INTRODUCTION
Due to the frequent changes in influenza surface antigens, the influenza strains that make up the seasonal trivalent influenza vaccine (TIV) are reviewed annually by the World Health Organization (WHO) [1], and an annual programme of vaccination is required. In the UK, an annual dose of unadjuvanted TIV is recommended as universal vaccination for all persons aged ⩾65 years. Selective annual vaccination is also offered to those aged from 6 months to 64 years in a clinical risk group [2] and, for the first time in 2011/2012, to all pregnant women [2].
In recent years, a number of studies have been conducted across Europe to estimate the annual vaccine effectiveness (VE) of TIV as soon as possible after the start of the influenza epidemic, and to monitor it through the season [Reference Kissling and Valenciano3, Reference Valenciano4]. In the UK, a variety of study designs, test-negative case-control [Reference Pebody5] or cohort [Reference Hardelid6] studies in primary care, are used. VE is estimated against a number of clinical and microbiological end-points, including consultations for influenza-like illness, lower respiratory tract infection and laboratory-confirmed influenza in primary care. The purpose of these studies is to evaluate the effectiveness of the seasonal TIV (or monovalent pandemic vaccine) against the circulating influenza strains each year and to inform the targeting of complementary or alternative public health measures (e.g. use of antivirals) for groups who may be less well protected by the vaccine. In addition, along with other similar VE studies from different geographical settings, the findings help inform the WHO decision each year on the composition of the annual TIV for the following season [1].
However, despite the fact that the objective of the annual influenza immunization programme is to protect those most at risk of serious illness or death following influenza infection [2], to date there have been only a few studies estimating VE against severe influenza end-points – mainly hospitalization [Reference Hellenbrand7–Reference Puig-Barberà10]. In the 2010/2011 influenza season, the first post-2009 pandemic season, the UK experienced a rapid increase in the daily number of severe cases with confirmed or suspected influenza A(H1N1)pdm09 occupying critical care beds [Reference Bolotin11]. This changing epidemiology of influenza cases highlighted the importance of establishing additional systems and methodologies to measure VE for preventing influenza requiring critical care admission, as well as studies using hospitalization or death as an end-point.
Following a successful pilot in 2010/2011, in 2011/2012 new surveillance systems for severe influenza infection resulting in intensive care unit (ICU) or high dependency unit (HDU) admission were established in England and Scotland. These were designed to monitor and estimate the impact of seasonal influenza in the population, and to describe the epidemiology of severe disease resulting in critical care admission [Reference Bolotin11]. These systems provided an ideal source of individual-level case data for a VE study against severe influenza end-points. In both countries, detailed, high-quality primary-care-based influenza vaccine uptake monitoring systems to General Practitioner (GP) practice level are also well established, which provide a source of comparative data on TIV uptake in the general population. The existence of these two data sources provides the potential to rapidly estimate VE against severe influenza within an influenza season using the screening method.
The aim of the study was to estimate the VE of the 2011/2012 TIV against severe influenza resulting in ICU admission in England and Scotland using the screening method.
METHODS
Data collection: cases
Severe influenza surveillance systems
We identified cases of severe influenza admitted to ICUs/HDUs from severe influenza surveillance systems in England and Scotland. In 2011/2012, the UK Severe Influenza Surveillance System (USISS sentinel) collected individual-level data on confirmed influenza cases admitted to ICUs or HDUs in a sentinel network of 36 hospitals selected from 158 eligible acute trusts by stratified random sampling. In Scotland in 2011/2012, the Scottish ICU Influenza Surveillance System collected individual-level data on confirmed influenza cases admitted to ICUs or HDUs from all 30 hospitals with intensive care provision across all 14 NHS boards.
All patients that met the following criteria were included in the study: cases aged > 6 months admitted to an HDU or ICU between week 40/2011 and week 20/2012 in one of the USISS participating sentinel hospitals in England, or any hospital in Scotland, with laboratory-confirmed influenza. Patients that were not registered with an English or Scottish NHS GP were excluded.
For each case, data on age, sex, date of onset of influenza-like illness, specific clinical risk group status (the presence or absence of chronic respiratory disease, chronic heart disease, chronic kidney disease, chronic liver disease, chronic neurological disease, diabetes or immunosupression) and pregnancy status was extracted from the hospital surveillance systems.
