INTRODUCTION
Diarrhoea is a leading cause of morbidity and mortality in children aged <5 years [Reference Bryce1], but much less is known about the burden of disease in older children, adolescents, and adults. Past efforts to estimate the burden of disease have relied on combining limited morbidity and mortality data. Mortality estimates were obtained from countries where vital registration data were available. Morbidity estimates were generated from limited outbreak surveillance data [Reference Flint2, 3]. Although these methods provide important information they only address mortality in countries that use standard medical certification for the cause of registered deaths and thus do not include many low- and middle-income countries. For morbidity, outbreak surveillance or physician reports do not detect all episodes of community-acquired diarrhoea. In addition, routine surveillance systems are not in place in most of the developing world where evidence has shown that diarrhoea incidence is greatest and mortality remains one of the top five causes of death in young children [Reference Kosek, Bern and Guerrant4, Reference Boschi-Pinto, Velebit and Shibuya5].
The 2004 Global Burden of Disease update estimates that diarrhoeal diseases account for a reported 72·8 million disability adjusted life years (DALYs) (4·8% of all DALYs) which encompasses the 2 163 000 diarrhoea deaths and 4.6 billion diarrhoea episodes each year [3]. The original methods for deriving these estimates were developed as part of the 1990 Global Burden of Disease Report based on a systematic literature review of childhood diarrhoea from 1980 to 1990, but have not been updated since then and separate estimation based on data from older children and adults has never been incorporated [Reference Murray and Lopez6]. We conducted a systematic review of diarrhoea morbidity and mortality in children aged ⩾5 years, adolescents, and adults. To our knowledge this is the first systematic literature review designed to summarize diarrhoea incidence rates in all regions of the world and diarrhoea mortality rates in developing countries for these age groups.
METHODS
Systematic review
We searched PubMed/Medline, CAB abstracts, System for Information on Grey Literature in Europe (SIGLE), and all World Health Organization (WHO) regional databases for studies published from 1 January 1980 to 31 December 2008 using all combinations of the following search terms: diarrhoea, morbidity, incidence, prevalence, mortality, etiology, cause of death, and gastroenteritis. The objective of the search was to identify all papers reporting diarrhoea specific morbidity and mortality rates that met our inclusion and exclusion criteria. We also included unpublished studies for which full reports including a clear description of methods and results were available and when authors permitted inclusion of results in this analysis. We included studies published in all languages which contained data with at least 12 months of surveillance for morbidity or of recall for mortality studies to rule out seasonality bias. For the morbidity analyses we included prospective studies with a recall period of <2 weeks and cross-sectional surveys with a recall period of <4 weeks conducted in more developed countries. For the mortality analyses we included only studies with at least 20 total deaths in persons aged ⩾5 years and limited the studies to those conducted after 1980. We excluded studies conducted in special populations, such as travellers, cancer patients, HIV-positive patient populations and conflict zones, and studies of generalized gastroenteritis symptoms, such as nausea or vomiting without differentiating incidence of symptomatic diarrhoea. Because diarrhoea in adults is not widely studied and there is not a standard definition of self-reported community diarrhoea, we included all definitions of symptomatic diarrhoea. We also excluded studies designed to detect antibiotic-associated diarrhoea or hospital-acquired diarrhoea, individual case reports, and outbreak studies.
We conducted the initial searches in all databases and combined the search results dropping all duplicate titles by using RefWorks Reference Manager [7]. We then screened all titles for relevance and all abstracts of papers passing the initial title and abstract review. We trained two abstractors to read the full papers of all remaining studies in order determine final eligibility for morbidity or mortality analyses. Any paper excluded after reading the full manuscript was logged and the reasons for exclusion were documented. We developed a standardized data abstraction sheet that included all relevant study characteristics and results. Both data abstractors completed the abstraction sheet for each included study; we then cross-checked the abstraction sheets and rectified any differences to create a final database of included studies.
Morbidity analytical methods
The objective of the morbidity analyses was to generate diarrhoea incidence rates by age and sex for all regions of the world. Only two studies conducted prospective daily diarrhoea surveillance, therefore to meet this objective we approximated diarrhoea incidence rates using 1-, 2-, and 4-week prevalence rates per 100 person-years (p-yr) assuming prevalence approximates incidence when the duration of the episode is short (typically <3 days). Because studies report various age categories we created standardized age categories and generated incidence rates for each year of age based on the individual study data. We then generated a mean incidence rate for each study according to our standardized age bands. For example, if a study reported diarrhoea incidence to be x for age 10–12 years and y for age 13–14 years, we calculated the mean for age band 10–14 years to be (3x+2y)/5. Many studies did not provide narrow age bands, therefore overall incidence rates were applied to all ages included in the study. For example, if a study reported an incidence rate of z for all ages ⩾15 years, this rate was then applied to the 15–19 years age band and all subsequent ages. We then aggregated the data from multiple narrow age bands to generate three broad age categories, 5–14, 15–54, and ⩾55 years for regions of the world where we had at least one representative study. We generated median incidence rates, by broad age band, and calculated the interquartile range (IQR) (3rd quartile minus 1st quartile) for each age band within each region. Age-specific incidence rates were then applied to the 2005 reference population for each age within the age band to generate regional and global estimates for total annual diarrhea episodes in all persons aged ⩾5 years [8].
In this review we included studies published as early as 1980 including data from the mid-1960s to the mid-2000s. We plotted the categorical age-specific incidence rates for each study based on the year of data collection. We then fitted an unweighted polynomial curve to the individual data points within each age band. Although we used strict inclusion and exclusion criteria for this review, there is still much variability in studies with regard to sample size, length of recall and variation in definition of diarrhoea. Unfortunately we did not identify an adequate number of studies to assess how these variables influence the precision of the estimated incidence rate. Typically studies are weighted according to sample size, but this assumes equality and consistency with regard to all other aspects of the study design. Because these studies have great variability, we chose not to weight studies by sample size when fitting the polynomial curve.
Mortality analytical methods
The objective of the mortality review was to summarize age- and sex-specific diarrhoea specific mortality rates for regions of the world where there are inadequate or non-existent vital registration data. To meet this objective we applied the same age standardization technique described above for incidence rates to the diarrhoea mortality rates to generate standardized age strata. We generated regional median mortality rates based on all available data in each region for 11 discrete age categories in 5-year increments until age 25 years and then in subsequent 10-year age bands until age 85 years.
