INTRODUCTION
Shiga toxin-producing Escherichia coli (STEC) can cause serious illness and pathology because of their ability to produce potent cytotoxins. Persons who ingest STEC may be asymptomatic, or develop illness ranging in severity from mild diarrhoea to haemorrhagic colitis, and in some cases life-threatening haemolytic uraemic syndrome (HUS). Cattle and other ruminant animals such as sheep, goats, and deer are considered the primary reservoir of STEC. The infectious dose is thought to be very small and STEC are often spread by ingesting contaminated food items that are not subsequently cooked to temperatures adequate to kill the bacteria. Person-to-person transmission, direct animal contact, and waterborne transmission, either from contaminated drinking water or recreational water, are other exposure routes [Reference Nataro and Kaper1].
STEC were first recognized as a foodborne threat in 1982 when two outbreaks of haemorrhagic colitis associated with eating undercooked hamburgers occurred in the USA [Reference Riley2]. The outbreak agent was identified as E. coli O157:H7; subsequently numerous outbreaks of this organism followed [Reference Rangel3–12]. There are also many non-O157 serogroups known to cause illness; the most common non-O157 serogroups implicated in STEC infections in the USA include O26, O45, O103, O111, O121, and O145 [Reference Brooks13]. Several studies have suggested similar frequencies between O157:H7 and non-O157 E. coli infections [14–Reference Lathrop, Edge and Bareta18]. However, non-O157 STEC infections are more likely to escape detection because plating on sorbitol-MacConkey agar does not reliably detect non-O157 STEC and screening of stool specimens with a Shiga toxin enzyme immunoassay (EIA) is not universally performed at clinical laboratories. Moreover, outbreaks of non-O157 STEC in the USA are uncommonly recognized and not well described.
On 22 August 2008, a case cluster of hospitalized children with bloody diarrhoea and severe abdominal cramping was reported to the Oklahoma State Department of Health (OSDH). Subsequent investigation identified E. coli O111:NM as the agent and a popular buffet-style restaurant in a rural area of northeastern Oklahoma as a common exposure. This report summarizes all aspects of the epidemiological investigation and response.
MATERIALS AND METHODS
Epidemiological investigation
Case definitions and finding
Case definitions were developed for confirmed, probable, and suspect infections as well as confirmed and probable HUS (Table 1) from restaurant patrons dining during 10–24 August 2008, the outbreak period. A secondary case was defined as a person with no restaurant exposure and close contact to a case with onset of diarrhoeal illness meeting one of the definitions above within 10 days of the index case.
Table 1. E. coli O111:NM outbreak case definition classifications – Oklahoma, 2008
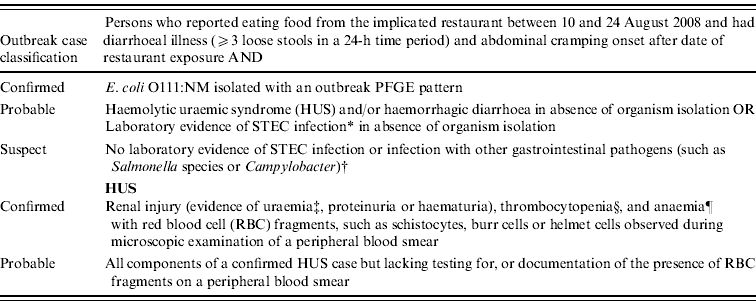
* Laboratory evidence equivalent to positive enzyme immunoassay test (Meridian Premier EHEC) of stool-inoculated broth specimen and positive polymerase chain reaction test for stx1 and/or stx2 genes on broth pellet.
† Persons with a history of chronic diarrhoea or colitis were excluded as suspect case-patients.
‡ Uraemia was defined as having a serum creatinine level ⩾1·0 mg/dl in children aged <13 years, or a serum creatinine level ⩾1·5 mg/dl in individuals aged ⩾13 years.
§ Thrombocytopenia was defined as a platelet count <150 000×109/l.
¶ Anaemia was defined as having at least one haemoglobin level <13 g/dl, for males, or <12·0 g/dl, for females.