Primary-care records
We obtained the 2011/2012 TIV vaccination history of cases, including date of administration, by sending a standard data collection proforma by post to the patient's GP in England, and through direct access to primary-care vaccination records in Scotland. In addition, the clinical risk group status and the date of onset of influenza-like illness for each case were also confirmed against primary-care records. In the small number of cases where the primary-care records contained information on comorbidities not documented in the surveillance systems, the primary-care records were considered to contain the most complete information. In the small number of cases where the patient consulted their GP prior to hospitalization for the presenting illness and there was a discrepancy between date of illness onset in the primary-care records and in the surveillance system, the primary-care records were considered to be the most accurate source of data on date of illness onset.
Adults and children aged ⩾13 years were considered as vaccinated against influenza if they received vaccination with the 2011/2012 TIV >14 days before disease onset. Children aged from 6 months to <13 years were considered fully vaccinated against influenza if they received vaccination with the 2011/2012 TIV >14 days before disease onset and had previously received influenza vaccine, or they received vaccination with the second dose of 2011/2012 TIV >14 days before disease onset that season.
Data collection: reference population
Data on population vaccination coverage was obtained from routine vaccine uptake monitoring systems. In England, the Health Protection Agency (HPA) Department of Health (DH) Influenza Immunization Uptake Monitoring Programme (ImmForm) [Reference Gates12] collected cumulative weekly TIV uptake data from the registered GP population in England between 1 September 2011 and 31 January 2012 (inclusive), the period of time during which UK GPs implement the seasonal influenza vaccination programme. ImmForm comprises a weekly automated collection from a sentinel group of GP practices in England, with 50·3% of all (n = 8205) general practices providing immunization services reporting in week 40/2011, rising to 66·9% of all GP practices in week 4/2012. In addition to the sentinel group of GP practices involved in weekly reporting, ImmForm also conducts a monthly mandatory survey of all GP practices in England, with a response rate at end of season of 99·5% in 2011/2012. The data on weekly vaccine uptake from the sentinel group of practices for the final week of each month is very similar to the data received for the end of month mandatory vaccine uptake data from all practices, with the vaccine uptake differing by a median of 0·6% (range 0·1% for week 4/2012 to 3·9% for week 43/2011). Thus the sentinel system in England provides accurate real-time weekly estimates of population vaccine uptake. In Scotland, Health Protection Scotland (HPS) collected cumulative weekly TIV uptake data from the registered GP population in Scotland from 1 October 2011 to March 31 2012 (inclusive). The Scottish seasonal influenza vaccine uptake monitoring system comprises a weekly automated collection from all GP practices, with 37% of practices submitting data in week 40/2011, increasing to 96% of practices in week 52/11 and 98% of practices in week 4/2012. The code set used for this data extract (PRIMIS) is the same as that used to gather vaccine uptake information via ImmForm in England.
Data on population vaccination coverage by age group (6 months to <2 years, 2 years to <16 years, 16 years to <65 years, ⩾65 years), specific clinical risk group (presence or absence of chronic heart disease, chronic renal disease, chronic liver disease, chronic neurological disease, diabetes, immunocompromised), pregnancy status and geographical area [Strategic Health Authority (SHA) in England, and Scotland] and week was extracted directly from the English and Scottish routine vaccine uptake monitoring systems, with the numerator being the number of individuals vaccinated in each subgroup, and the denominator being the number of GP-registered eligible individuals in each subgroup. SHAs are the current regional administrative health service structures in England, and cover geographical areas of population size from about 2·5–7·5 million; Scotland has a population of just over 5 million. The combined weekly and cumulative TIV vaccine uptake for England and Scotland by influenza vaccine risk group was calculated by weighting the vaccine uptake in each group in each country by the proportion of the study population in each group from each country.
The Health Protection Agency has approval under Section 60 of the Health and Social Care Act (now subsumed into the National Information Governance Board for Health and Social Care with Section 60, now Section 251 of the NHS Act 2006) to process confidential patient information for the purpose of monitoring the effectiveness and safety of vaccination programmes, so additional ethical approval for the study was not required.
Data management
Individual case data were entered into a password-protected Access database. The aggregate ImmForm and HPS extracts were downloaded into a Microsoft Excel database. Data were analysed in Stata v. 12 (StataCorp., USA).
Statistical analysis
The crude VE was computed as:
where PPV is the proportion of the reference group vaccinated at week 4/2012 (vaccine coverage in the reference group), and PCV the proportion of cases vaccinated [Reference Farrington13]. Exact binomial 95% confidence intervals (CIs) were calculated for the crude VE. Analysis was stratified by influenza vaccine target groups: age ⩾65 years, and age <65 years in risk groups (including pregnancy).
