Garcia2].
The Brazilian programme for surveillance and control of canine VL (CVL) uses two serological techniques for diagnosis, i.e. screening conducted with the Dual Path Platform rapid test® (DPP-TR) immunoassay and a confirmatory test, the enzyme-linked immunosorbent assay (ELISA) [3]. One of the disadvantages of serological tests is the possibility of false-positive results due to cross-reactivity with other species of the family Trypanosomatidae, and even phylogenetically distant microorganisms [Reference Sherlock1, Reference Ikeda-Garcia2, Reference Sundar and Rai4].
Although the gold standard technique is based on the demonstration of the parasite obtained from biological material punctures of liver, lymph nodes, spleen and bone marrow biopsy or scraping of skin, these techniques are invasive, causing risk to the animal and being impractical in public health programmes in which a large number of animals are evaluated in a short period of time [5]. Different molecular methods for diagnosis of leishmaniasis can be used, which are more commonly employed based on polymerase chain reaction (PCR). Other more sophisticated methods such as fluorescence hybridization, sequencing and real-time PCR are available for the identification of the parasite, but these techniques are expensive, time-consuming and mainly used by research centres and/or specific services in endemic areas [Reference Reithinger and Dujardim6].
PCR has been performed for diagnosis of canine leishmaniasis with its potential for parasite detection having greater sensitivity and specificity compared to microscopy or culture, particularly in samples with low numbers of parasites [Reference Cruz7], or collected less invasively as peripheral blood [Reference Martín-Sánchez8, Reference Leite9].
In this context, the present study aimed to compare the performance of PCR for the diagnosis of genus and species of Leishmania with two serological techniques: the screening test (rapid test DPP-TR immunoassay) and confirmatory assay (ELISA) used in the national programme for control of CVL in two regions with different epidemiological profiles of canine leishmaniasis.
MATERIALS AND METHODS
Blood samples
Two regions (cities) of distinct epidemiological profile were studied: (i) Teresina (Piauí), located in northeastern Brazil, which has been endemic for L. chagasi infection for decades, and (ii) Campinas (São Paulo), located in southeastern Brazil, which has no record of autochthonous human cases, and is an isolated area that was chosen for analysis because there had been a gradual increase in cases of canine leishmaniasis over the last 5 years (2009–2013) [Reference Presotto10, 11].
Samples from animals were collected by teams from the Centre for Zoonosis Control (CCZ), between September and October 2012 in Campinas and in January 2013 in Teresina. Of 195 samples obtained, 98 were from Teresina and 97 from Campinas. Thirty-four samples from each city were from dogs suspected of leishmaniasis with positive serology after screening, and the remaining samples were from other dogs with no suspicion of leishmaniasis, used as a negative control group. The samples were enrolled by the CCZ teams in accordance with criteria established by municipalities for epidemiological evaluation of CVL. All samples were derived from domestic animals.
The samples were obtained by puncture of the cephalic vein and placed in sterile polypropylene tubes with separator gel. The same medium was used for transportation of blood clots. Soon after completion of field collections, the tubes were centrifuged and the withdrawal of serum was performed (by CCZ serological tests).
Preparation of samples
For the dissolution of coagulum, 10 U streptokinase (200 μl) was added to each sample, and then incubated at 37°C for 18 h. After incubation the sample was centrifuged at 3000 rpm for 15 min at room temperature and the supernatant discarded.
DNA extraction
DNA extraction was performed using a commercial kit (Dneasy Blood and Tissue kit, Qiagen, Brazil) according to the manufacturer's instructions.
PCR for genus and species
Identification of Leishmania spp. was performed using Leish-150 [5′-GGG (G/T) AGGGGCGTTCT (C/G) CGAA-3′] and Leish-152 [5′-(C/G)(C/G)(C/G)(A/T)CTAT(A/T)TTACACCAACCCC-3′] primers [Reference Passos12, Reference Gomes13] and L. chagasi species identification was performed using RV1 (5′-CTTTTCTGGTCCCGCGGGTAGG-3′), and RV2 (5′- CCACCTGGCCTATTTTACACCA-3′) primers [Reference le Fichoux14, Reference Gomes15].