Active surveillance for case finding included the following: (1) alerting all hospitals, laboratories, physicians, and health departments in Oklahoma through the state's health alert network system and requesting prompt reporting of suspect cases; (2) reviewing hospital medical records of patients who had a diarrhoeal illness recorded by a nearby metropolitan syndromic surveillance system; (3) posting an Epidemic Exchange (Epi-X) alert for case finding in out-of-state residents; (4) contacting dining companions of cases to determine illness status; and (5) releasing multiple OSDH media releases to advertise a toll-free number for restaurant patrons to call.
Case-control study
To identify potential transmission vehicles and other risk factors, a case-control study was conducted. Controls included dining companions of cases, persons identified by credit card or cheque receipts and persons who contacted the OSDH as a result of media releases and did not report any gastrointestinal symptoms between 10 August and date of interview. All case and control interviews were conducted during the period 22 August–24 September 2008. Because the majority of case-patients ate lunch and dinner during the weekend of 15–17 August, cases and controls dining during these mealtimes and dates were included in the study. A standard questionnaire administered to case-patients and controls collected data on demographics, symptomatology, medical treatment and food item selections with respective quantities. For the food exposure analysis of persons aged <13 years, all available controls were included in the study. For exposure analysis of persons aged ⩾13 years, controls were randomly selected and limited to four per case.
Catered event cohort study
The restaurant catered an event off-site on Saturday, 16 August. Based on a list of attendees from the event organizer, a retrospective cohort study was conducted using a standard questionnaire to identify illness status and food items consumed by attendees. Event attendees who had also dined at the restaurant during the outbreak period were excluded from the analysis.
Statistical methods
Epidemiological analyses were performed with SAS version 9.1 (SAS Institute Inc., USA). The Wilcoxon rank-sum test was used to compare median ages. Daily attack rates were estimated using denominator data extracted from a restaurant patronage log. We calculated odds ratios and 95% confidence intervals for the association between illness and each exposure variable. For exposures with <5 responses, Fisher's exact test was performed. In both studies, analysis of food items was restricted to confirmed and probable cases compared with controls. Persons selected for the case-control study were limited to those who reported only one dining exposure throughout lunch and dinner hours at the restaurant during the weekend of 15–17 August. Multivariate logistic regression was performed in a backward stepwise fashion including all statistically significant variables identified during univariate analysis of exposures for persons aged ⩾13 years by gender. For all statistical tests, a P value <0·05 was considered significant.
Environmental investigation
The environmental investigation included multiple restaurant inspections beginning on 23 August 2008 and collection of environmental swabs (28 August and 17 September) of various surfaces of the food preparation and serving areas and in restrooms; six food items were sampled for microbiological testing. The restaurant's water system was evaluated on 25 August and water samples from filtered and unfiltered faucets within the restaurant were tested. Water samples from a private well on the premises were collected for analysis on 27 and 29 August. Water samples were tested for the presence of total coliforms and E. coli using standard methods [Reference Clesceri, Greenberg and Eaton19]. Well-water specimens were plated for bacterial culture, isolation, and identification. On 5 November, two 10-litre samples of well water were collected for PCR testing by the U.S. Centers for Disease Control and Prevention (CDC) Waterborne Diseases Laboratory.
All restaurant employees were interviewed using a standard questionnaire regarding their work schedule, recent history of gastrointestinal illness, and specific job responsibilities, including foods handled, cleaning duties, and handwashing practices. Submission of two stool specimens collected at least 24 h apart was required from all employees reporting recent diarrhoeal illness.
Microbiological investigation
Faecal specimens collected from potential outbreak-associated cases were submitted by hospitals to the Oklahoma Public Health Laboratory (PHL) and were screened for routine bacterial pathogens, including STEC. The specimens were simultaneously inoculated into the following standard medias (all produced by Remel, USA): MacConkey agar, Hektoen agar, sheep-blood agar, sorbitol-MacConkey agar (SMAC), cefaperazone-vancomycin-amphotericin B (CVA) agar, and GN broth under standard atmospheric conditions at 35–37°C for 18–24 h.
