INTRODUCTION
Recreational exposure to surface water may have negative health effects when water quality is microbiologically poor, possibly resulting in outbreaks of disease. Generally, when cases of presumptive recreational water-related illness are reported, symptoms are mild and non-specific [1]. Moreover, these reports are often about small groups of affected people or individual cases. Often, epidemiological investigations have not been performed and water-quality data referring to the estimated time of exposure of cases are lacking or are inadequate. These factors make it difficult to attribute reported cases of presumptive waterborne illness to recreational water contact. However, surveillance activities may help to characterize the epidemiology of the illness and identify trends in aetiological agents. Further, major deficiencies in providing safe recreational water may be identified and the collected data may be used to support interventions that should prevent illness in the future.
Systematic surveillance of waterborne disease outbreaks in the USA has revealed numerous outbreaks associated with untreated recreational water or swimming pools over the years. From 1991 to 2006, 138 waterborne disease outbreaks related to untreated recreational water were reported [Reference Barwick2–Reference Yoder9]. The majority of these outbreaks involved cases of gastroenteritis (36–89%), cases of neurological conditions such as meningoencephalitis and meningitis caused by Naegleria fowleri (5–38%) or skin conditions related to schistosomes (5–21%). Compilation of outbreaks of infectious intestinal disease in the UK from 1992 to 2003 has identified five outbreaks related to untreated recreational water, involving recreational river use and exposure to fountains [Reference Smith10]. Both in the USA and the UK, Cryptosporidium, Giardia and norovirus were frequently reported aetiological agents.
The Netherlands does not have a nationwide US- or UK-like passive surveillance system that reports clinically affected cases attributed to waterborne infectious disease. This lack of systematic surveillance in The Netherlands previously resulted in a lack of insight into the frequency of presumptive recreational waterborne outbreaks as well as in the severity of disease, the number of patients and the types of pathogens involved. To fill this information gap an epidemiological surveillance system was set up in 1991 to keep a record of health complaints associated with untreated recreational water reported by the general Dutch population to the responsible authorities.
This paper gives an overview of the types and numbers of outbreaks that were reported and studied from 1991 to 2007. Based on the data obtained and the observed trends in the number of outbreaks, new insights into disease burden in The Netherlands resulting from exposure to untreated recreational water are presented. Moreover, factors associated with the occurrence of disease outbreaks and the requirements for proper investigation and prevention of such outbreaks are highlighted.
METHODS
Definitions
Recreational waterborne disease outbreaks were defined as water exposures in which two or more persons were epidemiologically linked to untreated recreational water by location of exposure, time and illness [Reference Yoder9]. Single cases of leptospirosis and wound infections were included in the outbreak counts; such infections affect individuals rather than groups of people.
Time was not limited to exposure on one single day, but could be within the range of 1–5 days. Family or household members that reported similar symptoms after simultaneous exposure to the same recreational water were all included as patients in the same outbreak; there were no reports of secondary cases.
Outdoor recreational water settings included all official bathing sites in untreated fresh and marine water in The Netherlands for which water-quality data were reported to the European Commission. In 2007, there were 641 official bathing sites, of which 555 were inland waters and 86 were coastal (North Sea) waters. From 1990 to 2007, the Dutch population (approximately 16 million) made on average 8·7 million daytrips to coastal beaches and 5·7 million daytrips to inland beaches per year. About 80% of these daytrips took place during summer. Population and daytrip data were retrieved from the website of Statistics Netherlands (http://statline.cbs.nl) where official national statistics are available for policy-makers and scientific research.
Only two of the reported outbreaks occurred outside the official bathing season (one in October 2002, one in April 2005) and these were included.
Additionally, outbreaks associated with exposure to an untreated recreational water body that was not designated as an official bathing site were reported; these outbreaks were also included, but comprised only 3% of the total reported.
Surveillance specifically addressed illness related to exposure to untreated recreational waters and therefore data concerning chlorinated swimming pools were not collected.
Data collection
In The Netherlands, by law, the 12 provinces are the authorities responsible for bathing-water quality; as such they provide information about bathing-water quality to the public through leaflets, service telephone numbers and the internet, and encourage the public to report back any issue concerning bathing sites, whether this is about aesthetics, suspected bathing water-related health complaints or water quality. However, the public may also report health complaints related to bathing water to one of the public health services (due to merging the number gradually declined from 63 in 1991 to 32 in 2007). Occasionally, general practitioners notify public health services of increased numbers of cases of presumptive waterborne illness.