To adjust for major confounders, the appropriate vaccine coverage was matched to cases at an individual level based on age group (6 months to <2 years, 2 years to <16 years, 16 years to <65 years, ⩾65 years), specific clinical risk group (chronic respiratory disease, chronic neurological disease, chronic heart disease, chronic renal disease, chronic liver disease, chronic neurological disease, diabetes, immunosuppression), pregnancy status and geographical area (SHA in England, and Scotland) and week of onset. The vaccine coverage data was offset by 14 days to provide an estimate of weekly effective influenza vaccine uptake taking into account the 14 days required to develop immunity.
To obtain the adjusted VE estimates with 95% CIs, logistic regression was conducted with the outcome variable as the vaccination status of the case (1 = vaccinated, 0 = unvaccinated), and with an offset for the log-odds of the matched coverage. The linear model used was
where P is the probability of vaccination in the case, PPV the matched coverage and c a constant parameter to be estimated. VE was then calculated as 1 – exp(c), since exp(c) = [P/(1–P)]/[PPV/(1–PPV)].
RESULTS
Description of cases
Forty-six cases were reported to the English USISS sentinel hospital surveillance scheme during the study period. Forty-three cases were eligible for inclusion in the study; the remaining three were excluded because they were not registered with an NHS GP (two cases), or were aged < 6 months (one case). Seventeen cases were reported to the Scottish ICU surveillance system; all 17 were eligible for inclusion in the study. Thirty-three (55%) of the cases were due to influenza A(H3N2), 18 (30%) were due to untyped influenza A, eight (13%) were due to influenza B and one (2%) was due to influenza A(H1N1)pdm09.
Vaccination status was available for 56/60 cases (93%). In those aged ⩾65 years, 75% of cases were reported to have been vaccinated with seasonal 2011/2012 TIV > 14 days prior to symptom onset, and for those aged < 65 years in clinical risk groups or pregnant, 83% were reported to have been vaccinated (Table 1). None of the cases had received TIV in the 14 days prior to symptom onset.
Table 1. Vaccination status against seasonal influenza 2011/2012 >14 days prior to symptom onset of cases reported to severe influenza surveillance systems in England and Scotland in 2011/2012
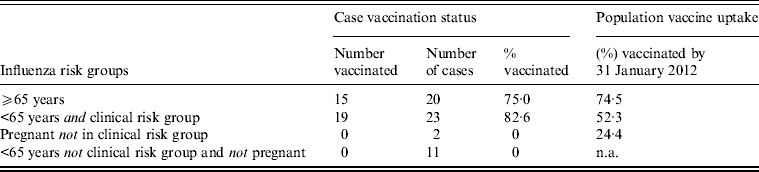
n.a., Not applicable.
Description of reference population
The combined population vaccine coverage for England and Scotland by week 4/2012 for the GP-registered population aged ⩾65 years was very similar to the proportion of cases vaccinated in that age group. In contrast, the population vaccine coverage for those aged <65 years in clinical risk groups was substantially lower than the proportion of cases vaccinated (Table 1).
Vaccine effectiveness
The crude VE estimate was −2% (95% CI −259 to 64·4) for those those aged ⩾65 years, and −189% (95% CI −780 to −11) for those aged < 65 years in a clinical risk group (including pregnancy).
After adjusting for age group, specific clinical risk group, country and week of illness onset, the adjusted VE estimate was below zero for those aged ⩾65 years, with wide 95% CIs that crossed zero. The upper limit of the 95% CIs for VE was below zero for all cases, and for those aged < 65 years in a clinical risk group (Table 2).
Table 2. Adjusted vaccine effectiveness (VE) by logistic regression, adjusting for age group, specific risk group, week and Strategic Health Authority (SHA)
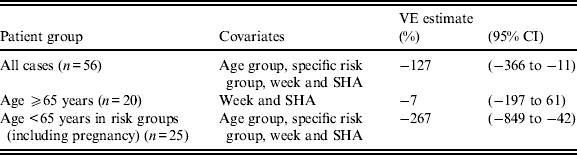
CI, confidence interval.
DISCUSSION
This study of VE against severe influenza resulting in ICU admission in England and Scotland has two key findings: first, both the crude and adjusted VE estimates for prevention of severe influenza leading to ICU admission in those aged ⩾65 years were below zero, which is consistent with other studies suggesting that the 2011/2012 TIV was poorly protective at preventing severe influenza in this age group; second, both the VE point estimate and the upper limit of the 95% CIs for those aged <65 years in risk groups was below zero, which suggests that there may still be significant residual confounding using this methodology in this age group, even after adjusting for all measurable confounders.
