INTRODUCTION
Ventilator-associated pneumonia (VAP) is widely considered to be the most serious device-associated infection (DAI) in the intensive-care unit (ICU) setting [Reference Arabi1, Reference Rosenthal, Guzman and Orellano2]. According to studies from developed [Reference Safdar3] and developing [Reference Arabi1, Reference Rosenthal4] countries, the most important clinical consequences attributable to VAP are increased mortality rates, significant morbidity, and increased length of stay in hospital [Reference Rosenthal4, Reference Bouadma, Wolff and Lucet5]. From an economic perspective, VAP is also responsible for significant increases in healthcare costs, as reported in both developed and developing countries in Latin America [Reference Safdar3, Reference Rosenthal4], but not yet in studies from India.
The burden posed by VAP has not been systematically analysed at international level, particularly in limited-resource countries [Reference Arabi1] where most hospitals do not implement basic infection control programmes, resulting in a general unawareness of the incidence of VAP [Reference Arabi1]. As reported by the International Nosocomial Infection Control Consortium (INICC) in pooled studies [Reference Rosenthal6–Reference Rosenthal9], and in particular from India [Reference Mehta10], the rates of VAP were found to be 3–5 times higher than reported from developed countries.
For analytical purposes, the World Bank classifies economies as low, middle, or high income. Since 1 July 2011 low-income is defined as an average income of US$1005 or less per year; lower-middle as US$1006–3975; upper-middle as US$3976–12275; and high-income above this figure. Low- and middle-income economies are commonly referred to as developing economies.
The influence of socioeconomic level over DAIs in developing countries has been assessed in two studies [Reference Rosenthal11, Reference Rosenthal12]. The first, conducted in paediatric ICUs, showed that lower middle-income countries had higher VAP rates than upper middle-income countries [9·0 vs. 0·5/1000 mechanical ventilator (MV) days] [Reference Rosenthal11] and rates were highest in ICUs of academic hospitals compared to private or public hospitals (8·3 vs. 3·5 VAP/1000 MV days) [Reference Rosenthal11]. Similarly for neonatal ICU patients, the VAP rates in academic hospitals significantly exceeded those in private or public hospitals (13·2 vs. 2·4 and 4·9 VAP/1000 MV days) [Reference Rosenthal12]. Regrettably, there are no published studies from developing countries on this issue in adult ICUs.
It has been shown that the incidence of VAP in developed countries can be substantially reduced by more than 30% through basic but effective measures, such as systematic surveillance, hand hygiene (HH) compliance, semi-recumbent positioning, early removal of endotracheal tubes, maintenance of endotracheal cuff pressure and continuous subglottic suctioning [Reference Coffin13]. By contrast, the importance of measuring patient infection risks and prevention practices remains greatly under-recognized in developing countries [Reference Arabi1, Reference Rosenthal14] and specifically such programmes have not been evaluated in India, the second most populous country in the world, with a population of about 1·3 billion [Reference Arabi1]. Launched in 2002 internationally [Reference Rosenthal6–Reference Rosenthal9], the INICC has supported hospitals in limited-resource countries in performing surveillance and reducing healthcare-associated infection rates. These hospitals contact INICC and receive forms and manuals with guidance to implement effective surveillance and infection control programmes. INICC also provides administrative and scientific support to upload, process, analyse and develop charts and tables with the collected data.
With the aim of reducing VAP rates in adult ICUs of INICC member hospitals in India, we implemented a novel multidimensional infection control programme that comprised six specific interventions: (i) a practice bundle, (ii) education, (iii) outcome surveillance, (iv) process surveillance, (v) feedback on DAI rates and (vi) feedback on the performance of infection control practices. The bundle integrated in this approach for VAP reduction is based on the guidelines published by the Society for Health Care Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA), which provide feasible and cost-effective infection control measures [Reference Coffin13].