A downloadable standard report form is available to provinces and public health services to facilitate collection of relevant information regarding reported health complaints. The report form elicits information on the implicated bathing site, date, time and type of exposure, onset, type and severity of symptoms, contact with a physician, and possible other exposures that may have caused the symptoms. Provinces and public health services are not obliged to use the report form and are free in their choices on how to follow-up reported health complaints.
The National Institute for Public Health and the Environment annually asked provinces and public health services to provide overviews of the outbreaks and single cases of illness associated with untreated recreational water use they were notified of during the most recent preceding bathing season (1 May to 1 October). Provinces and public health services were requested to complete a standard form and subdivide the outbreaks and single cases they encountered into six categories: (I) gastroenteritis, (II) skin, (III) ear conditions, (IV) eye conditions, (V) leptospirosis and (VI) other health complaints. The request was usually made in October–November, followed by a reminder in December–January.
From 2004 onwards, provinces and public health services were additionally asked to report a presumptive outbreak as soon as they were notified of at least 10 cases presenting the same symptoms that occurred after exposure to the same bathing site. This direct approach enabled prompt investigation of presumptive outbreaks through questioning patients, examination of clinical samples and analysis of water samples for the presumed aetiological agent.
During the bathing season, water quality at official bathing sites was tested fortnightly for compliance with standards for faecal indicator bacteria according to the European Bathing Water Directive [11, 12] as outlined in Table 1. Compliance data were obtained from the reports annually published by the European Commission whereas information on water quality during outbreaks was retrieved from outbreak reports.
Table 1. Faecal indicator parameters and compliance criteria for untreated recreational water according to European Bathing Water Directives 76/100/EC [11] and 2006/7/EC [12]
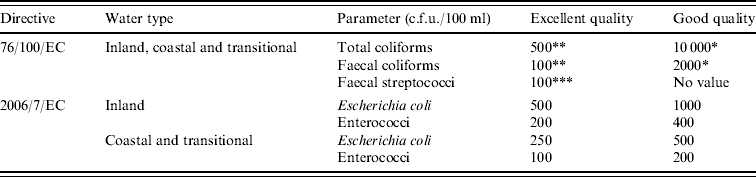
76/100/EC:
* Imperative, 95% of samples compliant; ** guideline, 80% of samples compliant; *** guideline, 90% of samples compliant.
2006/7/EC: Classification based upon 95-percentile evaluation of data from four subsequent bathing seasons, comprising of at least 16 samples.
Information about the weather during the bathing seasons and summers of 1991–2007 was obtained from the Royal Netherlands Meteorological Institute (http://www.knmi.nl).
Data analysis
Data analysis was done using Excel 2003 (Microsoft Corporation, USA). The number of reported outbreaks and single cases of leptospirosis and wound infections was corrected for double reporting (by both province and health service) and for reports that concerned one patient only.
Outbreak classification
Outbreaks were classified according to the Centers for Disease Control and Prevention (CDC) classification scheme for waterborne disease outbreaks that classifies outbreaks according to the strength of evidence implicating water as the transmission route, based on availability of epidemiological and water-quality data [Reference Yoder9]. There are four outbreak categories (Table 2).
Table 2. Classification criteria for waterborne disease outbreaks [Reference Yoder9]
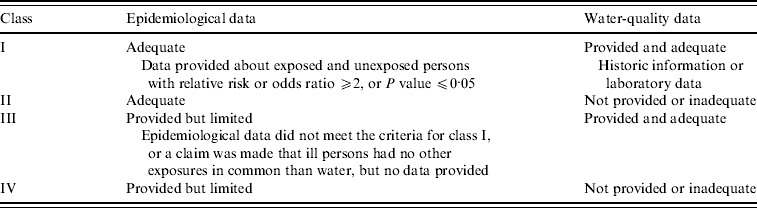
Outbreaks that were reported without any details regarding water quality and epidemiology and that were suspected to be recreational water-related illness only because people reported health complaints shortly after exposure to a specific body of water were classified as class IV. Cases of leptospirosis that were clinically confirmed and accompanied by observations of (traces of) rats in and around the implicated water were classified as class III. Similarly, outbreaks of presumptive cercarial dermatitis (swimmers' itch) supported by the finding of snails that shed Trichobilharzia cercariae were classified as class III. All reports on water quality providing indicator or pathogen counts or indicating that water quality was in compliance with European bathing-water legislation were considered adequate.