All clinical and environmental specimens were screened for the presence of Shiga toxin (Stx) with an EIA using a commercial kit (Premier EHEC®, Meridian Bioscience, USA). All Stx-positive specimens had individual colonies picked and biochemically identified by API 20E (bioMérieux Inc., France). Isolates from all specimen types that were identified as E. coli were then tested by real-time polymerase chain reaction (PCR) for the presence of Shiga toxin genes stx1 and stx2. Any isolates testing positive for stx1, stx2, or both were forwarded to the CDC for serotyping and characterization of other toxin genes, and analysed by pulsed-field gel electrophoresis (PFGE) at the PHL. PFGE dendrograms were created in BioNumerics (Applied Maths, USA) to determine relatedness of isolates.
RESULTS
Epidemiological investigation
Of 1823 persons interviewed, 341 (18·7%) met the outbreak case definition; 16% confirmed, 29% probable and 55% suspect. The median age of all 341 case-patients was 51 years, 66% were female (Table 2). Eighty-seven percent dined once at the restaurant during the outbreak period. Twenty-one (6%) case-patients attended the catered event. There were no persons classified as an outbreak case that attended both the catered event and ate at the restaurant. An additional 264 individuals who ate at the restaurant or attended the catered event reported illness that did not fully meet the clinical criteria of one of the outbreak case classifications, and were not included in the study or outbreak summary.
Table 2. Frequency of selected characteristics in E. coli O111:NM cases – Oklahoma, August–September 2008
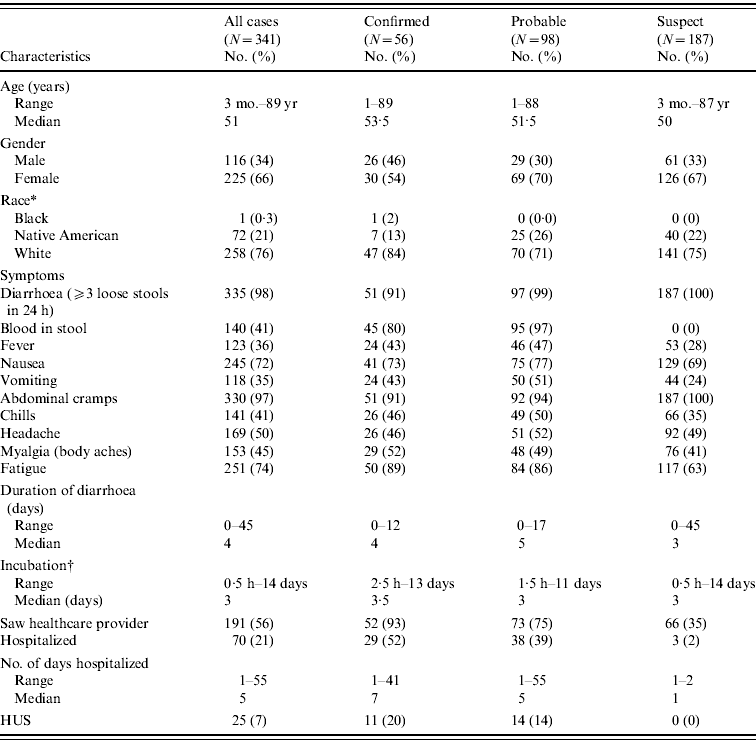
HUS, Haemolytic uraemic syndrome.
* Race was unknown for 10 (3%) cases.
† Incubation periods were defined as interval from time of exposure (consumption of restaurant food) to time of onset of first gastrointestinal symptom; calculations restricted to cases with singular dining event at the implicated restaurant during the outbreak period or catered event attendance.
Sixty-five percent of case-patients dined the weekend beginning Friday, 15 August (Fig. 1) and the highest estimated attack rate (AR) occurred that day (AR 9·0%), followed by Saturday, 16 August (AR 8·3%), and Sunday, 17 August (AR 6·8%); the overall outbreak AR was about 5%. Confirmed cases had restaurant exposures beginning 15 August continuing until 24 August when the restaurant was closed. Illness onsets of all cases extended from 10 August to 5 September; the earliest onset date of a confirmed case was 18 August (Fig. 1). The median incubation period was 3 days (Table 2). For confirmed cases, incubation periods ranged from 2·5 h to 13 days. Nine percent of confirmed and probable cases had incubation periods <1 day or >10 days. The most prevalent symptoms were diarrhoea, abdominal cramping, fatigue, nausea, headache, myalgia, and blood in stools. Duration of diarrhoea ranged from <1 day to 45 days (median 4 days). Seventy (21%) case-patients were hospitalized and one death occurred. The fatality was a 26-year-old male with no known underlying medical conditions, who developed severe haemorrhagic colitis beginning 20 August. He was hospitalized a day later and died on 24 August from complications of HUS. Twenty-five (7%) outbreak cases developed HUS (Table 2); the median HUS patient age was 46 years (range 1–88 years) with adults comprising 60% of the HUS cases. Length of hospitalization ranged from 1–55 days (median 5 days).