METHODS
Setting and study design
This quasi-experimental, prospective cohort study was conducted in 21 adult ICUs of 14 INICC member hospitals, in 10 cities in India. Each hospital has actively participated in the INICC surveillance programme for at least 1 year, each with an infection control team comprised of at least one medical doctor with formal education and background in infectious diseases, internal medicine, and/or hospital epidemiology, and infection control professionals (ICPs). The study period was 7 years and 3 months, from July 2004 to October 2011, and was divided into baseline and intervention periods. The Institutional Review Board (IRB) at each hospital approved the study protocol. Patient confidentiality was protected by codifying the recorded information, making it only identifiable to the infection control team.
Baseline period
The baseline period included only the performance of outcome and process surveillance and lasted for 3 months. This period allowed for monthly receipt of case report forms at INICC headquarters in Argentina from participating centres, validation, queries, computer analysis, and communication of results and feedback of performance to participants. The sample size of patients and duration of data collection during the baseline period was sufficient to compare these parameters with the intervention period. From a statistical perspective, this was addressed by considering the changes in rates over time. The relatively short baseline period may impact the standard error of our estimates but this was not thought to bias the results as there were no systematic differences between the two groups. Finally, it was necessary to begin the intervention period as early as possible in order to achieve the desired results within the time-frame.
Intervention period
The intervention period started in the fourth month of participation. This was a cohort study, and for that reason, each ICU joined the INICC programme at different times. Thus, by the time the impact of the INICC intervention was analysed, we had ICUs with different lengths of participation in intervention periods with an average duration (±s.d.) of 23·33 ± 16·86 (range 6–76) months.
INICC multidimensional approach
The INICC multidimensional approach included the following practices: (1) bundle for VAP prevention, (2) education, (3) outcome surveillance, (4) process surveillance, (5) feedback on VAP rates, and (6) performance feedback of infection control practices [Reference Rosenthal, Maki and Graves15].
Bundle for VAP prevention
The bundle included the following elements:
(1) Adherence to HH guidelines [Reference Coffin13].
(2) Maintenance of patients in a semi-recumbent position (30–45° elevation of the head of the bed) [Reference Dellinger and Vincent16].
(3) Performance of daily assessments of readiness to wean and use of weaning protocols [Reference Coffin13].
(4) Performance of regular oral care with an antiseptic solution [Reference Tantipong17].
(5) Use of non-invasive ventilation whenever possible and minimization of the duration of ventilation [Reference Coffin13].
(6) Preferable use of orotracheal instead of nasotracheal intubation [Reference Coffin13].
(7) Maintenance of an endotracheal cuff pressure of at least 20 cm H2O [Reference Rello18].
(8) Removal of the condensate from ventilator circuits [Reference Coffin13] and keeping the ventilator circuit closed during condensate removal [Reference Coffin13].
(9) Change of the ventilator circuit only when visibly soiled or malfunctioning [Reference Coffin13].
(10) Avoidance of gastric overdistention [Reference Coffin13].
(11) Avoidance of histamine receptor 2 (H2) blocking agents and proton-pump inhibitors [Reference Coffin13].
(12) Use of sterile water to rinse reusable respiratory equipment [Reference Coffin13].
(13) We performed direct observation of HH compliance, duration of ventilation, and ventilation ratio use, using a structured observation tool at regularly scheduled intervals [Reference Rosenthal, Maki and Graves15].
Education
The INICC chairman trained the principal and secondary investigators at most of the participating hospitals. In addition, investigators were provided with instruction manuals and training tools, and their updates, which describe how to perform surveillance and complete surveillance forms according to the guidelines published by the SHEA and the IDSA [Reference Coffin13]. Investigators had regular email and telephone access to a support team at the INICC Central Office in Buenos Aires, Argentina, charged with responding to all queries within 24 h. The INICC chairman further reviewed all queries and responses.
INICC surveillance methods
The INICC surveillance programme included two components: outcome surveillance (DA HAI rates and their adverse effects, including mortality rates) and process surveillance (adherence to HH and other basic preventive infection control practices) [Reference Rosenthal, Maki and Graves15]. Investigators were required to complete outcome and process surveillance forms at their ICUs, which were then sent for their monthly analysis to the INICC headquarters office in Buenos Aires.