RESULTS
Outbreaks
From 1991 to 2007, the National Institute for Public Health and the Environment received 1055 reports of untreated recreational water-related illness from provinces and public health services; 313 (30%) of these included only one patient and were therefore not included in data analysis. The remaining 742 outbreaks were mainly comprised of skin conditions and gastroenteritis, distantly followed by ear conditions, and mixed outbreaks of gastroenteritis and skin conditions. Leptospirosis was not frequently reported and eye conditions were rare (Table 3, Figs 1, 2).
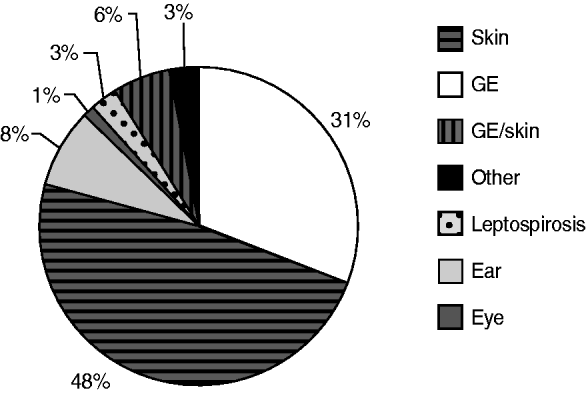
Fig. 1. Waterborne disease outbreaks associated with untreated recreational water reported in The Netherlands from 1991 to 2007, subdivided in types of health complaints, displayed as a percentage of the total.
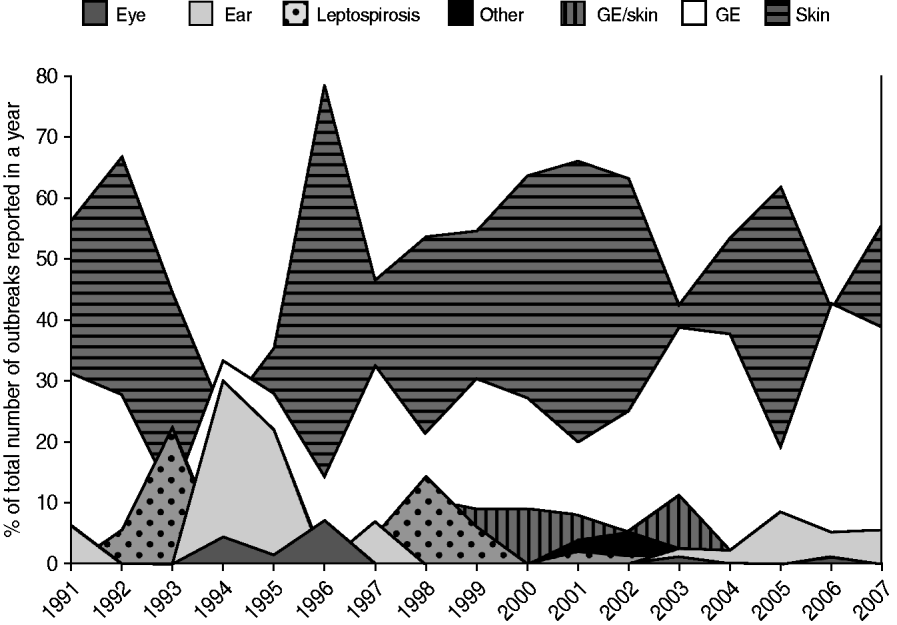
Fig. 2. Trends in the number of waterborne disease outbreaks associated with untreated recreational water reported in The Netherlands from 1991 to 2007.
