Introduction
COVID-19, the illness caused by SARS-CoV-2 virus, has a range of flu-like clinical manifestations, including cough, fever, fatigue, myalgia and shortness of breath [Reference Rothan and Byrareddy1]. Although resembling a common cold, this infection can cause serious respiratory illness, such as pneumonia and acute respiratory distress syndrome, especially in high-risk individuals [Reference Huang2]. Diagnosis of COVID-19 is usually based on the detection of SARS-CoV-2 by a reverse transcription polymerase-chain reaction (RT-PCR) testing of oropharyngeal or nasopharyngeal swabs, tracheal aspirate or bronchoalveolar lavage samples [Reference Pascarella3]. Patients with moderate or severe COVID-19 are usually hospitalised for observation and supportive care, since there is currently no specific treatment [Reference Sanders4]. Early in the pandemic, the presence of non-respiratory symptoms, such as gastrointestinal and smell and taste disorders, was widely reported [Reference Nobel5, Reference Beltrán-Corbellini6]. Due to this, attention to COVID-19 patients with non-classic symptoms has been heightened, as well as their predictive value for test positivity [Reference Nobel5, Reference Jin7, Reference Tostmann8].
The Portuguese National Epidemiological Surveillance System (SINAVE) is an electronic epidemiological surveillance system of all nationally notifiable diseases [9]. Notification is exclusively and compulsorily online, where clinicians and laboratories report each suspect case in a clinical and laboratorial disease-specific form, respectively [10]. The COVID-19 case report form was created in January 2020, and includes a clinical section with symptoms and signs for inquiry, based on international guidelines. The first diagnosed case of COVID-19 in Portugal was on 1 March, a symptomatic male with fever, cough and myalgia, and a recent travel history to a country with an active COVID-19 outbreak [11].
This study aims to better understand the clinical presentation of COVID-19 cases at the time of notification in Portugal, in order to update surveillance components. We aimed to identify which symptoms, at the time of notification, were associated with a positive RT-PCR result for SARS-CoV-2 among suspected cases in mainland Portugal between 1 March and 1 April 2020.
Case definition
For surveillance purposes, the national case definition aligned with guidance from the World Health Organization and the European Centre for Disease Control and Prevention (ECDC) [12, 13]. The criteria for testing until 8 March were: the presence of fever and/or cough and/or shortness of breath, in addition to an epidemiological link with a confirmed case or recent travel history to an affected country (the list of countries was frequently updated by the Portuguese Directorate-General of Health (DGS), according to the COVID-19 risk of transmission). On 9 March, the criteria for SARS-CoV-2 testing were widened to include hospitalised cases with severe pneumonia and no other apparent cause. On 26 March, Portugal entered the mitigation phase, and the criteria for testing were further expanded to include all cases of acute respiratory distress syndrome with cough or fever [13].
Methods
We extracted retrospective surveillance data on all COVID-19 suspected cases notified to SINAVE until 1 April 2020. Socio-demographic data were collected from each case report form, being automatically fed by the National Patient Record System. Data on clinical symptoms and signs were reported by clinicians on each case report form. An automatised procedure using structured query language algorithm was used to clean and merge both clinical and laboratory SINAVE databases. Posteriorly, a team of clinicians and epidemiologists cleaned, deduplicated, reviewed and cross-checked the data on each suspect case notification. Because completeness of the symptom variables ranged from 33.7% to 46.6%, we selected only those notifications with complete information on symptom variables for analysis.
Cases were defined as individuals with an RT-PCR-positive test result for SARS-CoV-2 virus and compared to controls, defined as all notifications on individuals with an RT-PCR-negative test result for SARS-CoV-2. Controls were chosen as we wanted to better understand the clinical presentation of COVID-19 cases compared to those with similar clinical presentation. No matching methods were used. All suspected cases that met the test criteria and were in mainland Portugal during the study period were tested and included in this study, since COVID-19 suspected cases notification in SINAVE is compulsory nationally. All 12 symptoms and signs included in the clinical notification of COVID-19 were selected as predictors (fever as tympanic temperature equal or above 38 °C, cough, shortness of breath, headache, myalgia, joint pain, fatigue, sore throat, chest pain, diarrhoea, nausea and abdominal pain). The dependent variable was the RT-PCR test result. The χ 2 tests were used to compare notified cases on sex, age group, health region and presence of comorbidities, defined as a binary variable on having a prior diagnosed chronic medical condition. Symptoms were first analysed using unconditional univariate logistic regression, assessed by calculating odds ratios and 95% confidence intervals. For model selection, a backward stepwise method was performed. To correct for possible confounding, we started with all variables of the study, a multiple adjustment and carried out a multivariate logistic regression. Variables with a P-value <0.001 in univariate analysis were included in the initial model. The best-fit prediction model was selected by choosing the one with the lowest Bayesian Information Criterion (BIC) score. Analyses were performed using STATA v.16 (Statacorp, Texas, USA).