Outcome surveillance
Outcome surveillance included rates of VAP/1000 MV days, microorganism profile and antimicrobial resistance, length of stay, and mortality rates in the participating ICUs. The definitions applied for surveillance were those developed by the U.S. Centers for Disease Control and Prevention for the National Health Safety Network (CDC NHSN) programme [Reference Horan, Andrus and Dudeck19]. Additionally, INICC methods were adapted to the limited-resource setting of developing countries to take account of socioeconomic status [Reference Rosenthal, Maki and Graves15]. The Abdominal Surgery Impact Scale (ASIS) score was used instead of the APACHE II score due to budgetary limitations of participating ICUs [Reference Garner20].
Definitions
VAP was diagnosed in a mechanically ventilated patient when a chest radiograph showed new or progressive infiltrates, consolidation, cavitation, or pleural effusion. The patient also had to meet at least one of the following criteria: new onset of purulent sputum or change in character of sputum, organism cultured from blood, or isolation of an aetiological agent from a specimen obtained by tracheal aspirate, bronchial brushing or bronchoalveolar lavage, or biopsy [Reference Horan, Andrus and Dudeck19].
Process surveillance
Process surveillance was designed to monitor compliance with easily measurable, key infection control measures and HH at each participating ICU. Due to budgetary limitations, only the following components of the bundle were monitored.
(1) HH compliance by HCWs was determined by monitoring practices by the hospital's ICP during randomly selected 1-h observation periods, three times a week. Although staff were aware of the monitoring, they did not have prior knowledge of the precise times of the observation periods [Reference Rosenthal, Maki and Graves15]. ICPs were trained to monitor and record HH compliance through direct observation according to the ‘Five Moments for Hand Hygiene’ as recommended by the World Health Organization (WHO) which describes monitoring of HH: (1) before patient contact, (2) before an aseptic task, (3) after body fluid exposure risk, (4) after patient contact, and (5) after contact with patient surroundings [Reference Sax21] .
(2) Compliance with VAP preventive measures were recorded 5 days a week on a form that evaluated adherence to infection control procedures by HCWs and monitored (i) maintenance of patients in a semi-recumbent position (30–45° elevation of the head of the bed), (ii) compliance with nebulizer without turbidity; (iii) tubing without condensation, (iv) tubing without mucus, (v) absence of pharyngeal lake, and (vi) compliance with smooth enteric nourishment.
Feedback on VAP rates
The INICC Central Office team communicated monthly analysis reports (graphs, charts, etc.) to participating hospitals of outcome surveillance data on VAP rates, and microbiology profile. These data were posted in prominent locations in the ICU to increase awareness of patient outcomes and enable staff to focus on key issues and apply specific strategies to reduce DA HAI rates. The effectiveness of this approach had been demonstrated in previous INICC studies in limited-resource settings [Reference Rosenthal22, Reference Rosenthal23]. Final reports on the surveillance and infection control interventions were similarly communicated to all staff.
Validation of reported ventilator-associated rates
Outcome surveillance forms for individual patients allowed validation of reported VAP rates as they included key clinical and microbiological criteria of infection. Internal validation of forms was performed by investigators at the participating centre to ensure relevant infection criteria were accurately recorded for each case. External validation at INICC headquarters, reviewed, and entered patient data on the reported infection into the INICC's database following discussion of queries with the submitting centre. Finally, consistency analyses of the database were performed to ensure matching of data entered and medical records.