Table 3. Waterborne disease outbreaks associated with untreated recreational water reported in The Netherlands from 1991 to 2007
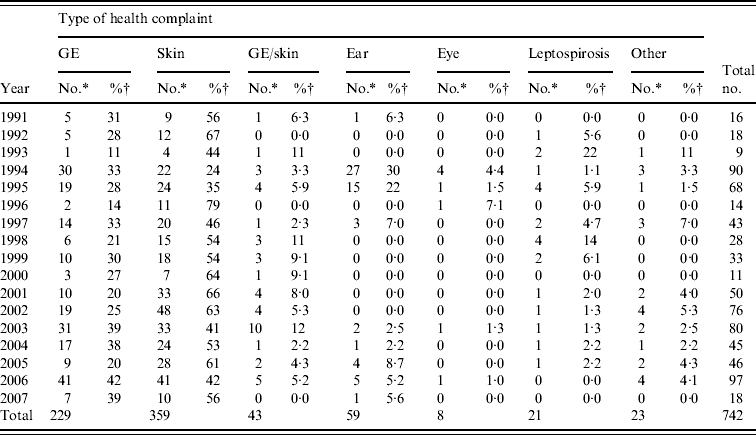
GE, Gastroenteritis; GE/skin, gastroenteritis and skin complaints.
* Number of outbreaks in which two or more patients were involved.
† Percentage of the total number of outbreaks reported in a year.
Directly reported outbreaks
From 2004 to 2007, a total of 42 outbreaks were directly reported during the bathing season; 24 (57%) comprised skin conditions, 10 (24%) gastroenteritis and five (12%) otitis externa. There was one query about Weil's disease, one report of wound infections and one report of a patient that suffered from a phototoxic reaction of unknown aetiology. Thirteen outbreaks were investigated extensively. In six outbreaks of presumptive cercarial dermatitis snails and water were examined for the presence of Trichobilharzia, which was detected in three outbreaks [Reference Schets16]. During three outbreaks of gastroenteritis, water samples were tested for enterovirus, norovirus and Salmonella. All water samples were negative, while vomit samples from patients, available in one outbreak, contained various norovirus variants [Reference Duizer17]. There was no conclusive evidence that cases in these gastroenteritis outbreaks were waterborne rather than the result of person-to-person transmission. In two outbreaks of otitis externa, water samples from the implicated lakes and ear swabs from patients contained P. aeruginosa. P. aeruginosa numbers were low in two lakes [2–37 colony-forming units (c.f.u.)/100 ml] whereas a third lake was heavily contaminated (311–736 c.f.u./100 ml). From a third outbreak, clinical samples were not available and water samples did not contain P. aeruginosa.
Two laboratory-confirmed cases of wound infections and one case of an ear infection caused by Vibrio alginolyticus instigated analysis of samples from the North Sea in which all three patients had swum. Both V. alginolyticus and V. parahaemolyticus were found. The total Vibrio spp. concentration in the water samples was 2×104 to 2×105 c.f.u./l [Reference Schets18].
Classification of outbreaks
Over three-quarter of the outbreaks were classified as class IV (Table 4) because water-quality data were not provided and epidemiological investigation of the outbreak was not performed.
Table 4. Classification of waterborne disease outbreaks associated with untreated recreational water reported in The Netherlands from 1991 to 2007 according to CDC criteria [Reference Yoder9]
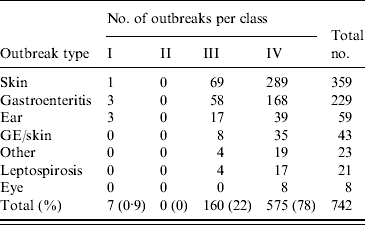
GE/skin, Gastroenteritis and skin complaints.
The 160 outbreaks classified as class III were mainly comprised of outbreaks of skin conditions or gastroenteritis (Table 5). For 71% of outbreaks of skin conditions the nature of the symptoms induced examination of water and/or snails for the presence of the parasite Trichobilharzia, which was detected in 65% of the tests. Outbreaks of gastroenteritis frequently (76%) made the responsible authorities test water for the presence of faecal indicator bacteria and compliance with European bathing-water legislation, sometimes supplemented with analysis for pathogens such as Salmonella and enteric viruses. However, 86% of these outbreaks could not be explained by poor water quality or detection of pathogens. Outbreaks of ear complaints almost exclusively (94%) instigated analysis for P. aeruginosa, which was indeed detected in 75% of the tests. There were no class II outbreaks (Table 4) and <1% of the outbreaks were classified as class I (Tables 4, 6).