RESULTS
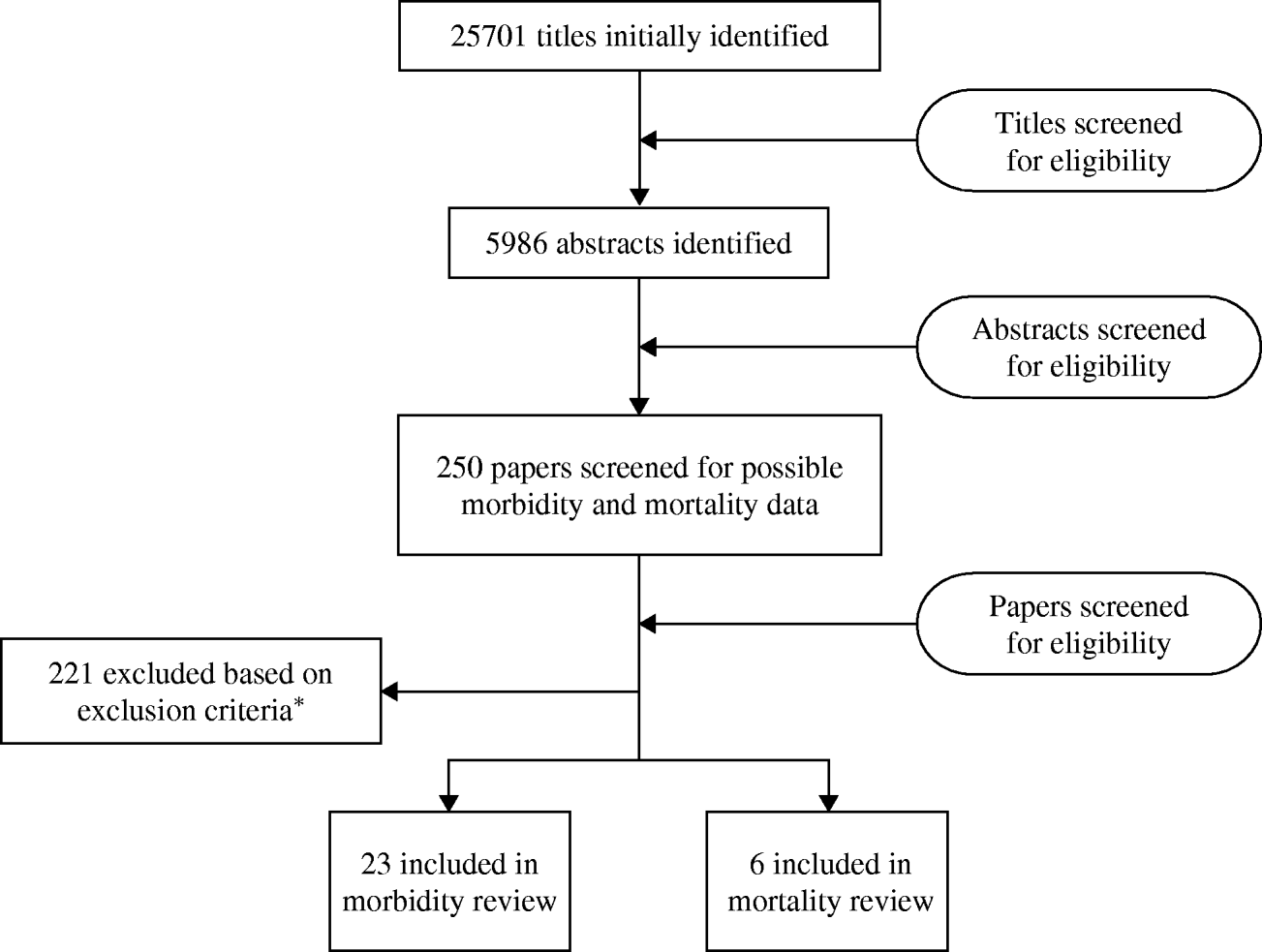
We identified at total of 25 701 titles during the initial systematic review with contributions from all databases (Fig. 1). After initial review of titles and abstracts and complete review of more than 250 papers, we included 23 papers [9–Reference Do30], (Liu, unpublished observations) that met our inclusion and exclusion criteria for the morbidity review and six papers [9, Reference Etard31–Reference Anwar35] which met our inclusion and exclusion criteria for the mortality review.
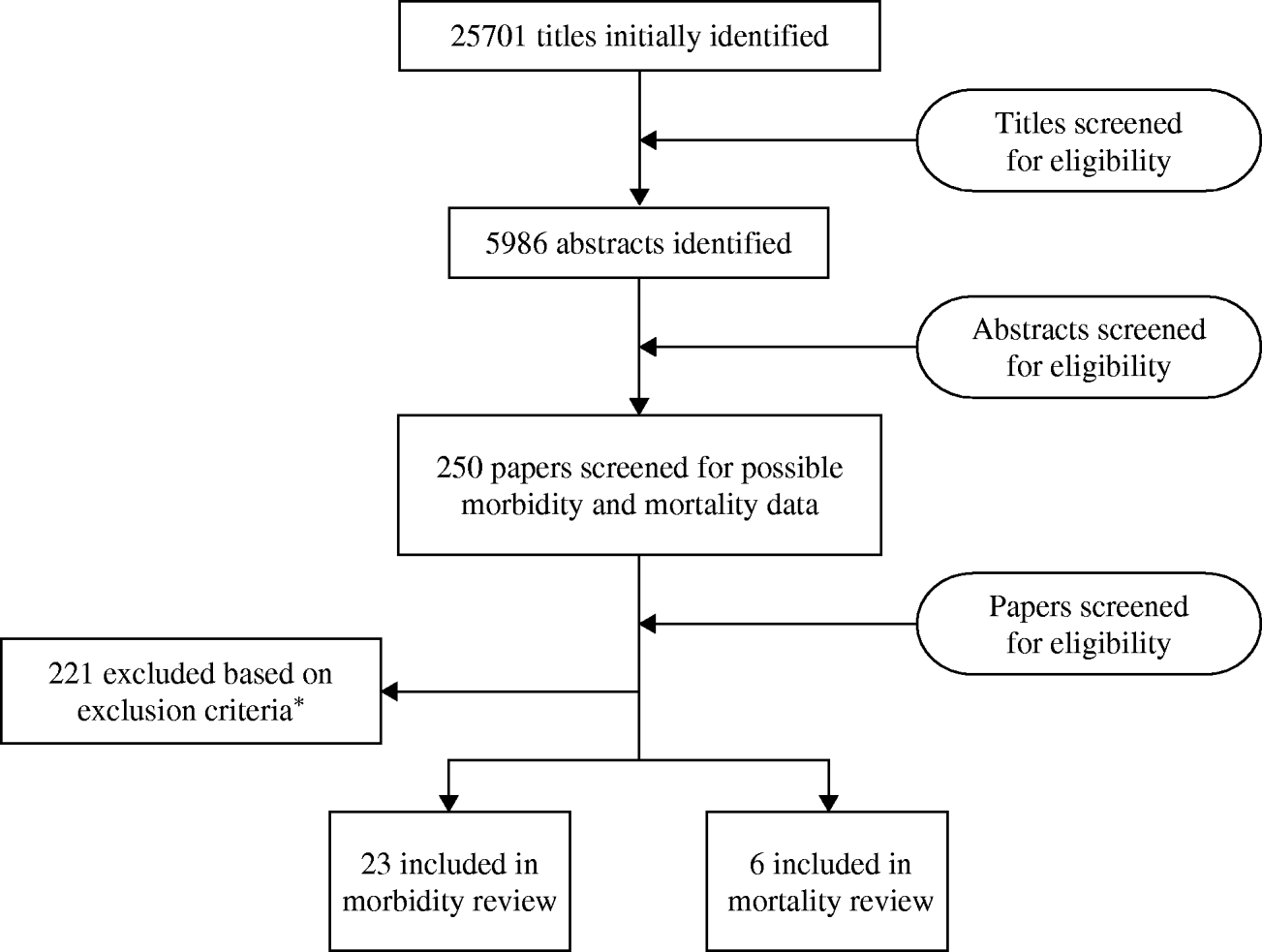
Fig. 1. Results of the systematic review. * Inclusion criteria: any children aged >5 years and adults; all languages; prospective studies with at least 12 months of surveillance or cross-sectional surveys conducted over a period of a year. Exclusion criteria: studies conducted among travellers, special populations (e.g. cancer patients, only