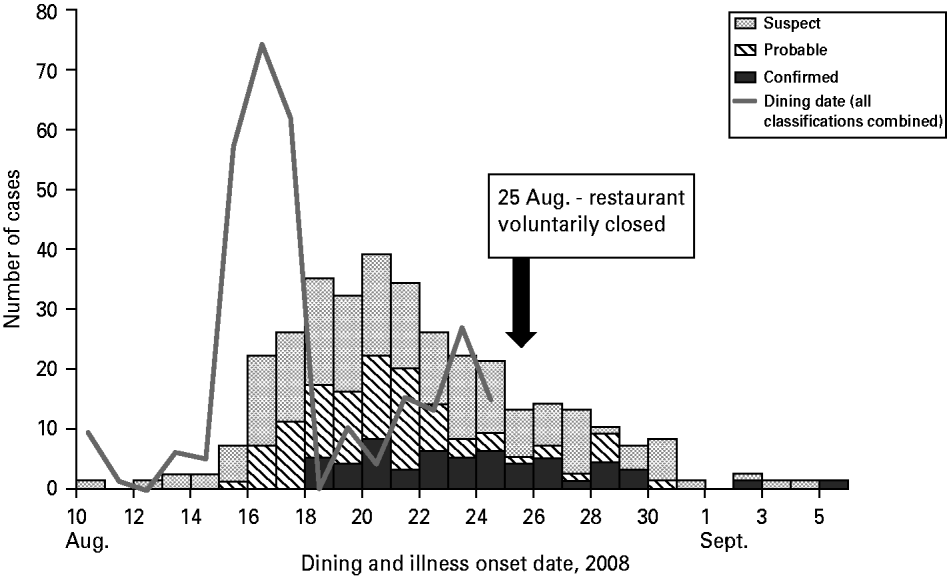
Fig. 1. Restaurant exposure dates [N=297; cases who ate at the catered event (n=21), reported multiple dining dates, reported exposure on a day the restaurant was not open, or were an ill employee are not included (n=23)] and onset of illness by case classification [N=341; symptom onset dates of restaurant workers meeting outbreak case definitions included 8/20 for one probable case and 8/12, 8/17, 8/17, and 8/20 for four suspect cases], E. coli O111:NM outbreak investigation – Oklahoma, August–September 2008.
Additional cases
Five persons who had E. coli O111:NM with the outbreak PFGE pattern isolated from stool, but no direct restaurant exposure, were identified during the outbreak. Three were contacts associated with an outbreak case-patient and attributable to secondary transmission. The fourth person was a child of a well mother and sibling who dined at the restaurant. The child's father and another sibling, neither of whom dined at the restaurant also developed diarrhoeal illness, but stool cultures were not obtained. No restaurant food was reportedly taken home. The fifth individual visited the community on 15 August but had no recognized exposure to the restaurant or to case-patients.
Case-control study
Ninety-six confirmed and probable case-patients dined at the restaurant during 15–17 August. Of 384 controls selected, 56% were female compared to 66% of cases (P=0·047). Age distribution of cases and controls did not significantly differ. Univariate analysis of 96 cases and 384 controls, indicated that 8/87 food exposures were statistically associated with illness; however, only fried chicken, mashed potatoes, or any dessert were consumed by ⩾50% of the cases. Because buffet item preferences tended to vary between children and adults, case-patients and controls were stratified into three age groups. Food and beverage exposure analysis of 11 cases and 12 controls aged <5 years indicated that mashed potatoes [odds ratio (OR) 15·0, 95% confidence interval (CI) 1·3–865], macaroni and cheese (OR 37·3, 95% CI 2·96 to >999), and any type of dessert (OR 2·2, 95% CI 1·3–4·00) were statistically associated with illness and could have accounted for over 90% of illnesses in very young children. Of children aged 5–12 years, 11 cases and 25 controls were available for analysis; no food or drink items were statistically associated with illness. Findings from univariate analysis of persons aged >12 years and stratified by gender are summarized in Table 3. For males, eating ham was significantly associated with illness although ham was only consumed by 36% of cases. For females, ten individual food items were significantly associated with illness, and all of the items fell into the categories of desserts, salads or salad toppings. Six of the food items were consumed by <30% of females.