Table 5. Specified outbreaks types for class III outbreaks and tests done to identify aetiological agents
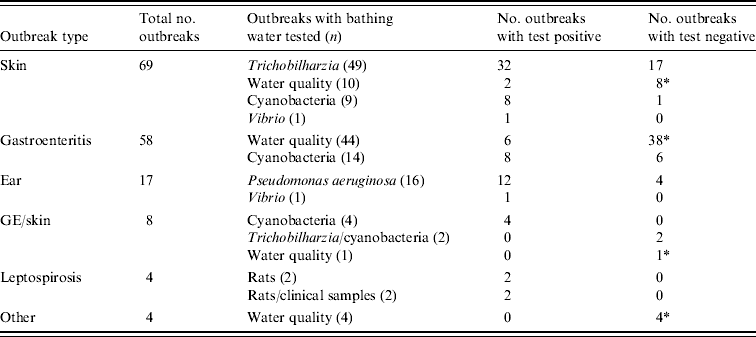
Table 6. Details of class I outbreaks
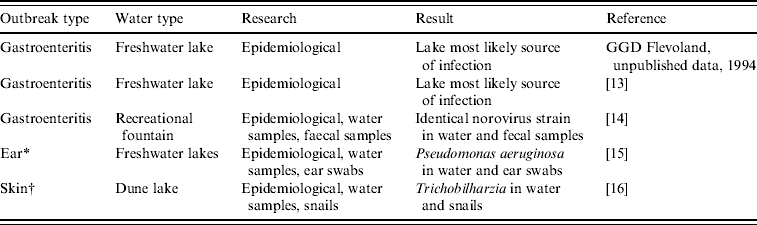
* Three simultaneously occurring outbreaks of otitis externa, included in one matched case-control study.
† Outbreak of cercarial dermatitis.
Patients
For 155/742 (21%) outbreaks the number of patients involved was not reported in absolute numbers: ‘several’, ‘more than one’ and ‘tens’ were common indications. In assessing the total number of patients involved, for those outbreaks the number of patients was set at two, although there could have been (many) more. As a result of this approach, the total estimated number of patients involved in the 742 outbreaks was at least 5623. Most patients suffered from skin (40%) or gastrointestinal (34%) conditions, followed by ear conditions (18%). Patients that had gastroenteritis and skin complaints (5%), eye conditions (0·3%), leptospirosis (0·4%) or other health complaints (2%) comprised only fractions of the total number of patients (Table 7).
Table 7. The minimum number of patients involved in waterborne disease outbreaks associated with untreated recreational water reported in The Netherlands from 1991 to 2007
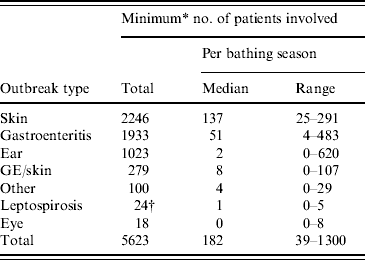
GE/skin, Gastroenteritis and skin complaints.
* For 21% of the outbreaks the number of patients involved was not reported in absolute numbers; for those outbreaks the number of patients was set at two although there could have been more, resulting in an estimated minimum total number of patients involved.
† Total number of patients reported.
Bathing sites
The disease outbreaks reported from 1991 to 2007, and the reports of cyanobacterial scums from the same period, related to about 60% of the Dutch bathing sites (n=385). Several sites (128 inland waters, one coastal waters) were involved in outbreaks in more than one bathing season; the frequency of recurrence ranged from 2–10 times. Recurrent sites were of all sizes and did not only include small-water bodies. Generally, recurrent sites were involved in more than one type of health complaint, suggesting different contamination sources, although in some cases a particular type of health complaint prevailed over the other(s). Twenty-six (4%) sites were involved in the same type of health complaint for several years, with a maximum of four; skin conditions (n=15) and gastroenteritis (n=6) most frequently recurred. A high percentage of compliance with European bathing-water legislation did not correlate (r=0·1) with a low number of outbreaks reported (Fig. 3).