HIV-positive individuals); case reports; hospitalized-acquired diarrhoea or antibiotic-associated diarrhoea; studies lacking appropriate information regarding study population; reports of outbreaks; prospective morbidity studies with recall beyond 2 weeks; and cross-sectional studies with recall beyond 4 weeks; mortality studies with fewer than 20 deaths.
Morbidity
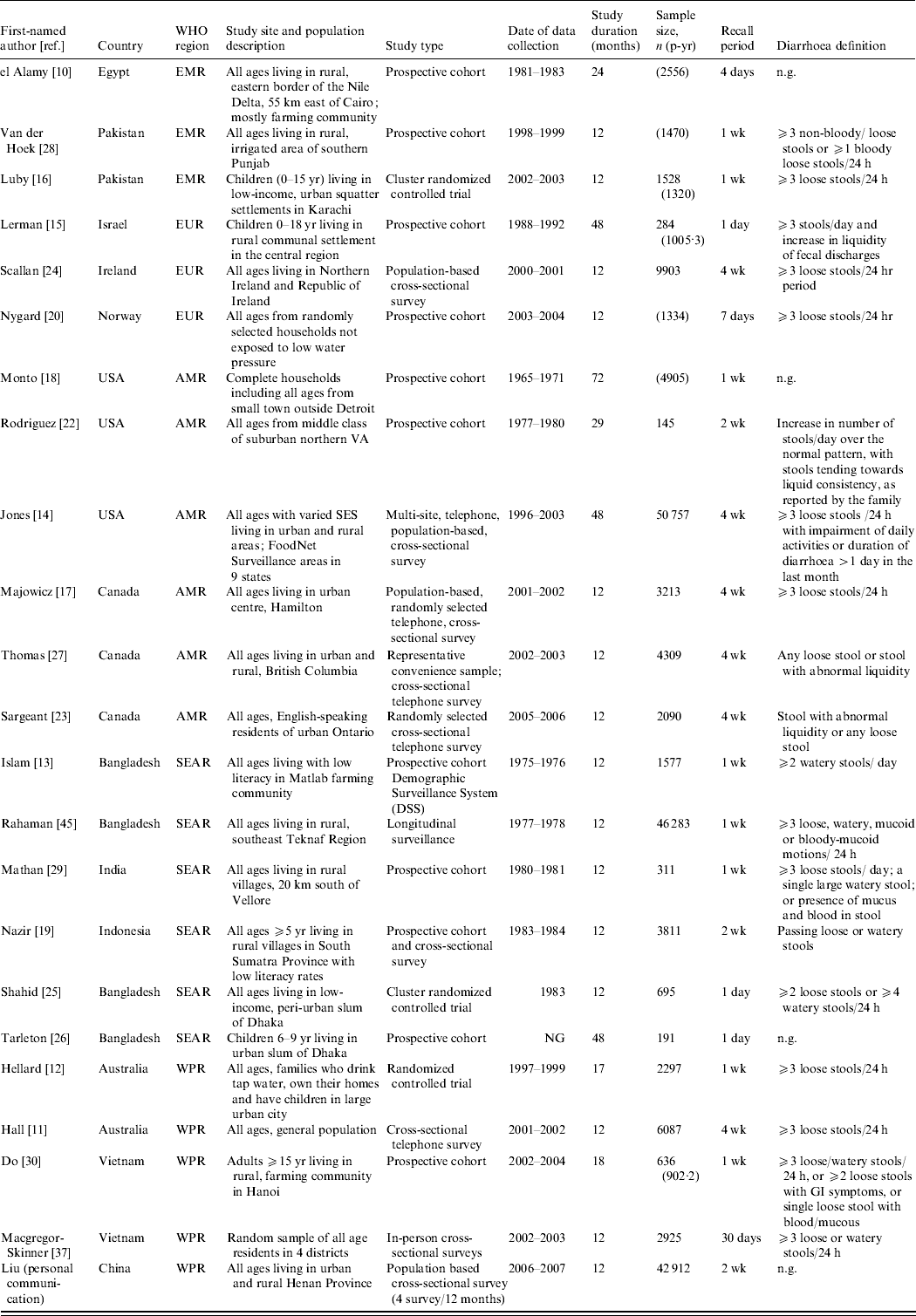
Study characteristics of all papers included in the final morbidity analyses are described in Table 1. The 23 studies were conducted in 14 different countries from five WHO regions. No studies were identified from Africa (AFR) or Central/South America. The studies included all ages starting at age 5 years, but many studies provided either very wide age bands or no age bands. None provided incidence rates by sex, therefore we were not able to identify possible differences in sex-specific morbidity rates.