Table 3. Bivariate analysis of restaurant buffet items consumed 15–17 August by persons aged >12 years by gender, E. coli O111:NM outbreak investigation – Oklahoma, 2008
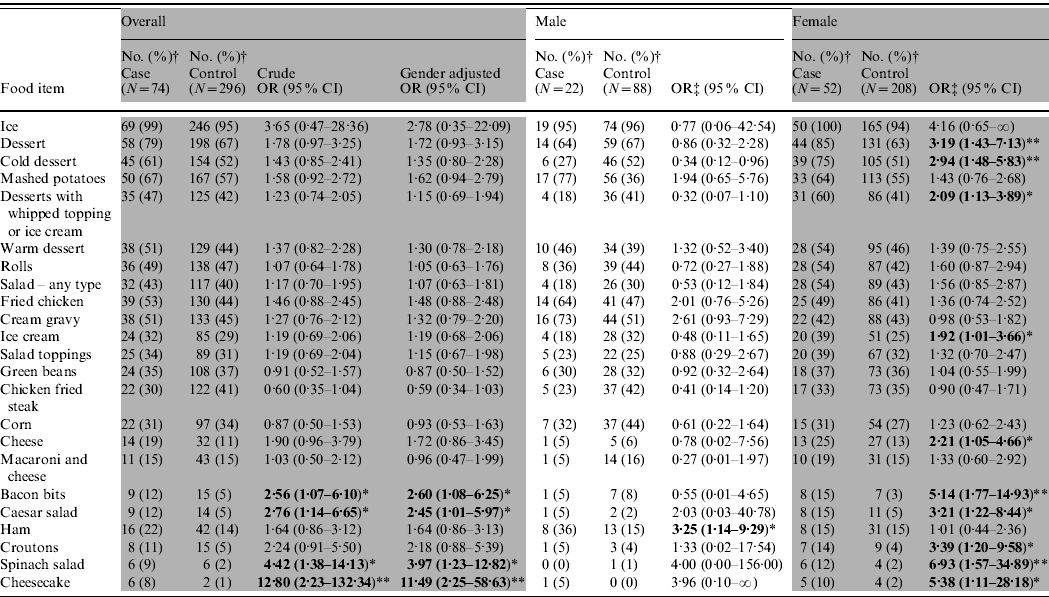
OR, Odds ratio; CI, confidence interval.
† Unknown exposures to individual food items are not included in the denominator, so percentages vary.
‡ Fisher's exact test performed for exposure items in which at least one cell had <5 responses.
* P<0·05, ** P<0·01.
Each food exposure that showed statistical significance in the univariate analysis of females aged >12 years was included in multivariate analysis; only spinach salad [adjusted OR (aOR) 6·6, 95% CI 1·7–25·2) and desserts (aOR 2·9, 95% CI 1·3–6·6) remained independently and significantly associated with illness. Only 12% of women reported eating spinach salad and 10% ate the only individual dessert that remained statistically associated with illness in the logistic regression model (cheesecake).
Catered event cohort study
Twenty-one (9·1%) cases were identified in 232 persons attending the catered event; two were confirmed cases, three were probable, and 16 were suspect. One case-patient was hospitalized. Ill persons did not significantly differ from other event attendees by age or gender. Dates of illness onset ranged from 16 to 30 August. In a univariate analysis of all 21 case-patients and 166 well persons, four food items were associated with illness, including chicken (OR 3·6, 95% CI 1·01–12·6), tabouli salad (OR 3·3, 95% CI 1·3–8·6), ham (OR 2·9, 95% CI 1·06–8·0), and watermelon (OR 2·6, 95% CI 1·01–6·7). When ill persons were restricted to the five confirmed and probable case-patients, only watermelon remained significantly associated with illness (OR 20·5, 95% CI 2·7–∞). All confirmed and probable case-patients in the cohort reported eating watermelon compared to only 37 (24%) of 154 well persons.