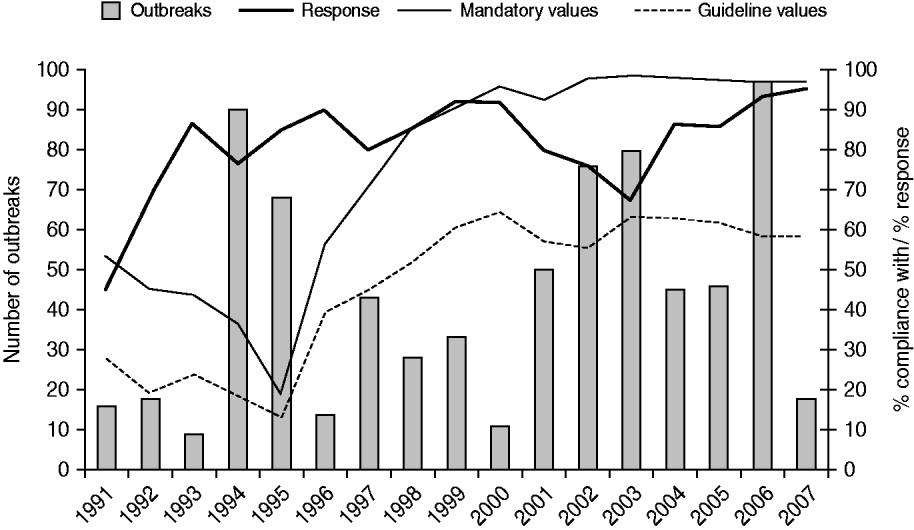
Fig. 3. The number of waterborne disease outbreaks associated with untreated recreational water reported in The Netherlands from 1991 to 2007, the percentage of authorities that responded to the request for information about such outbreaks, and compliance with mandatory and guideline values for faecal indicator bacteria in European bathing-water legislation [11, 12].
Response
On average, 82% of the provinces and health services responded to the information request. Poorer responses were obtained in the early years (1991–1992), but also in 2003 when by mistake the reminder was not sent to non-responders (Fig. 3). Non-responding was random; there was no consistent pattern of non-responding provinces or public health services. About 44% (range 17–80%) of the responding authorities encountered reports of health complaints associated with untreated recreational water during bathing seasons.
Weather
The number of outbreaks reported per bathing season was strongly variable and ranged from nine to 97, with a median of 43. The number of outbreaks reported per bathing season did not increase with increasing numbers of provinces and public health services responding to the information request (r=−0·02), but was, however, on track with the weather during summer, particularly during the summer holidays (July–August) (Fig. 4). The average monthly temperature in June, July and August and the number of summer days (i.e. days with a maximum temperature of ⩾25°C) and tropical days (i.e. days with a maximum temperature of ⩾30°C) in a summer were strongly associated with the number of outbreaks (r=0·8–0·9).
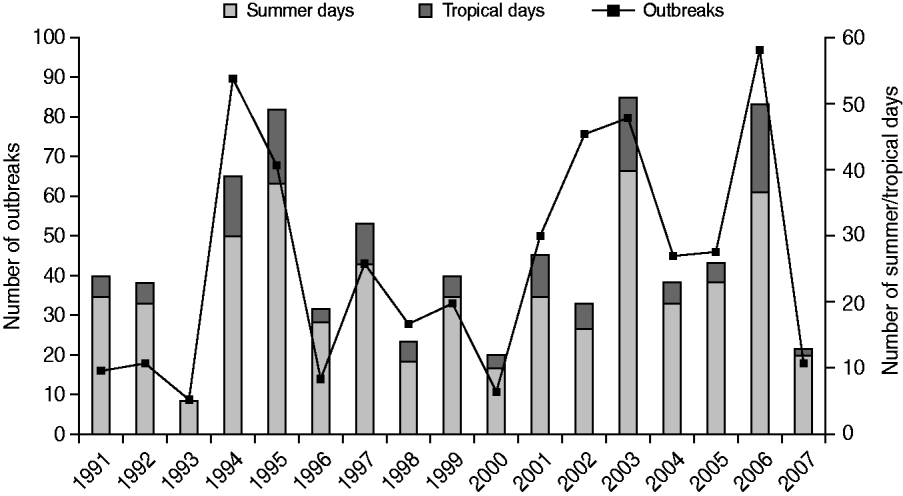
Fig. 4. The number of waterborne disease outbreaks associated with untreated recreational water reported in The Netherlands from 1991 to 2007 in relation to the number of summer days (i.e. days with a maximum temperature of ⩾25°C; www.knmi.nl) and tropical days (i.e. days with a maximum temperature of ⩾30°C; www.knmi.nl) in a summer.