Statistical methods
Patients' characteristics were compared using Fisher's exact test for dichotomous variables and unmatched Student's t test for continuous variables. Ninety-five percent confidence intervals (95% CI) were calculated using VCStat (Castiglia). Relative risk (RR) ratios with 95% CIs were calculated for comparisons of rates of VAP using Epi Info v. 6 (CDC, USA). P values <0·05 by two-sided tests were considered significant. Two types of analysis were conducted to evaluate the impact of interventions on VAP rates. First, the data of the baseline period were compared with the intervention period using RR, 95% CI and P value. Second, Poisson regression was used to analyse progressive VAP rate reduction data between baseline rates and 6-month follow-up intervention periods. For this comparison, we used as baseline data only those hospitals that contributed to follow-up in that period (i.e. excluding from the baseline those hospitals that had longer periods of follow-up). Poisson regression was also used to account for random effects of clustering of VAP rates within hospitals across time periods. These models were estimated using Stata v. 11.0 (StataCorp., USA). For this analysis we used incident rate ratio, 95% CIs, and P value.
RESULTS
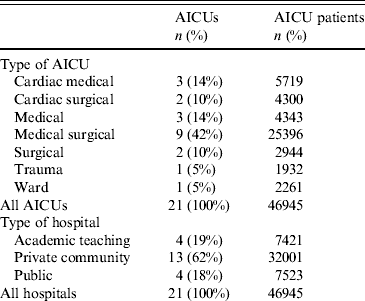
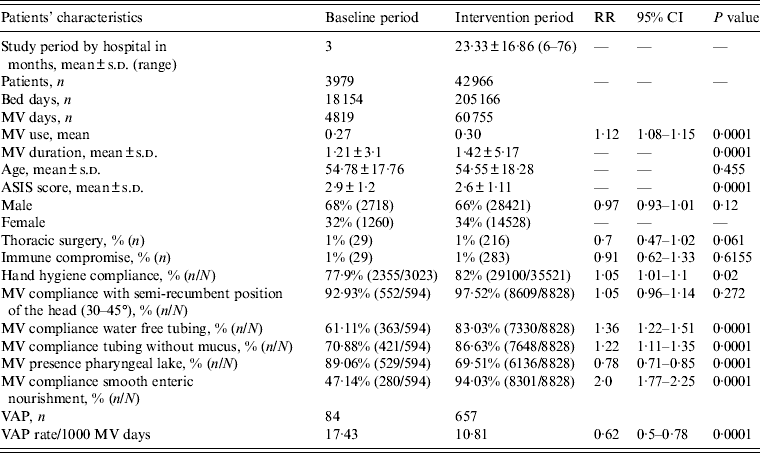
During the study period, 46 954 patients, hospitalized for 223 320 days, in 21 adult ICUs (AICUs) from 10 cities in India were enrolled in the study, with a total of 65 574 MV days (Tables 1 and 2). Age, gender, patients with thoracic surgery and immunocompromised patients were similar during both periods. ASIS mean score was lower during the intervention period. By contrast, MV use mean and MV duration mean were higher during the intervention period (Table 2). Surveillance showed that compliance with preventive measures improved significantly during the intervention period with improvements in HH of 5%, compliance with tubing without water by 36%, tubing without mucus by 22%, provision of smooth enteric nourishment by 100%, the presence of pharyngeal lake decreased by 22%. Maintenance of patients in a semi-recumbent position (30–45° elevation of the head of the bed) and compliance with nebulizer without turbidity were high during the baseline period and remained similar during the intervention period (Table 2).
Table 1. Characteristics of participating AICUs by speciality and type of hospital

AICU, Adult intensive care unit.
Table 2. Patient characteristics, hand hygiene compliance, compliance with care bundle, device use, and VAP rates, in the baseline and intervention periods

RR, Relative risk; CI, confidence interval; MV, mechanical ventilator; s.d., standard deviation; ASIS, average severity of illness score; VAP, ventilator-associated pneumonia.
Bed days are the total number of days that patients are in the intensive care unit during the selected time period.
MV days: the total number of days of exposure to mechanical ventilation by all of the patients in the selected population during the selected time period.
MV use ratios were calculated by dividing the total number of MV days by the total number of bed days.