Aggregated data were collected in the scope of national epidemiological surveillance, requiring no supplementary ethical clearance. Confidentiality and anonymity were protected, as no individual cases are identifiable in this analysis.
COVID-19 suspected cases
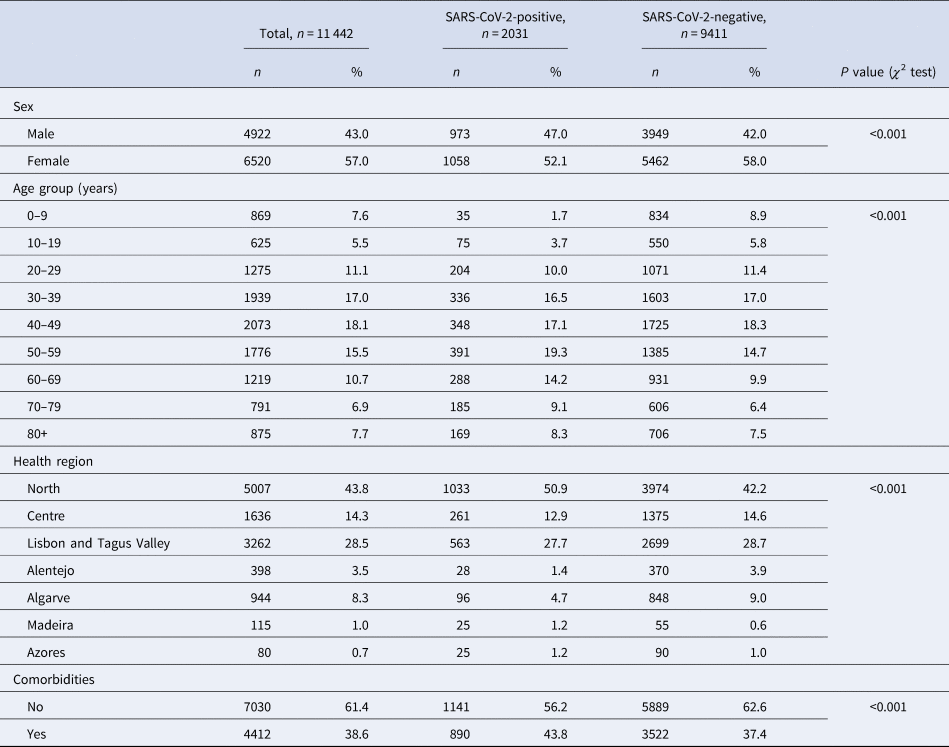
From 51 726 COVID-19 suspected cases notifications, 11 442 had sufficiently complete symptom data for analysis: 2031 (15.96%) tested positive for SARS-CoV-2. Among all COVID-19 suspected cases, 56.98% were female (Table 1), although this proportion was slightly lower among cases (52.1%, P < 0.001). Median age of controls was 43 years (s.d. ± 22.6), while among cases was 50 years (s.d. ± 19.8). The North region was the most affected area, with circa 50% of test-positive cases. A larger proportion of individuals in the SARS-CoV-2 test-positive group had comorbid conditions (P < 0.001).
Table 1. Description of COVID-19 notifications with complete data, as of date of notification, by SARS-CoV-2 test result, Portugal, March–April 2020 (n = 11 442)
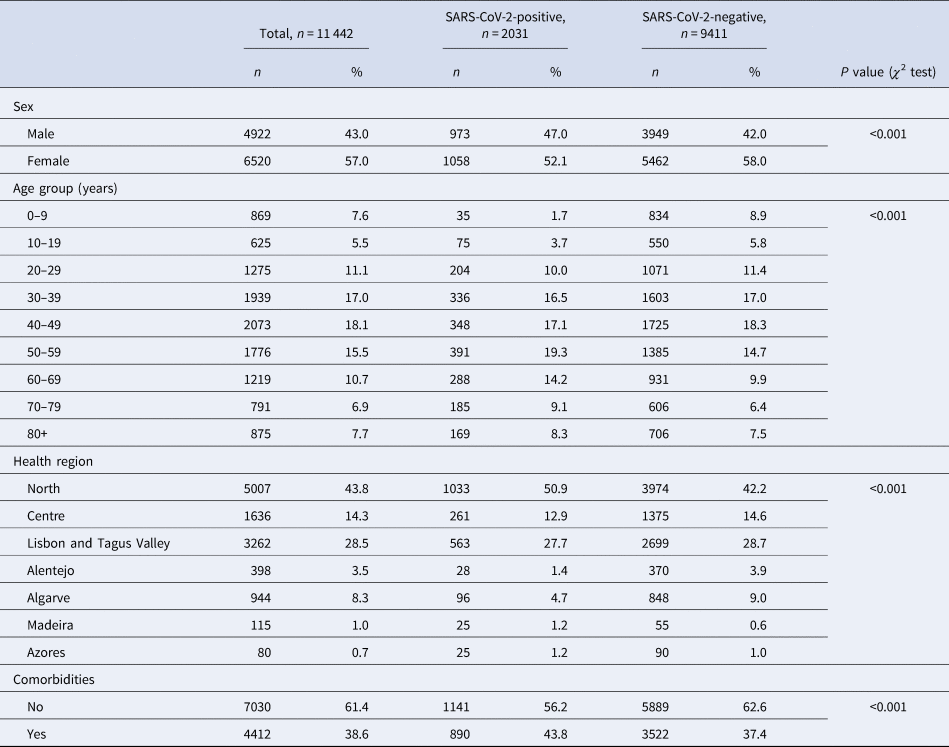
Cases and controls differed significantly on age group, sex, health region and presence of comorbidities. Because cases and controls were not matched, we included these variables in further analysis of symptoms for multiple adjustment.