Cyanobacteria
During the study period, there were 170 reports of cyanobacterial scums in recreational waters. Algal blooms generally occurred in lakes with high levels of eutrophication. In summers with a high average temperature, cyanobacteria caused more inconvenience because of (partial) closure of bathing sites. None of these 170 scum reports was accompanied by reports of cases of illness. Alongside the 170 scum reports, occasionally, some of the reported outbreaks of gastroenteritis or skin conditions were suspected to be related to exposure to cyanobacteria (Table 5). However, none of these was further investigated apart from testing the bathing water for the presence of cyanobacteria. To date, no disease outbreaks due to exposure to freshwater cyanobacteria have been observed in The Netherlands [Reference Van Riel, Schets and Meulenbelt19].
DISCUSSION
Outbreaks
The number of disease outbreaks associated with untreated recreational water use reported in The Netherlands from 1991 to 2007 is high compared to the number of such outbreaks reported in the USA during roughly the same period (1991–2006): 742 vs. 138. However, in the USA, CDC excluded all outbreaks that lacked any epidemiological data linking the outbreak to water from their Surveillance Summaries [Reference Yoder9] whereas, in The Netherlands, all outbreaks suspected to be recreational water-related were classified as class IV despite limited available data. Excluding these outbreaks, the remaining number (n=167) is still high compared to the number reported in the USA, particularly given the relative population numbers (The Netherlands 16 million; USA 305 million). First, this discrepancy may reflect that surveillance systems only detect a part of the waterborne disease outbreaks and merely depend on the preparedness of the public and the authorities responsible for bathing-water quality to report outbreaks. Compared to the USA, surveillance in a small country like The Netherlands, with more centralized public healthcare, is characterized by shorter communication lines which enable easy and direct reporting by the general public as well as by the responsible authorities. Second, some outbreaks may have been falsely attributed to recreational water because information on other possible common exposures was inaccurate, and as a result these exposures were wrongly excluded as cause of the outbreak. It is assumed that responsible authorities only reported outbreaks as recreational waterborne after checking other possible common exposures, as indicated on the standard report form provided, but there were no means to verify this for all outbreaks retrospectively.
The true disease burden due to recreational water contact in The Netherlands is probably underestimated. Since illness is often mild [1], people do not seek medical attention and cases of illness may go unnoticed. Moreover, the majority of recreational waters are visited not only by the local population but also by people from outside the area (http://statline.cbs.nl). Possible cases of illness disperse when these people return home and as a result clustering of cases is not observed. Underreporting may, however, also occur when general practitioners accept mild recreational water-related illness as a fact, e.g. general practitioners in Dutch areas with abundant water consider elevated numbers of cases of otitis externa a common phenomenon in summer that does not require reporting to public health services [Reference Schets20].
Weather
High numbers of outbreaks were generally reported in warm summers, with the summer of 2002 being the only exception. This summer was warm and humid throughout, which probably accounted for many visits to recreational sites despite the lack of many summer and tropical days. During warm summers, the number of bathers may increase greatly which is likely to result in increased numbers of reports of illness independent of water quality. When water quality deteriorates, large groups of people become exposed. Large numbers of bathers may negatively affect water quality, particularly in smaller lakes with minimal water refreshment [Reference Graczyk21]. Moreover, when water temperatures increase, conditions become more favourable for common inhabitants of fresh and coastal waters that flourish at higher water temperatures, such as P. aeruginosa and Vibrio spp. Weather conditions may also favour proliferation of cyanobacteria [1] and Trichobilharzia [Reference Horák, Kolářová and Adema22] but abundance of these organisms is only recognized as a problem when many people (may) become exposed. These microorganisms, indigenous to ecosystems and only causing problems at elevated water temperatures, may play a more profound role in causing waterborne outbreaks in the future, as a result of climate change. If water temperatures increase due to global warming, these organisms may be present in high concentrations during more prolonged hot periods which may lead to increased exposure. Exposure may also increase when higher temperatures encourage more people to visit recreational lakes at a higher frequency [Reference Schijven and de Roda Husman23, Reference Semenza and Menne24].