Table 1. Characteristics of studies included in the morbidity analyses
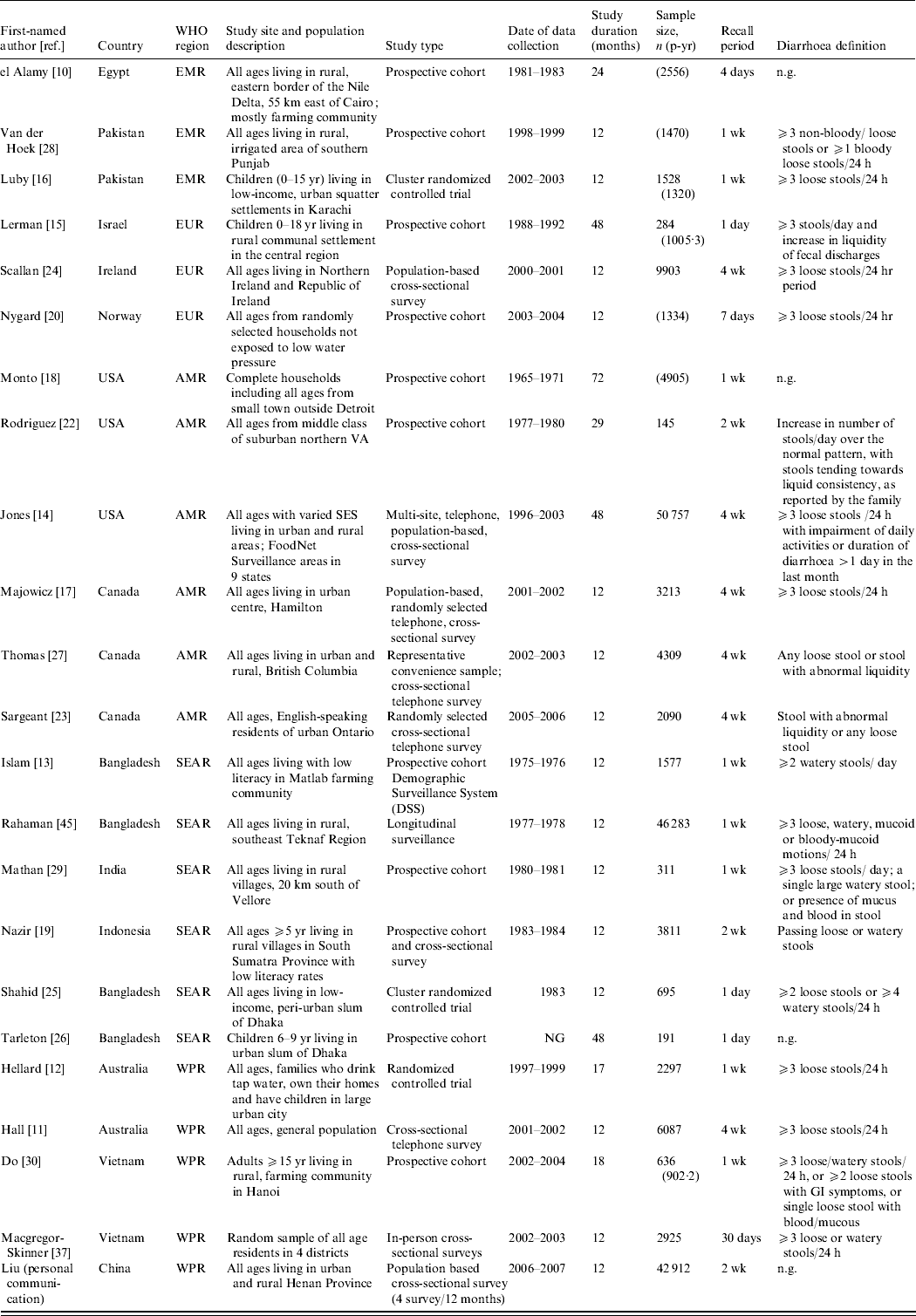
EMR, Eastern Mediterranean region; EUR, Europe; AMR, Region of the Americas; SEAR, South and South East Asia region; WPR, Western Pacific region; n.g., not given, SES, socioeconomic status; GI, gastrointestinal.
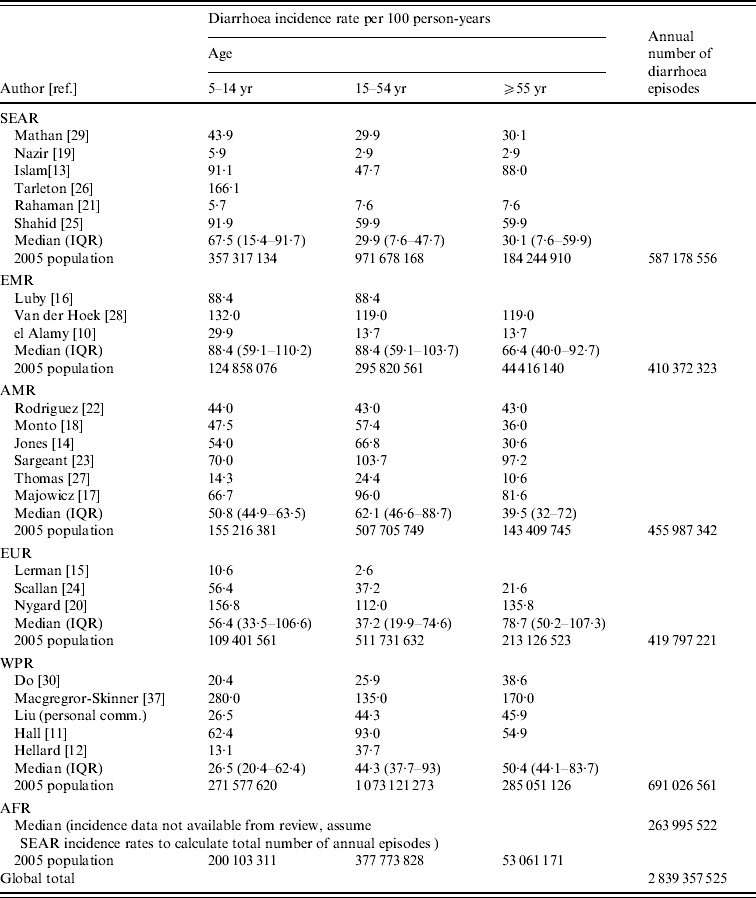
We calculated diarrhoea incidence rates by age and region (Table 2) and found that for older children and adolescents, aged 5–14 years, the highest median rate was found in the Eastern Mediterranean region (EMR) (88·4/100 p-yr). This estimate is based on three studies in the region. The lowest regional incidence rate was observed in the Western Pacific region (WPR) (26·5/100 p-yr). For adolescents and adults, aged 15–54 years, the highest median morbidity rate was again observed in the EMR (88·4/100 p-yr). The lowest morbidity rate for ages 15–54 years was observed in the South and South East Asia region (SEAR) (29·9/100 p-yr). For adults aged ⩾55 years, the highest reported median incidence rate was found in Europe (EUR) (78·7/100 p-yr) and the lowest was observed in the SEAR (30·1/100 p-yr). Applying the annual incidence rates to the 2005 [36] population in each region, we estimated more than 2.8 billion episodes of diarrhoea per year in children aged ⩾5 years, adolescents and adults. The largest burden occurs in the WPR where there are an estimated 691 million episodes of diarrhea each year in this age group.