During the baseline period, 4819 MV days, with a mean MV use of 0·27 was recorded. There were 84 VAPs and an incidence density of 17·43 VAP/1000 MV days. Merging all data of the intervention period, during the implementation of the multidimensional infection control programme, we recorded 60755 MV days, for a MV use mean of 0·30. There were 657 VAPs and an incidence density of 10·8 VAP/1000 MV days (RR 0·62, 95% CI 0·5–0·78, P = 0·0001). These results show a 38% VAP rate reduction (Table 2).
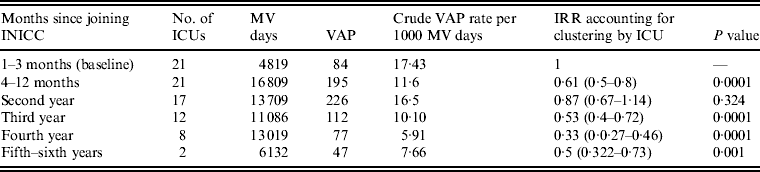
The random-effects Poisson regression showed that compared to baseline VAP rates for the 3 months before the intervention, VAP rates were reduced by 39% after 9 months of participation. This rate was further reduced by 13% during the second year, by 47% during the third year, by 67% during the fourth year and by 50% during the fifth and sixth years (Table 3).
Table 3. VAP rates stratified by length of participation of ICU

VAP, Ventilator-associated pneumonia; ICU, intensive care units; INICC, International Nosocomial Infection Control Consortium; MV, mechanical ventilator; IRR, incident rate ratio.
Pseudomonas, Acinetobacter spp. and Klebsiella were the predominant microorganisms isolated during both sampling periods and accounted for >80% of all bacterial isolates. During the baseline period, only 2·4% of VAPs were detected without microbiological sampling and this rose to 18·6% in the intervention period.
DISCUSSION
A comparison of the baseline rate of VAP found in this study (17·43/1000 MV days) shows that it was almost tenfold higher than the USA 1·8 VAP rate/1000 MV days determined by the CDC NSHN [Reference Dudeck24], and more than twofold higher than the 6·8/1000 rate determined by KISS [Reference Geffers and Gastmeier25].
In comparison with VAP rates from other developing countries, our VAP baseline rate was lower than the first international INICC report published in 2006 (24·1 VAP/1000 MV days) [Reference Rosenthal6], but similar to the second, third, and fourth INICC reports published in 2008 (19·5 VAP/1000 MV days) [Reference Rosenthal7], 2010 (13·16 VAP/1000 MV days) [Reference Rosenthal8], and 2012 (15·8 VAP/1000 MV days) [Reference Rosenthal9]. Within the scope of other studies from India, the VAP rates were similar to the baseline rate reported here. A multicentre study in 12 ICUs of seven hospitals in 2007 found an overall rate of 10·46 VAP/1000 MV days [Reference Mehta10], and similarly, in 2009, a rate of 15·87 VAP/1000 MV days in a tertiary-care hospital was reported [Reference Joseph26].
Previous INCC studies have shown that implementation of the six-component multidimensional approach for VAP listed in the Methods section resulted in significant reductions in rates of VAP in Argentina (51·28 vs. 35·50 VAP/1000 MV days) [Reference Rosenthal, Guzman and Crnich27], and in China, where a 79% cumulative VAP rate reduction was recorded [Reference Tao28]. Similarly high reductions were found in pooled VAP rates in paediatric ICUs (31%) [Reference Rosenthal29], neonatal ICUs (33%) [Reference Rosenthal30] and AICUs (56%) [Reference Rosenthal31] in limited-resource countries.
Patients' characteristics, such as age, gender, thoracic surgery and immunocompromised status, showed similar intrinsic risk in both study periods although the ASIS mean score was lower in the intervention period. By contrast, MV use mean and MV duration mean were higher during the intervention period indicating increased patient intrinsic risk.