Bathing-water legislation
European standards for bathing-water quality are based on faecal indicators aimed at protecting bathers from exposure to pathogens of faecal origin, either human or animal. In The Netherlands, compliance with faecal indicator imperative values has largely increased since the early 1990s and is generally high and stable at >90% since the late 1990s [25]. Despite high compliance with European bathing-water legislation, suggesting good bathing-water quality with respect to faecal contamination, outbreaks of gastroenteritis have frequently been observed and comprised 31% of all outbreaks reported from 1991 to 2007. These observations support the results of studies that have demonstrated the presence of enteric pathogens such as Cryptosporidium and Giardia in recreational waters in the absence of (high numbers of) faecal indicators [Reference Graczyk26, Reference Schets27], suggesting that monitoring for faecal indicator parameters and striving for compliance with standards set by the European Commission may not sufficiently protect bathers from exposure to enteric pathogens. Moreover, more than half (56%) of the outbreaks reported from 1991 to 2007 in The Netherlands were comprised of skin and ear conditions caused by pathogens of non-faecal origin such as Trichobilharzia, P. aeruginosa and Vibrio spp. European bathing-water legislation does not address any of these pathogens, although the revised Bathing Water Directive has introduced the so-called bathing-water profiles which characterize bathing sites physically, geographically and hydrologically, and identify possible contamination sources of both faecal and non-faecal origin [12].
Investigation of outbreaks
The majority of the reported outbreaks were not further investigated. Since most of the outbreaks involved a limited number of patients, this practice seems valid. However, when large groups of cases are reported, further investigation is wanted, particularly for the prevention of disease in the future. The evidence required to attribute an outbreak to exposure to a certain body of water is, however, difficult to obtain. Outbreaks of skin and ear conditions were more often supported by the detection of the presumed aetiological agent than outbreaks of gastroenteritis. These waterborne pathogens of non-faecal origin are part of the normal aquatic flora and therefore more likely to be detected. Moreover, outbreaks of gastroenteritis may be waterborne, but may also arise from other exposures, like the consumption of contaminated food; faecal contamination sources may be diffuse or temporal and microorganisms causing gastroenteritis generally do not proliferate in the aquatic environment. Thus, faecal contamination is difficult to detect, particularly when water sampling is triggered by reported health complaints, introducing a delay of at least a few days, during which time pathogen numbers may already have decreased due to dispersion, dilution or die-off. Detection of all waterborne pathogens may be hampered by the lack of sensitivity of detection methods and inhomogeneous distribution of the pathogens in the water, and there is a time interval between swimmer exposure and actual microbiological testing of water samples. The presence or absence of a pathogen in an analysed sample does not prove the presence or absence of the pathogen at the time of exposure while test results do not reflect the current water quality and therefore, attribution of an outbreak to recreational water exposure cannot be solely based on microbiological monitoring data, but also needs additional support from epidemiological data.
To improve and expedite outbreak investigations, the relevant authorities and researchers could coordinate their responsibilities and possible actions in advance. The basic design of an epidemiological study may be prepared beforehand, including the development of standard questionnaires that minimize possible bias in data collection, analysis and interpretation. In environmental investigations, the identification of contamination sources is expedited when sufficient laboratory capacity is reserved in advance [Reference Craun28].
Public health impact
The limited number of bathing sites in The Netherlands with recurrent outbreaks of the same illness (4%) and the low frequency of recurrence (2–4 times) at these sites suggest that, generally, water-quality problems resulting in disease outbreaks were due to incidental (faecal) contamination events or environmental conditions that favoured the growth of indigenous pathogens, which were the presumed aetiological agents in the majority of the outbreaks. Since these pathogens generally belong to natural ecosystems, measures taken to eliminate them may disturb ecosystems which may be in conflict with the European Water Framework Directive [29], and thus undesirable. Therefore, preventive measures to protect public health merely include identification of all possible contamination sources and high-risk situations combined in regularly updated bathing-water profiles [12], and alertness for changes that might have a negative effect on water quality and provision of adequate and updated information to the public. People should be informed of the possible negative health impacts of swimming in surface water, although it should be stressed that illness is generally mild and self-limiting, and the positive health effects of swimming should be emphasized.
The major findings of this long-term study may also apply to situations internationally and be of value for public health workers in other parts of the world where climatological and social behavioural circumstances are comparable to those in The Netherlands.
ACKNOWLEDGEMENTS
This research was performed by the order and for the account of the General Directorate for Environmental Protection, Ministry of Housing, Spatial Planning and the Environment, The Netherlands. The authors appreciate the input of the employees at provinces and public health services.
DECLARATION OF INTEREST
None.