Table 2. Median diarrhoea incidence by age group and world region
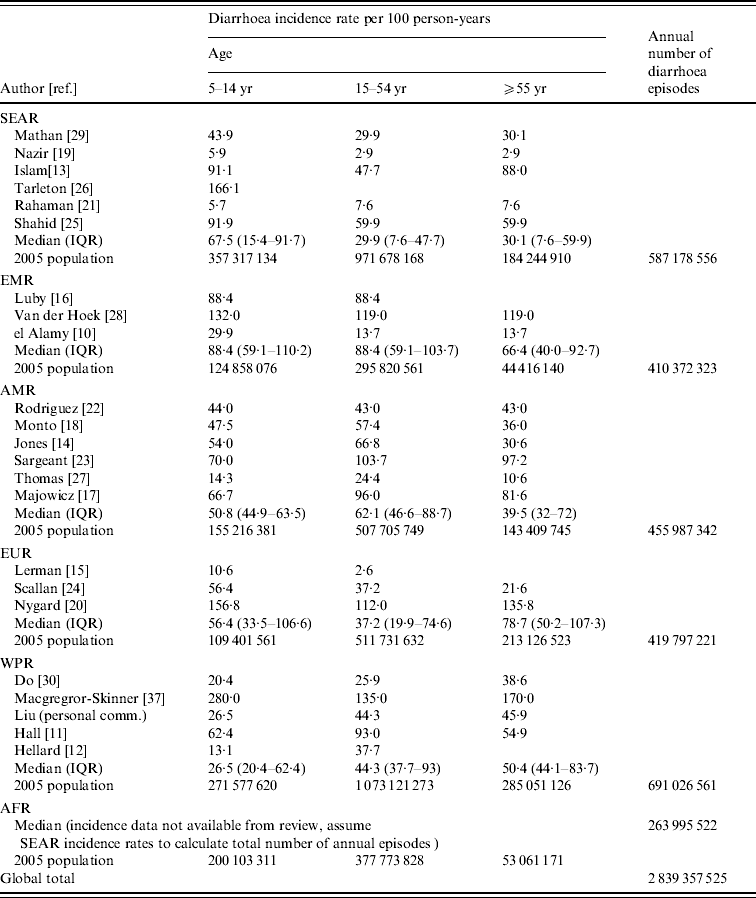
SEAR, South and South East Asia region; EMR, Eastern Mediterranean region; AMR, Region of the Americas; EUR, Europe; WPR, Western Pacific region; AFR, Africa; IQR, Interquartile range.
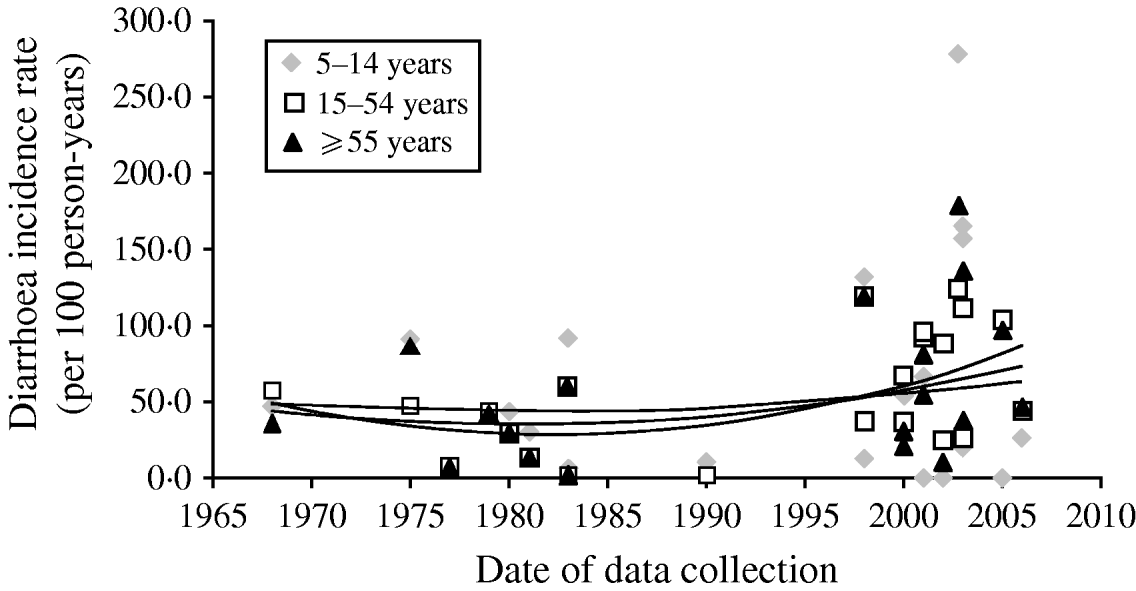
Overall there has been little change in diarrhoea morbidity over time as can be observed in Figure 2. Although the shape of the curve is suggestive of a slight increase this may be the result of many more additional studies since the late 1990s. We excluded one study which emerged as an outlier from the trend lines. In this Vietnamese study, the authors reported incidence rates of 280/100, 135/100, and 170/100 p-yr for ages 5–14, 15–54, and ⩾55 years, respectively [Reference Macgregor-Skinner37]. Overall, although there are not adequate data to suggest an increase in morbidity, the data do indicate that rates have at least remained constant rather than declining over the last 30 years in persons aged ⩾5 years.