Environmental investigation
The establishment was out of compliance with five regulations associated with hot and cold holding of foods, food storage, labelling and storage of toxic items, and cleanliness of food contact surfaces. The restaurant did not have written protocols or schedules for cleaning the kitchen, buffet, dining, or bathroom areas. A diluted bleach solution was used to clean surfaces and food spills, but there was no established method for monitoring the concentration.
Of 60 restaurant employees, 16 reported some symptoms of illness immediately preceding or during the outbreak period resulting in an employee AR of 27%. However, no employees met confirmed case definition criteria and only five (8%) employees reported symptoms that met the probable or suspect case definitions. Symptom onset dates of the 16 ill employees ranged from 9 to 27 August. Thirteen (81%) of 16 symptomatic employees reported beverage and food handling duties that included preparation of all hot foods and various desserts and assembly of salads for menu orders during their shifts; four reported working while experiencing diarrhoea. Five restaurant staff worked on-site at the catered event preparing and serving food items; one reported working while ill. Some foods for the catered event were prepared in advance at the restaurant by other workers.
Sixteen stool specimens were collected from 24 August to 1 September from nine restaurant employees and submitted to the PHL. All were negative by culture and Shiga toxin EIA. Similarly, STEC organisms were not isolated from tested food items or surface swabs.
The restaurant owners disclosed that their private well had been accessed briefly on 10 August to supply water to the restaurant when a sudden interruption of the municipal water system occurred during a lunch period of high volume patronage. The private well was the sole water source for a few hours on this date, but was not accessed again once the municipal water service was restored. The well was physically located on the restaurant property, which is positioned on a major road on the outskirts of a small rural community. Pasture land with livestock adjoins the property on the rear aspect of the restaurant. Well water samples collected on 27 and 29 August were positive for total and faecal coliforms. Numerous types of bacteria, including Proteus, Klebsiella, Serratia, Enterobacter, Pseudomonas, and Pantoea species were cultured from the well-water samples. E. coli isolates were also identified, but none were Stx-producing or serogrouped as O111. PCR testing by the CDC Waterborne Diseases Laboratory also failed to detect the presence of E. coli O111.
Microbiological investigation
The PHL tested 166 clinical stool specimens, of which 68 (41%) screened Shiga toxin positive by EIA. Sixty STEC isolates from 58 patients were recovered, plus two additional isolates from out-of-state case-patient specimens were received from another public health laboratory. All STEC isolates were sorbitol fermenting. All but two of the isolates carried both the stx1 and stx2 genes; all carried the intimin gene. Serotyping by CDC indicated that all isolates were O111:NM. Six XbaI PFGE patterns were identified in the 62 isolates. The primary XbaI outbreak pattern was seen in 50 (81%) patient isolates. Three other XbaI patterns displayed only subtle differences and were considered to be closely related to the primary outbreak pattern. One isolate had additional variations from the primary outbreak pattern; nonetheless, the source patient did have restaurant exposure and was considered to be outbreak-associated. The sixth identified PFGE pattern was shared by isolates from two clinical specimens, but no epidemiological links to the restaurant outbreak were determined.
DISCUSSION
To our knowledge this is the largest community outbreak of E. coli O111 on record. Several potential vehicles of introduction and contributing factors for spread within the restaurant were explored, including a primary contaminated food item, an infected food handler, contaminated well water, and cross-contamination from restaurant surfaces or equipment harbouring the organism. Multiple specimens representing these potential vehicles were obtained for laboratory testing, but E. coli O111 was not isolated by culture or identified by molecular methods in any of them. The epidemiological findings suggest that foodborne transmission of E. coli O111 through various food items – either contaminated directly by an infected food handler's hands or by cross-contamination from food preparation equipment, counter surfaces, or storage areas – occurred at the restaurant.