The start of the 2011/2012 influenza season in the UK was unusually late, with the peak Royal College of General Practitioners (RCGP) influenza-like illness consultation rate in England and Wales occurring in week 7/2012 [14]. The season was also exceptionally mild, with peak clinical consultation activity at its lowest level since the RCGP scheme was established in 1966 [14]. The main influenza virus circulating was influenza A(H3N2) [14]. The late mild season led to a much lower than expected number of confirmed influenza ICU/HDU cases reported to the severe influenza surveillance systems, with 80% fewer cases reported to USISS sentinel in 2010/2011 compared to 2011/2012, despite the fact that the sentinel system expanded from 19 trusts to 36 trusts during this period. The small number of observed cases led to wide CIs around the VE estimates. Despite this, the finding of a VE estimate for prevention of severe influenza resulting in critical care admission below zero in those aged ⩾65 years is consistent with evidence from other studies that the 2011/2012 TIV was poorly protective against the dominant circulating A(H3N2) strain of influenza. Studies of the effectiveness of the 2011/2012 TIV against less severe influenza end-points demonstrated low overall levels of protection, and waning of protection during the season [Reference Pebody15]. In the UK, an end of season 2011/2012 test-negative case-control study of patients consulting in primary care estimated an adjusted VE of TIV against A(H3N2) influenza of 23% (95% CI −10 to 46), dropping to 12% (95% CI −31 to 41) for those vaccinated >3 months before illness onset [Reference Pebody15]. The early estimates from the European iMOVE multicentre test-negative case-control in 2011/2012 also found a low adjusted VE for laboratory-confirmed influenza (43%), with wide CIs which could not be distinguished from no VE [Reference Kissling and Valenciano16]. These studies postulate that the reason for the poor VE observed in 2011/2012 may be partly due to the impact of waning immunity during a late influenza season, and partly due to the increasing proportion of viruses circulating later in the season that showed a reduced match with the A/Perth/16/2009 component of the 2011/2012 TIV [1]. This reduced strain matching led the WHO to recommend a change in the composition of the forthcoming 2013/2014 vaccine A(H3N2) strain [1].
The negative VE estimate obtained for those aged <65 years in risk groups, with upper 95% CIs below zero is unlikely to be a robust finding, and implies that, despite adjusting for age group, specific risk group, week of illness onset and SHA, there is probably still significant residual confounding by indication in this group using this methodology. Confounding by indication, or negative confounding, occurs when those more likely to develop severe complications are more likely to be vaccinated than those at less risk, thus leading to a reduced estimate of VE [Reference Hak17]. In those aged <65 years in risk groups, who are subject to selective rather than universal vaccination, it is indeed likely that those with more severe underlying comorbidities within each specific risk group are more likely to have been vaccinated than those with less severe comorbidites. Although the USISS intensive care surveillance systems do not collect data on the severity of underlying comorbidities, data on the number of comorbidities for each case were available. Interestingly, just over half (12/23) of cases aged <65 years in risk groups had more than one underlying medical condition that could increase their risk of severe influenza, and also their likelihood of vaccination. As the vaccine uptake monitoring systems which provided data for the reference population do not collect data on multiple comorbidities, it was not possible to adjust for this in the logistic regression. Alternative explanations of negative bias are less likely. Bias can be introduced into VE studies if case ascertainment is not independent of vaccination status, which can occur if access to healthcare influences both the ascertainment of the outcome and the chances of being vaccinated. However, in this study severe influenza requiring admission to ICU/HDU is such a serious outcome that it is unlikely to be influenced by access issues, and vaccine status information was gathered independently from primary care, after reporting of ICU cases by secondary care. The alternative explanation for the negative VE estimates in those aged <65 years, i.e. that receipt of influenza vaccine could lead to severe influenza outcomes, is highly unlikely given the clear suggestion of residual confounding, the lack of biological plausibility for such an explanation, and the lack of previous evidence that receipt of influenza vaccine predisposes to severe influenza outcomes.
The screening method is a well recognized method for providing crude estimates of VE [Reference Farrington13]. It has the advantage of being a relatively simple and rapid method for estimating VE using routinely available data, which makes it particularly attractive for the annual estimation of influenza VE during the influenza season [Reference Legrand, Vergu and Flahault18]. The method relies on accurate estimates of population vaccine coverage [Reference Chen and Orenstein19] and assumes that the cases are drawn from the same population as the population vaccine coverage data [Reference Mary-Krause, Mary and Valleron20]. The influenza primary-care-based vaccine monitoring systems routinely established in England and Scotland provide timely and accurate measures of influenza vaccine uptake by week, age group, clinical risk group and SHA, covering >65% of general practices in England for the sentinel weekly uptake surveys, and >95% of practices for the end of season cumulative vaccine uptake survey. The case definition used for this study ensured that the cases were drawn from the same population as the population vaccine coverage data.