The control of cross-reactivity to other species of the genus Leishmania was performed by allele-specific primers (LU-5A (5′-TTTATTGGTATGCGAAACTTC-3′) and LB-3C [5′-CGT(C/G)CCGAACCCCGTGTC-3′]) for the species L. braziliensis [Reference Gomes15, Reference Harris16]. L. braziliensis showed the highest frequency for cutaneous leishmaniasis in the study areas and could be present as infection in animals besides other cross-reactants [17].
In the present study, GAPDH-F (5′-AGGCTGAGAACGGGAAACTT-3′) and GAPDH-R (5′-ATTAAGTTGGGGCAGGGACT-3′) primers [Reference Kullberg18] were used as endogenous controls for dog samples.
For each experiment, in a separate tube, the same reaction was performed for: (i) negative control (ultrapure water); (ii) positive control for the genus Leishmania spp.; (iii) positive control for L. chagasi; (iv) each sample to be analysed. The control DNA was extracted from promastigote culture from the Adolfo Lutz Institute (SP – MHOM/BR/1972/LD), and was used as an endogenous control.
For reactions of gene amplification a commercial kit (GoTaq® Hot Start Green Master Mix; Promega, Brazil) was used.
For amplification of DNA we used a Mastercycler gradient thermocycler (Eppendorf, Brazil). The cycling amplification reaction, to determine the presence of L. chagasi, was initiated with denaturation at 94 °C for 5 min followed by 30 cycles with the following conditions: 94°C for 30 s, 60°C for 30 s and 72°C for 30 s, with a final extension step at 72°C for 5 min. The amplification reaction originated a fragment of 145 bp.
The DNA amplification reactions for L. braziliensis and Leishmania spp. were as follows: initial denaturation at 95°C for 5 min, 30 cycles of 95°C for 45 s, 58 °C or 55°C for 45 s and 72°C for 45 s with a final extension at 72°C for 5 min. The reactions generated fragments of 146–149 bp and 120 bp for L. braziliensis and Leishmania spp., respectively.
All reactions were performed by adding the endogenous control in the same conditions for each primer. The DNA fragment for the endogenous control size was 911 bp.
The result of the reaction was visualized on agarose gel (2%) in Tris-Boro-EDTA (TBE) buffer and stained with 0·5 mg/ml ethidium bromide. The DNA fragments were visualized on a transilluminator and the sizes estimated by comparison with fragments of 100 bp marker.
Traditional diagnosis
The screening serological (DPP-TR) and confirmatory (ELISA immunoenzymatic assay) tests used followed the protocols previously established by the Ministry of Health of Brazil [19], modified by Joint Technical Note 48/2011 in each centre of zoonoses [3].
Data analysis
PCR was performed to identify genus and species of Leishmania and was compared to screening and confirmatory tests (DPP-TR and ELISA). We evaluated the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the methods using SAS v. 9·2 (SAS Institute Inc., USA). All values for sensitivity, specificity, PPV and NPV are given with their 95% confidence intervals. The study describes the absolute and percentage values for each test performed. Complementary evaluation using Cohen's kappa test was performed according to the criteria of Fleiss et al. [Reference Fleiss, Levin and Paik20]. This test was conducted to determine the statistical concordance between the tests performed in the two cities.
RESULTS
During the study period, 195 canine blood samples were collected, 97 (49·74%) from the city of Campinas and 98 (50·26%) from Teresina. Of these, 68/195 (34·87%) samples were positive by screening test, 34 from each city (34/195, 17·95%). The remaining 127 samples (65·13%), without suspicion of leishmaniasis, were used as negative controls: 63/195 (32·31%) dogs from Campinas and 64/195 (32·82%) dogs from Teresina. All dogs from Teresina were suspected of leishmaniasis but had no clinical signs of disease and the situation was the same for Campinas, except for one dog with suspected clinical signs that was positive by screening test and confirmatory PCR.
In Campinas, for the 34 cases with positive screening test, 30 cases were PCR positive and 20 were positive by confirmatory test. There was disagreement between ELISA and PCR in nine cases with positive PCR for both genera and for species L. chagasi, and negative by ELISA. In one case, confirmatory test was inconclusive by PCR and positive by rapid test. In Teresina, for the 34 cases with a positive screening test, a confirmatory test was positive for 23 samples and by PCR for only five (Table 1).