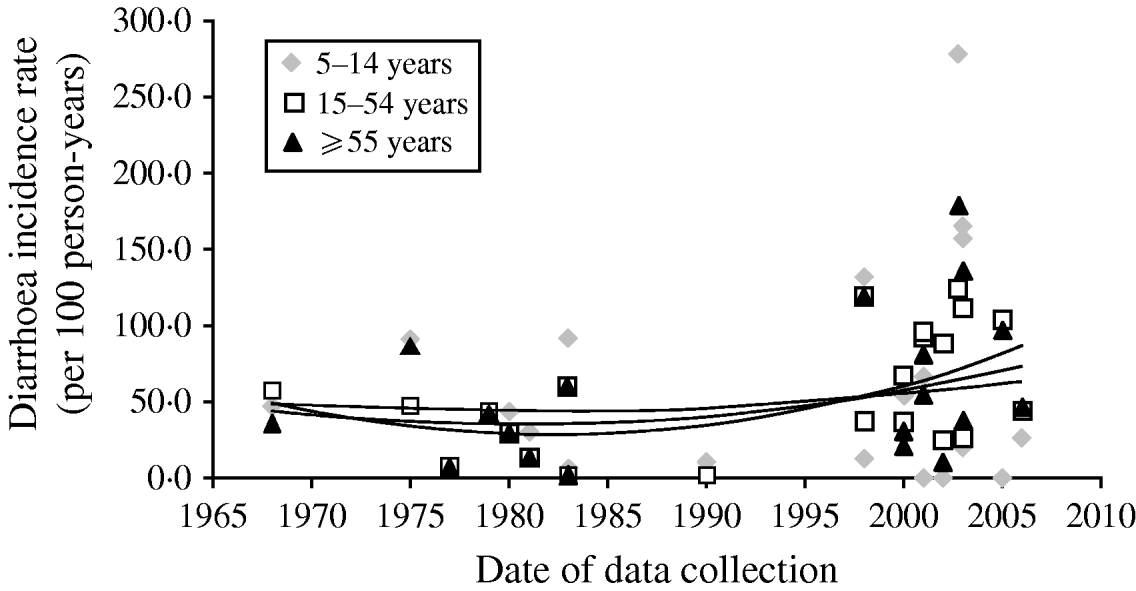
Fig. 2. Mean diarrhoea incidence rates and trend lines in stratified age groups for each included study (trend lines represent unweighted polynomial trend by age). Macgregor-Skinner was excluded from the trend lines as an extreme outlier [Reference Macgregor-Skinner37].
Mortality
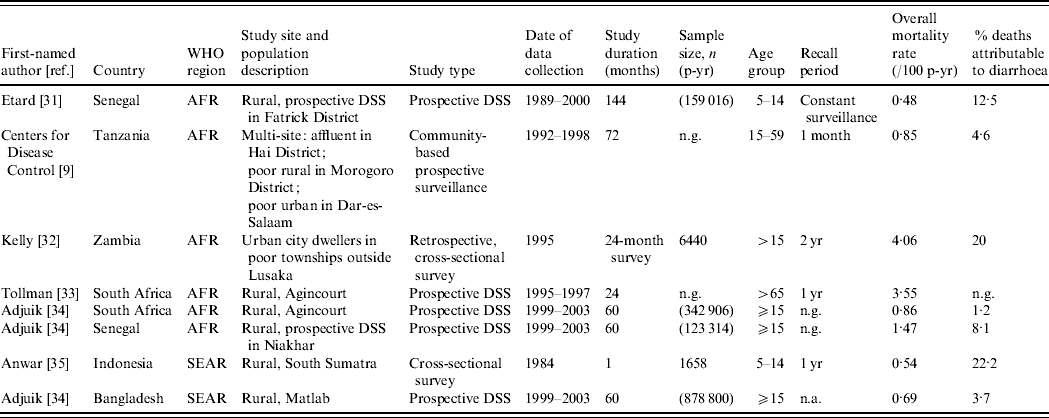
Table 3 presents the characteristics of studies included in the mortality analyses. We identified studies from six countries in two WHO regions, AFR and SEAR. Although the studies span the entire age range of interest, i.e. ⩾5 years, only two studies included children and adolescents aged 5–14 years [Reference Etard31, Reference Anwar35].
Table 3. Characteristics of studies included in the mortality analyses
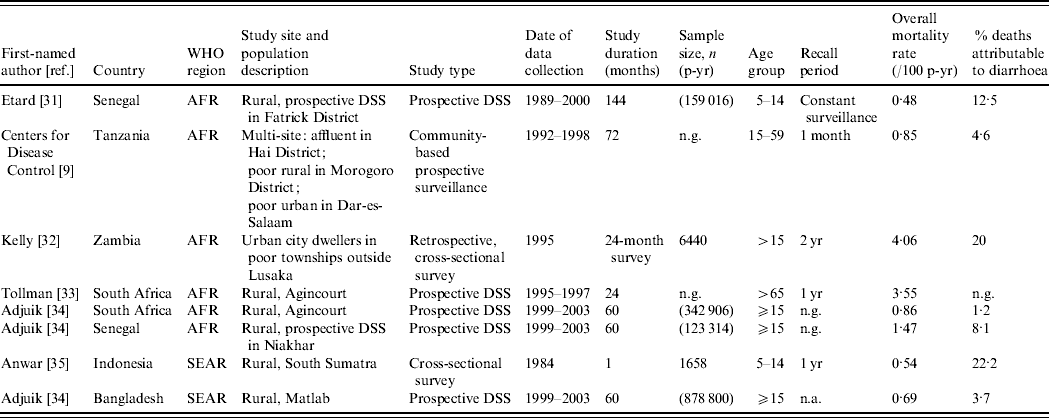
AFR, Africa; SEAR, South and South East Asia region; DSS, Demographic surveillance site; n.a., not available; n.g., not given.