In this study, the VE estimates obtained using the screening method are consistent with other findings of poor protection from the 2011/2012 TIV this season for those aged ⩾65 years [Reference Pebody15, Reference Kissling and Valenciano16]. It may be that applying the screening method to those subject to universal vaccination provides a feasible method for the annual estimation of seasonal influenza VE against severe influenza resulting in ICU/HDU admission in this age group, although the small numbers of cases in this study makes it difficult to draw firm conclusions at this stage. We recommend repeating this methodology for those aged ⩾65 years in future seasons to gain more experience of its performance. If this methodology does appear robust for those subject to universal vaccination, it may also be suitable for estimating severe influenza VE in school-aged children once the annual influenza vaccination programme is extended to this age group in the UK [21].
The finding of apparent significant residual confounding in those aged <65 years in risk groups suggests that this method is probably unsuitable for estimating VE in age groups where selective, rather than universal vaccination is recommended. Estimating VE against severe influenza resulting in ICU/HDU admission in those aged <65 years in risk groups remains an important objective; we need to explore alternative methods for estimating severe influenza VE in this group, ideally using individual-level controls to enable further adjustment for prognostic factors such as severity of underlying comorbidities. The test-negative case-control study design is being increasingly used for influenza VE studies [Reference Talbot8–Reference Puig-Barberà10, Reference Pebody15], and patients with acute respiratory infections requiring ICU admission who test PCR-negative for influenza, or alternatively those that are PCR-negative for influenza and PCR-positive for another respiratory virus, could provide a suitable control group. However, such patients are not currently captured by the existing severe influenza surveillance systems in the UK; identifying them would require hospitals to report all intensive care patients with acute respiratory infections and the results of their respiratory virus PCR tests, which would require a considerable increase in workload for reporting hospitals. This could reduce acceptability, leading to lower levels of reporting by participating hospitals and jeopardizing the quality and wider benefits of these surveillance systems. An alternative control group would be the community test-negative controls that are currently selected for the community VE studies that run each season [Reference Pebody15], as has been done in some earlier studies of VE against hospitalization due to influenza [Reference Foster22, Reference Legrand, Vergu and Flahault23]. However, such controls are likely to differ significantly from cases admitted to intensive care with severe influenza complications with regard to underlying conditions and risk of severe complications [Reference Foster22], which would again be very difficult to adjust for in the analysis. Indeed it may be that even using individual-level studies and techniques such as propensity scores to adjust for the conditional probability of vaccination [Reference Hak17] it may still prove difficult to completely adjust for confounding by indication in studies of VE against severe influenza requiring ICU admission in those subject to selective vaccination. It could be argued that a certain level of residual confounding is inevitable in such studies and that, if applied consistently on an annual basis, they are better used to assess the relative VE against severe influenza complications each year, rather than to provide an unbiased absolute VE estimate. In France, the screening method has been applied to estimate VE against severe influenza resulting in ICU admission across two seasons, 2010/11 and 2011/2012 [Reference Bonmarin24]. The French methodology is based on case vaccine uptake data obtained by patient recall, rather than from primary-care records, and is unable to adjust for age group in those aged <65 years, specific comorbidity or week of illness onset, and so is unlikely to lead to an accurate absolute VE estimates. However, a substantial decrease in VE was observed in 2011/2012 compared to 2010/2011, and the authors conclude that, as confounding should have a similar effect on results in both seasons, the screening method is useful for almost real-time monitoring of VE during the influenza season.
We conclude that our study design, which includes a more robust method of ascertaining case vaccination status, and the ability to adjust for specific comorbidity and week of illness onset as additional confounders, suggests that the degree of residual confounding in those aged <65 years in risk groups is too great to rely on the screening method for the estimation of severe influenza VE in those subject to selective vaccination, even for relative VE estimation. This highlights the need to develop alternative methodologies for estimating severe influenza VE in these groups. While residual confounding is less likely to have such an important impact in those aged ⩾65 years who are subject to universal vaccination, the small sample size in this study, resulting from the mild 2011/2012 influenza season in the UK, make it necessary to repeat this methodology in future seasons to fully establish its utility for estimating severe VE in this group.
ACKNOWLEDGEMENTS
We thank all the hospitals participating in the USISS and Severe Acute Respiratory Illness (SARI) surveillance systems, and all the primary-care providers in England and Scotland who provided data on the influenza vaccination status of their cases. We also thank Fateha Begum, Peter Gates and the ImmForm Team, who are responsible for the HPA/DH vaccine uptake monitoring system, and Beatrix von Wissmann and Paul Markač for validating and quality assuring the HPS vaccine uptake monitoring system.
DECLARATION OF INTEREST
None.