Table 1. Results of the screening and confirmatory tests distributed between Campinas and Teresina cities
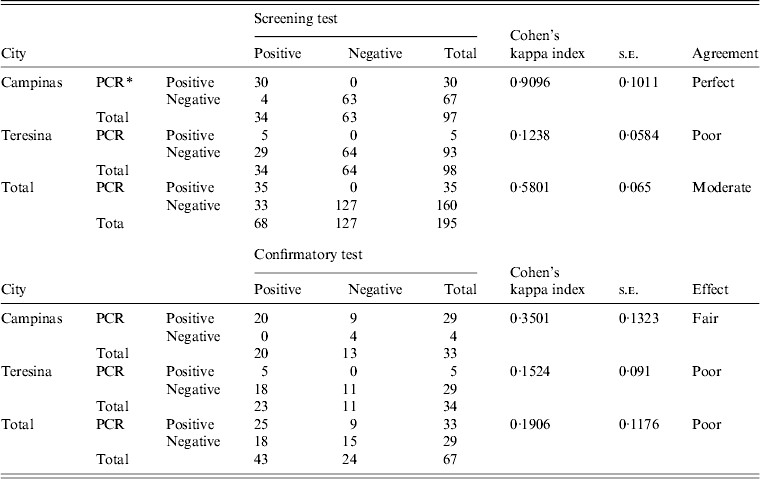
Screening test, DPP-TR immunochromatographic; confirmatory test, enzyme-linked immunosorbent assay (ELISA); s.e., standard error; PCR, polymerase chain reaction.
* The PCR was performed for the genus Leishmania and species L. chagasi.
Considering our data, Table 2 describes the performance of the diagnostic tests used in the study. Values in Table 2 were calculated from Table 1. Tables 3 and 4 show the comparisons of the methods used for the screening and confirmatory methods, respectively. Values in Table 3 and 4 were obtained based on absolute values presented in Table 1.
Table 2. Description of the performance of the three tests evaluated in Campinas and Teresina

Screening test, DPP-TR immunochromatographic; confirmatory test, enzyme-linked immunosorbent assay (ELISA); PCR, polymerase chain reaction.
* The PCR was performed for the genus Leishmania and species L. chagasi.
Table 3. Reference values of PCR compared with the screening test (rapid test DPP-TR immunoassay) for samples from Campinas and Teresina for leishmaniasis screening*
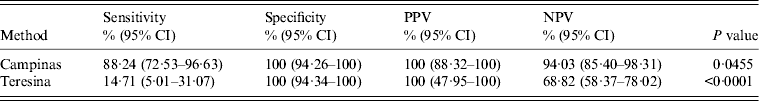
PCR, Polymerase chain reaction; CI confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Examination by McNemar test.
* The values for the sensitivity, specificity, PPV and NPV were performed considering the data shown.
Table 4. Reference values of PCR compared to the confirmatory test (ELISA) in samples from Campinas and Teresina for leishmaniasis screening*
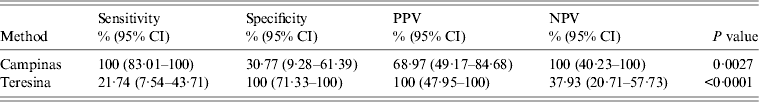
PCR, Polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Examination by McNemar test.
* The values for the sensitivity, specificity, PPV and NPV were performed considering the data shown in the Table 1.
PCR was compared considering the regions and the use of screening and confirmatory tests. When PCR was compared to the screening test a difference in sensitivity of the method in the two cities studied was observed (P = 0·0455 for Campinas, P < 0·0001 for Teresina). Campinas showed greater sensitivity for PCR (88·24%) compared to Teresina (14·71%).
Compared to PCR, the confirmatory test results were discordant between PCR and ELISA (P = 0·0027 for Campinas, P < 0·0001 for Teresina) for sensitivity, specificity, PPV and NPV in the two samples.
For Cohen's kappa index (0·9096), concordance was observed for both PCR and screening test in the Campinas samples. For the screening test in the Teresina samples and confirmatory test no concordance was observed in our data (Table 1).
All 195 samples were tested by PCR with primers for the species L. chagasi and L. (Viannia) brasiliensis. None of the samples tested was positive when tested with primers LU-5A and LB-3C [L. (V.) brasiliensis] and all 35 samples initially positive for Leishmania, were positive when tested with the primers RV1 and RV2 (L. chagasi).