The implementation of the multidimensional approach resulted in a significant improvement in process surveillance rates, HH compliance and elements of the care bundle. The position of the head in semi-recumbent position and compliance with nebulizer without turbidity remained similarly high and similar, respectively, during the whole study period. According to the literature, HH, lack of turbidity with nebulizer and head position are some of the key elements to reduce the risk of VAP [Reference Muscedere32].
Regarding the microorganism profile, the predominance of Klebsiella, Pseudomonas, and Acinetobacter spp. as the most common pathogens associated with VAP has previously been noted in India [Reference Jakribettu and Boloor33], and increasingly associated with multidrug resistance in hospitalized patients, especially in ICUs [Reference Joseph34].
This study has some limitations. The first is that our findings cannot be taken as representative of all AICU patients in Indian hospitals. However, it does demonstrate the value of a multidimensional approach for determination of VAP rates and their control of adverse effects in an AICU setting in a low-resource country. Second, we did not have the necessary resources to collect more data on process surveillance and measure compliance with all of the interventions examined and therefore we were unable to evaluate effects of specific interventions or other factors related to individual ICUs or hospitals. Additionally, due to budgetary limitations we were not able to analyse reduction in the time under MV, length of stay in ICU and hospital, mortality rate and costs. Finally, the setting of a 3-month baseline period may have been too short and might have overestimated the effect of interventions. However, we obtained a sufficient sample size during the baseline period with adequate confidence intervals for the baseline rates and this did not lead to a bias in the results obtained. In conclusion we have demonstrated a substantial reduction in VAP rates in the AICU setting in several Indian hospitals and confirm the effectiveness of this approach in low-resource settings. This was achieved despite higher patient intrinsic risk characteristics during the intervention period, through improved compliance with preventive measures and implementation of the multidimensional approach for VAP prevention trialled in infection control programmes in hospitals worldwide [Reference Rosenthal35]. Through the INICC network, investigators are freely furnished with training and methodological tools to perform outcome and process surveillance, and to implement effective infection prevention models for VAP. We extend an invitation to other hospitals in the developing world to participate in this project.
APPENDIX: Remaining co-authors of this study, members of the International Nosocomial Infection Control Consortium from India
A. Hegd, F. Kapadia (PD Hinduja National Hospital & Medical Research Centre, Mumbai); A. Bhakta, M. Bhattacharyya, S. Sen (Kolkata, AMRI Hospitals); A. GUPTA (Pushpanjali Crosslay Hospital, Ghaziabad); A. Poojary, G. Koppikar, L. Bhandarkar, S. Jadhav, N. Chavan, S. Bahirune, S. Durgad (Breach Candy Hospital Trust, Mumbai); T. Singhal, S. Shah (Kokilaben Dhirubhai Ambani Hospital, Mumbai); J. V. Divatia, R. Kelkar, S. Biswas, S. Raut, S. Sampat (Tata Memorial Hospital, Mumbai); B. N. Gokul, R. Sukanya, L. Pushparaj (Fortis Hospitals, Bangalore); K. Radhakrishnan (Amrita Institute of Medical Sciences & Research Centre, Kochi); S. Shetty, S. Binu, P. Pinto (Dr. L. H. Hiranandani Hospital, Mumbai); K. Subramani (Christian Medical College, Vellore) [www.inicc.org].
ACKNOWLEDGEMENTS
The authors thank the many healthcare professionals at each member hospital who assisted with the study surveillance; without their cooperation and generous assistance this INICC study would not have been possible: Mariano Vilar, Débora López Burgardt, Santiago Suárez, Denise Brito, Eugenia Manfredi, Luciana Soken, Dario Pizzuto, Ding Yuan, Julieta Sayar, and Isaac Kelmeszes who work at INICC headquarters in Buenos Aires; the INICC Country Coordinators and Advisory Board, who have so generously supported this unique international infection control network.
The funding for the activities carried out at INICC headquarters was provided by the corresponding author, Victor D. Rosenthal, and Foundation to Fight against Nosocomial Infections.
DECLARATION OF INTEREST
None.