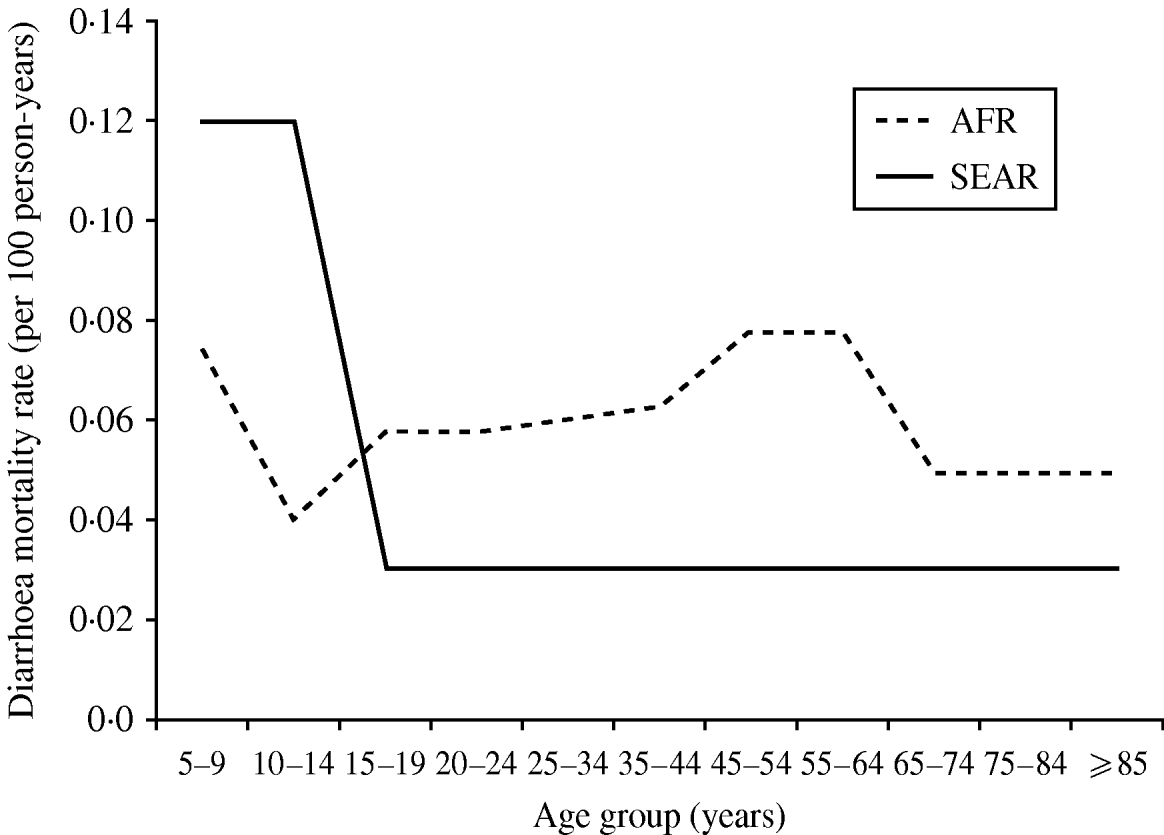
We observed that diarrhoea mortality decreases drastically as the child ages in Asia but is more consistent across the adolescent and adult lifespan in Africa (Fig. 3). In Africa, there were five studies contributing data for age ⩾15 years. In this age group the lowest diarrhoea mortality rate was found in South Africa (0·01/100 p-yr) and the highest in the rural poor in Tanzania (0·288/100 p-yr). In Asia one study provided data for age <15 years (0·12/100 p-yr) [Reference Anwar35] and one study provided data for age ⩾15 years (0·03/100 p-yr) [Reference Adjuik34].
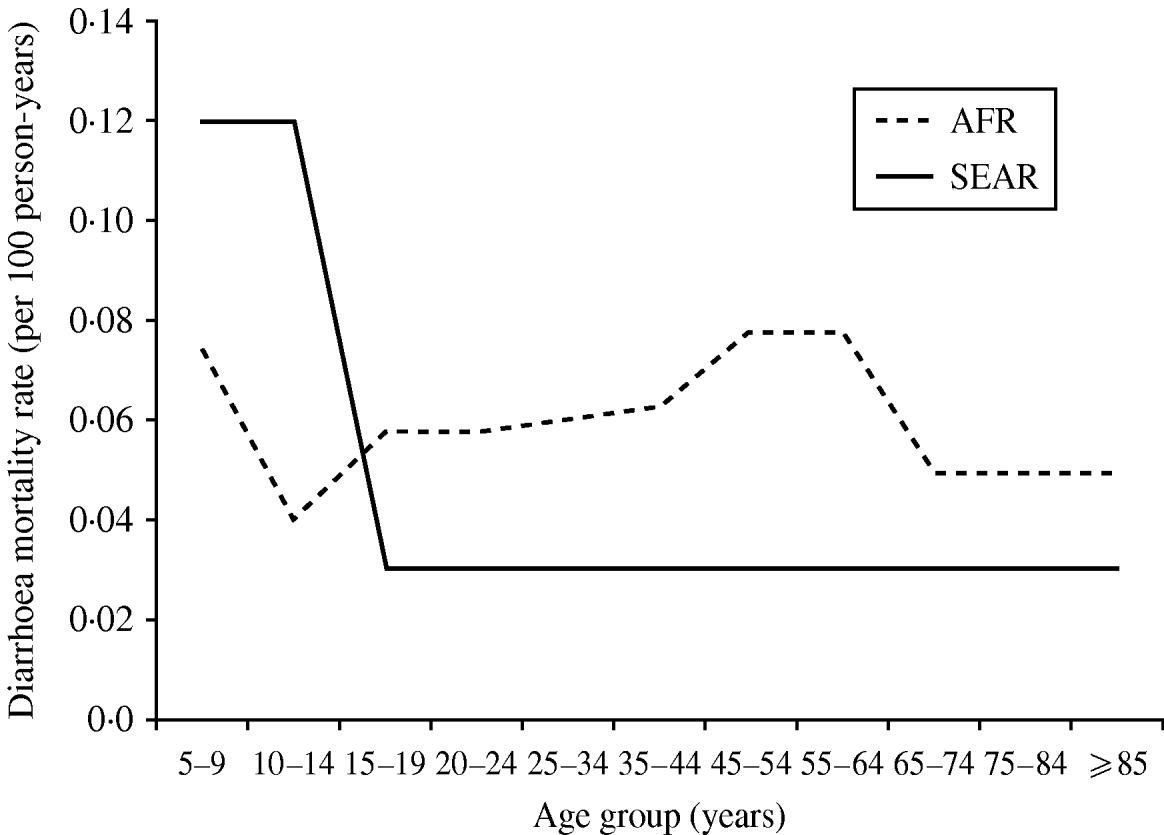
Fig. 3. Diarrhoea mortality rates by age and world region. AFR, Africa; SEAR, South and South East Asia region.