DISCUSSION
Different parasitological, serological and molecular techniques have been used in the investigation of CVL for diagnostics and monitoring [Reference Ikeda-Garcia2, Reference Reithinger and Dujardim6, Reference Gomes15, Reference Choudhry21–Reference Mohammadiha24]. Even with advances in the understanding of the complex epidemiology of leishmaniasis and the use of these diagnostic methods, the interpretation of the results remains open for discussion. In Brazil, the regional and epidemiological differences of the disease are important challenges to be considered for its understanding and control.
PCR is not widely used in epidemiological surveillance because of the high cost, demand for specific equipment, lack of standardization of technique, and need of trained staff. However, in research it is widely used and has the benefits of rapidity, high sensitivity and specificity [Reference Reithinger and Dujardim6, Reference Martín-Sánchez8, Reference Harris16, Reference Mohammadiha24, Reference Mathis and Deplazes25].
The PCR detection of CVL using peripheral blood has advantages: (i) the presence of circulating parasites are known to occur in dogs; (ii) this type of sampling is simple and less invasive compared to lymph node biopsy or aspirate or bone marrow aspiration; (iii) high sensitivity compared to conventional methods (serological and parasitological) and allows detection of asymptomatic dogs (and sometimes seronegative animals) [Reference Reale26–Reference Solano-Gallego29]; (iv) high PPV [Reference Solano-Gallego29]; and (v) allows longitudinal monitoring of infection [Reference Lachaud28]. However, as a limitation, PCR cannot be used as a unique diagnostic technique for the confirmation of disease, because a positive result confirms Leishmania infection, but not disease. A strong clinical suspicion, even with negative serology and positive PCR, confirms leishmaniasis [Reference Solano-Gallego29].
The performance of PCR is related to the extraction protocol and the primers chosen, in this work we used primers for genera (Leish-150 and Leish-152) and for the diagnosis of the species L. chagasi (RV1 and RV2). The primers used have shown excellent performance and are able to detect a parasite in 5–10 ml peripheral blood [Reference Reale26]. The high sensitivity of these primers derived from the kinetoplast (kDNA) is associated with targets with a high copy number, and conserved sequences in different species of Leishmania [Reference le Fichoux14, Reference Gomes15].
PCR was evaluated in canine samples in two cities with different epidemiological characteristics. The city of Teresina was considered to represent the spread of VL since the 1980s, associated with low socioeconomic conditions and poor sanitation and environmental changes, modulated by intense urbanization process, which favoured the vector's adaptation to the urban environment [Reference Costa, Pereira and Araujo30]. The city of Campinas has been monitored for the appearance of cases of CVL in a restricted area of the city between 2009 and 2013, where there is no record of autochthonous human cases [Reference Presotto10, 11].
To the best of our knowledge, we do not know of any studies comparing serological and molecular techniques in the diagnosis of VL in dogs from regions with different epidemiological profiles, and thus, we were interested in analysing the performance of PCR in a population that for decades has exhibited chronic infection compared to another region with the recent appearance of CVL.
Sensitivity was the measure with the greatest disagreement when comparing PCR and screening test (rapid test DPP-TR) and confirmatory test (ELISA) in both cities, where Teresina showed lower sensitivity (PCR screening 14·71%, PCR/ELISA 21·74%), and by contrast Campinas presented high sensitivity (PCR screening 88·24%, PCR/ELISA 100·00%). Similar results were found by Quinnell et al. [Reference Quinnell31], who compared the detection of Leishmania by PCR, serology and cellular immune response in dogs, reporting a variation in the sensitivity of PCR over the course of infection, higher (78–88%) from 0 to 135 days post-infection, and decreasing to about 50% after 300 days. The difference in sensitivity when we used a molecular technique in two regions with different epidemiological profiles can be linked to a probable profile, acute/sub-acute infection present in the canine population of Campinas, and predominantly chronic in Teresina.