While bacterial culture and Shiga toxin testing of submitted stool specimens did not identify an infected food handler, epidemiological findings are most consistent with foodborne transmission by an ill employee who continued to work, or by an asymptomatic food handler. Two employees, one with hostess duties and the other a food handler, reported working with diarrhoeal illness during 15–17 August. The food handler also assisted with the catered event on 16 August. Although cultures of stool specimens from both of these workers proved negative, specimen collection occurred 11–14 days after cessation of diarrhoea. Excretion of E. coli O157:H7 is generally limited to ⩽1 week but in some studies has ranged from 2–62 days [Reference Gallagher20, Reference Belongia21]. Little is available in the published literature regarding asymptomatic human carriage of E. coli O111; one study in Germany found that E. coli O111:H– accounted for 2% of asymptomatic STEC infections [Reference Wilson22]. Our investigation also found evidence of asymptomatic infections during this outbreak. Specifically, E. coli O111:NM infection with an outbreak strain was identified in a child with exposure to two family members who dined at the restaurant without developing clinical illness. Asymptomatic infections of E. coli O157:H7 in 6% of an exposed cohort have been reported [Reference Gallagher20].
The report that the restaurant's private well had been used briefly before the outbreak was of interest and well-water samples were collected for bacterial testing early in the investigation. Testing indicated environmental contamination and water quality that did not meet safe drinking water standards, but E. coli O111 was not cultured from serial water specimens nor was E. coli O111 DNA detected by PCR testing. Analysis of in-line water filters also failed to detect remnants of E. coli O111. Because the well water was reportedly used for all food and drink preparation during the emergency water interruption period on 10 August, a substantial number of cases of illness would be expected to have restaurant exposure on that date if the well was the principal way the bacteria entered the restaurant. The epidemiological data suggest that the outbreak escalated on 15 August, the same day that the first laboratory-confirmed cases of E. coli O111:NM infections reported restaurant exposure.
In a comprehensive review of 350 E. coli O157:H7 outbreaks occurring in the USA between 1982 and 2002, the transmission route for 52% was foodborne, 14% were spread person-to-person, 9% were waterborne, and 3% resulted from direct animal contact. For over 20% of the E. coli O157:H7 outbreaks in which a common exposure was determined, the major transmission route was not determined [Reference Rangel3]. Since few outbreaks of non-O157 STEC have been detected, less is known about the type and frequency of modes of spread of these STEC.
This outbreak had many features resembling a 1990 restaurant-associated outbreak of E. coli O157 in Scotland including a prolonged exposure period (>7 days), occurrence of secondary spread by asymptomatic individuals, and the inability to identify a single transmission source within the restaurant despite careful investigation [Reference Marsh23]. Characteristics of STEC such as their low infectious dose and long survivability in certain foods or in the environment under favourable conditions [Reference Baylis24, Reference Fukushima, Hoshina and Gomyoda25] may facilitate outbreaks, but the bacteria can be elusive to public health investigators. Only 10 outbreaks involving E. coli O111 have been reported since 1990 in the USA (R. Luna, CDC, personal communication). In 1999, the Texas Department of Health investigated an outbreak of 55 E. coli O111:H8 infections in cheerleading camp attendees [Reference Brooks26]. Epidemiological analysis suggested foodborne transmission via a salad bar or ice distributed in open barrels, but the outbreak organism was not cultured from ice, environmental surfaces, or food handler specimens. It was theorized in the Texas outbreak that risk-associated exposures varied over time. A food vehicle for E. coli O111 has only been conclusively determined in two previous outbreaks: dried fermented beef sausage [Reference Paton27] and unpasteurized apple cider [Reference Coronado28]. Other outbreaks of E. coli O111 have been attributed to person-to-person spread [Reference Boudailliez29], contaminated water [Reference Blanco30], and contact with calf faeces [Reference Smith31].
Despite extensive epidemiological and laboratory investigation, no single predominant transmission vehicle was identified in this restaurant-associated outbreak. Our investigation faced some limitations, which may have hampered our ability to determine how STEC were introduced into the restaurant setting and how spread of the bacteria continued within the restaurant. Food recall is frequently problematical when investigating a foodborne outbreak. Although food exposure information was obtained within several days to a few weeks of most persons' encounter with the restaurant, there were over 80 different food items offered on the buffet and many persons were unsure if they had selected a particular food item from the buffet options. Further, due to the large number of food items prepared by the restaurant, we elected to perform a preliminary epidemiological analysis to target certain dishes or ingredients for food testing. This decision resulted in a delay in food sampling and culture attempts. Finally, incomplete and delayed collection of stool specimens from restaurant employees reduced our ability to identify STEC colonization or infection in a food handler.