DISCUSSION
This is the first systematic review of diarrhoea morbidity and mortality in children aged ⩾5 years, adolescents, and adults designed to determine diarrhoea incidence rates for all regions of the world and diarrhoea mortality rates where vital registration data are lacking. We conducted an extensive literature search, identified 23 morbidity studies, and found that although there is variability by age, there is not a consistent pattern between regions as has been observed in children aged <5 years [Reference Boschi-Pinto, Lanata, Black and Ehiri38]. This review suggests that diarrhoea morbidity rates have remained constant in all ages since the 1980s in both developed and developing countries and estimates more than 2.8 billion episodes of diarrhoea per year in children aged ⩾5 years, adolescents, and adults. This is significantly greater than previous burden-of-disease estimates [3].
For young children diarrhoea mortality is one of the most important causes of death [Reference Boschi-Pinto, Velebit and Shibuya5]. In Asia, this risk remains high from ages 5–14 years, and then reaches a low and constant plateau throughout adolescence and adulthood while in Africa it declines less dramatically after age 5 years and remains relatively constant throughout the lifespan. Although extremely sparse especially for Asia, these data might be used to better inform adult mortality estimates for regions of the world lacking vital registration data. Previous estimates were based solely on data from regions of the world with vital registration data so although great caution must be exercised when interpreting these data, they add important information for regions of the world where adult cause-specific mortality evidence remains sparse.
We used strict inclusion and exclusion criteria to avoid potential biases in our estimates including seasonal variation and recall bias. All studies had at least 12 months of data collection to minimize seasonal bias. We recognize that this is a limitation because otherwise well-designed studies, such as outbreak studies, may have been excluded. For this reason, the estimates of diarrhoea morbidity from this review and analysis may underestimate the true burden and might be regarded as the minimum burden. Although we were not able to model diarrhoea seasonality for this global analysis at the local level, studies with seasonal data may be useful for national-level analyses where knowledge regarding site-specific diarrhoea seasonality would allow for extrapolation to annual estimates.
We limited the morbidity recall period to 2 weeks for developing countries and included studies with up to 4 weeks for more developed countries. There are no studies regarding appropriate recall period for diarrhoea morbidity in adults. Studies in children have shown that reported diarrhoea prevalence falls by half if a mother is asked to recall an episode after more than a week [Reference Byass and Hanlon39]. However, it has also been shown that literate mothers report higher diarrhoea morbidity rates for their children than illiterate mothers, despite decreased mortality rates in a more educated population [Reference Manesh40]. In this review we observed that cross-sectional surveys with 4-week recall in both developed countries and in Vietnam found higher rates of reported diarrhoea than many prospective studies, therefore it does not appear that a longer recall in these populations leads to an underestimate of morbidity. Alternatively, there may be over-reporting of morbidity by recalling episodes where more than 4 weeks have elapsed. Because we do not have prospective studies from similar settings to compare rates it is difficult to make assumptions with regard to potential reporting bias. Higher rates in more developed countries might be expected as children in developing countries experience higher diarrhoea rates and thus are actively building immunity at an early age whereas young children in more developed countries are exposed to fewer diarrhoea pathogens and may experience higher incidence rates later in life [Reference Malik, Bhan and Ray41]. Because appropriate studies from South and Central America were not identified, this may be a limitation for the estimate for diarrhoea morbidity for the Region of the Americas (AMR) where only data from the USA and Canada was available.
We found an increasing number of studies reporting morbidity data since the 1990s, including more in high-income countries with increased attention to surveillance of possible foodborne diseases. Unfortunately, many large-scale studies conducted in developed countries were excluded from our analysis because aggregate data of all gastroenteritis symptoms were reported without specific reference to diarrhoea symptoms [Reference Hellard12, Reference Wheeler42–Reference Payment44]. Although attempts were made to contact authors to provide the incidence of diarrhoea symptoms, in many cases this was not possible.
Overall we recovered limited data from which to derive regional morbidity and mortality estimates. For many regions of the world, such as Latin America and Africa we identified no morbidity studies, thus making the generalization of diarrhoea incidence rates in older children and adults in these regions difficult. The strict inclusion and exclusion criteria did not prove to discriminate against studies from any particular region, thus we are confident that the final database represents the best possible studies for estimating the burden of diarrhoea in older children and adults. The few data points limit the possibility of using more advanced modelling techniques to construct improved estimates for countries where data are missing. The mortality data are particularly scarce and it appears that very little research is being done outside of the Demographic Surveillance Sites (DSS) around the world to determine cause of death in adults in countries where civil registration of mortality does not exist. Improved cause-specific mortality estimates are crucial for the planning of health services, therefore every effort should be made to publish the results from surveillance sites as well as new efforts to establish vital registration systems in countries where current mortality data are scarce. Diarrhoea is often considered to be an important disease only in young children. However, we have shown that although mortality rates may have declined in the past 15 years, morbidity rates have remained constant and thus more aggressive efforts will be needed to ensure every person, both young and old, has access to clean water, sanitation facilities, and food sources free from contamination in order to minimize risk factors for diarrhoeal diseases.
ACKNOWLEDGEMENTS
This work was undertaken as a part of the Global Burden of Diseases, Injuries, and Risk Factors Study. A grant from the Bill and Melinda Gates Foundation supported the Study's core activities and partially supported the epidemiological reviews in this paper. This work was jointly funded by the World Health Organization – Foodborne Disease Burden Epidemiology Reference Group. The authors thank the members of the WHO Foodborne Disease Burden Epidemiology Reference Group (FERG) for their invaluable feedback and assistance in identifying unpublished data sources. We also thank the Melinda Munos, Hilda Ndirangu, and Ramya Amyakutty for help with the initial literature review and data abstraction. The results in this paper are prepared independently of the final estimates of the Global Burden of Diseases, Injuries, and Risk Factors Study.
DECLARATION OF INTEREST
None.