In the city of Campinas, there was better sensitivity between the screening test and PCR (34/30) and a greater disagreement between the confirmatory test compared to PCR (20/30). Another important result is the apparent low specificity of PCR obtained in Campinas (30·77%), which can be explained by the presence of nine samples with positive PCR confirmatory test (ELISA) negative. However, these nine cases were positive by the screening test. These results lead us to reflect on the accuracy of diagnosis of the CVL scheme implemented in Brazil, especially when considering a population of more recent infection, such as Campinas. In this population the screening test was more sensitive and specific than the confirmatory test, with reference to detection of the parasite DNA by PCR.
This explains the appearance of a significant number of samples possibly false negative by ELISA and the high sensitivity of PCR, similar to previous findings by Fallah et al. [Reference Fallah32], who showed that this technique (kDNA–PCR) is capable of detecting parasite DNA in samples of peripheral blood of seronegative ELISA infected dogs.
For Campinas samples, PPV of PCR was 100% compared to the screening test, suggesting that PCR can be an effective tool to detect active and recent infection, and NPV of PCR was 100% compared to the confirmatory test. In Teresina, the PPV of PCR was 100% compared to the screening and confirmatory tests. For NPV, the PCR was 68·9% compared to the screening test and 37% compared to the confirmatory test. Considering the low number of positive confirmatory tests, PCR is not useful in this sample.
Another important observation in our data is the concordance by Cohen's kappa index between the tests and cities. Considering our results, from the Campinas samples there was concordance between the screening test and PCR, but not for the confirmatory test. Taking into account the Teresina samples, the screening and confirmatory tests showed no concordance. This fact highlights two important factors: (i) the specific condition found in each region for leishmaniasis; (ii) the better information achieved by the screening test compared to the confirmatory test adopted in Brazil.
The accuracy of serological techniques used by the programme for monitoring and control of CVL is questionable [Reference Costa33, Reference Costa34], especially when canine euthanasia is indicated [19] when considering these animals as an important transmission link of leishmaniasis for humans. There is a lack of scientific evidence that supports the measure of elimination of seropositive dogs is related to a reduction of infection in humans [Reference Costa33–Reference Soares, de Mendonça and do Bonfim35]. This reflection is necessary when we consider the situation found in the epidemiological scenarios chosen for the development of this study. On the one hand we have Teresina, where an epidemic of human leishmaniasis has persisted for decades, coupled with a high prevalence of serologically positive dogs. However, according to our results, a small number of animals present circulating parasites, questioning their real value as reservoir. On the other hand, we have Campinas, where the infection spreads among the canine population with parasitaemia, widely detected in peripheral circulation, but causes no human clinical cases. This scenario allows us to highlight the complexity of the cycle of leishmaniasis, where we can find different patterns of infection, or preference vector, or even diversity of the virulence of the parasite for the canine and human host.
CONCLUSION
This study confirms the utility of PCR to analyse canine reservoirs, especially the detection of active infection, distinguishing the situation of dogs in endemic areas, where serological diagnosis is necessary for chronic disease. Together, serology and PCR can better assess the individual risk of each dog for transmitting the parasite. The two different scenarios presented in this study allow us to question the competence of these dogs for the transmission of VL to humans and the real need for euthanasia.
APPENDIX
Leishmaniasis Study Group
Andrea Vonzuben (Center for Zoonosis Control, Campinas, SP, Brazil); Angela T. Lauand Teixeira (Supervisory Laboratory of Parasitology, Unicamp); Celia Regina Mendes Sales (Laboratory of Parasitology, Unicamp); Christian Cruz Höfling (Center for Epidemiological Surveillance, Unicamp); Douglas Presotto (Coordinator Center for Zoonosis Control, Campinas, SP, Brazil); Fernando Luiz Lima de Oliveira (Municipal Health Foundation Teresina, PI, Brazil); Maria Clara Duarte Fregolente (Laboratory of Parasitology, Unicamp); Maria Luiza Moretti (Coordinator of the Center for Epidemiological Surveillance, Unicamp); Paulo Velho (Department of Clinical Dermatology, Unicamp); Selma Giorggio (Institute of Biology, Unicamp); Vera Lucia Pereira Chioccola (Instituto Adolfo Lutz, SP, Brazil); Marcelo de Carvalho Ramos (Associate Director of FCM, Unicamp).
ACKNOWLEDGEMENTS
The authors thank the laboratory of medical genetics from the State University of Campinas and Professor Carmen Silvia Bertuzzo for providing the PCR analysis.
DECLARATION OF INTEREST
None.