This unusually large E. coli O111 outbreak provided a unique opportunity to evaluate potential differences from E. coli O157:H7 outbreaks. The gastroenteritis symptom profile in ill persons and the proportion of cases progressing to HUS were similar to what is observed in O157 reports, as is the median incubation period of 4 days. However, a number of cases with incubation periods outside of the expected range of 1–10 days for STEC infections were identified. Of confirmed and probable cases, two had incubation periods of 11 days, one of 12 days, and another experienced a 13-day incubation period. Atypically short incubation periods were also observed. As no other enteric pathogens were cultured from clinical specimens, it is speculated that these variances in incubation period are attributable to dose response. It is also possible that the very large cohort in our outbreak provided an opportunity to evaluate the full clinical spectrum in persons infected with this strain of E. coli O111. Another feature in this outbreak was the median age of 51 years, which is considerably older than that reported during O157 outbreaks. Unlike patients with E. coli O157 infection, the highest proportion of case-patients requiring hospitalization and developing HUS were adults. Children aged <5 years are classically described as the highest risk age group for HUS and death as a complication of E. coli O157 infections [Reference Tserenpuntsag32, 33]. The fatality in our outbreak was a previously healthy 26-year-old male. These observations are probably reflective of the age distribution of restaurant patrons.
The strain of E. coli O111 recovered in this outbreak had not been previously isolated in Oklahoma. PFGE analysis revealed indistinguishable or closely related XbaI patterns in the majority of isolates suggesting that a predominant bacterial clone was involved in this outbreak. Whether the Oklahoma strains represent a newly emerged and more virulent STEC strain is unclear. Livestock rearing and grazing is prevalent in the community and regional area where the restaurant is located. The recognition of a spurious case of E. coli O111 infection during the restaurant-associated outbreak suggests that a low level of transmission of E. coli O111 generally goes unrecognized. STEC organisms are considered ubiquitous in nature, particularly in areas with high cattle density, so the potential for another E. coli O111 outbreak in the USA exists. Medical providers need to consider non-O157 STEC infections in their diagnostic work-ups of all acute enteric illness and HUS [34] and report to public health authorities for surveillance and outbreak monitoring.
ACKNOWLEDGEMENTS
Multiple persons contributed to the success of this outbreak investigation and we thank them for their time and talents. We especially thank the following members of the E. coli O111:NM Outbreak Investigation Team – Oklahoma State Department of Health: Kim Rayno, Becky Coffman, Anthony Lee, Renee Powell, Jolianne Stone, Tressa Madden, Travis Brown, Garry McKee, Rebekah Berry, Melannie Fields, Krystle Rodriguez, Rick Garner, Cara Crooks and Patricia Eakers-Jarrett; Mayes County Health Department: Kelli Rader, Ben May, Larry Bergner, Linda Axley, Becky Gipson, Teresa Pritchett, Darla Thompson, Sandy Schmidt, Josh Daily and Kay Back; Tulsa Health Department: Nicole Shaefli, Patrick Hilton, and Dave Cox; Centers for Disease Control and Prevention, National Center for Emerg-ing and Zoonotic Diseases: Samir Sodha, Colin Schwenson, Deborah Talkington, Jim Pruckler, Cheryl Bopp, Kathy Greene, Nancy Strockbine, Jonathan Yoder, and Vincent Hill; Oklahoma Department of Environmental Quality: Rick Austin, Chris Armstrong, Richard McDaniel, and Ted Whitten; and St Francis Hospital: Connie Bourne, DeAnna Osborn, and Mark Rowland. We especially thank Samir Sodha for his critical review of the manuscript and also express our gratitude to Barbara Neas for her consultation and assistance with biostatistical methodology and interpretation.
This study received funding from the Oklahoma State Department of Health.
DECLARATION OF INTEREST
None.