COVID-19 symptoms
Among test-positive cases, cough (73.0%, n = 1483), fever (59.7%, n = 1212), myalgia (43.9%, n = 891), headache (40.0%, n = 812) and fatigue (38%, n = 771) were most frequently reported (Table 2).
Table 2. Frequency and univariate association of COVID-19 symptoms with test outcome, among SINAVE notified cases, Portugal, March–April 2020 (n = 11 442)
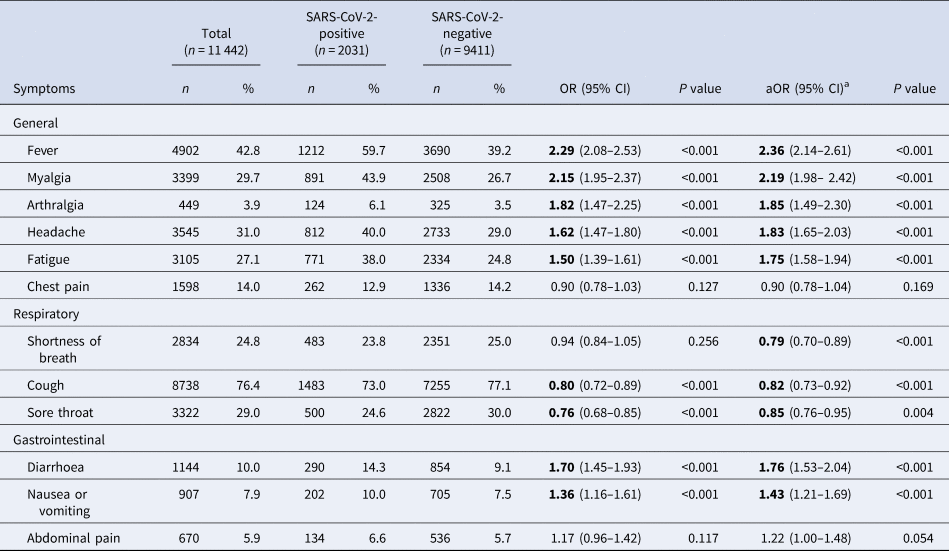
Bold significants P<0.05.
a Symptoms, age group, sex, health region and comorbidities adjusted.
At the time of notification, the presence of general symptoms such as fever, myalgia, arthralgia, headache and fatigue, as well as gastrointestinal symptoms (diarrhoea and nausea or vomiting) was positively associated with COVID-19. Symptoms of chest pain, shortness of breath and abdominal pain were not associated with a positive test result in the univariate analysis.
For symptoms included in the case definition (presence of fever or cough or shortness of breath), only cases presenting with fever (alone or in combination with other symptoms) were strongly associated with a positive test for SARS-CoV- 2 (crude odds ratio (OR) 2.29, 95% CI 2.08–2.53). Those presenting with cough, alone or in combination with other symptoms, were 20% less likely to test positive for SARS-CoV-2 virus (95% CI 0.72–0.89) and for those presenting only with shortness of breath, the association with positive test outcome was not statistically significant (crude OR 0.94, 95% CI 0.84–1.05).
After multiple adjustment for symptoms, as well as age group, sex and health region, the best-fit model did not retain nausea or vomiting, arthralgia and presence of comorbidities (Table 3). In this multivariate model, the presence of fever, myalgia, headache, fatigue or diarrhoea was strongly associated with the outcome of positive SARS-CoV-2 test result.
Table 3. Multivariate model on COVID-19 symptoms among SINAVE notified cases, Portugal, March–April 2020 (n = 11 442)
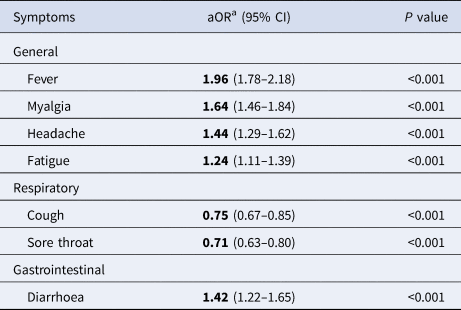
a Symptoms, age group, sex and health region adjusted.
General symptoms on this model were strongly associated with a positive test. Those who had fever were almost twice as likely to have a positive test as those presenting without fever (adjusted OR (aOR) 1.96, 95% CI 1.76–2.17). For respiratory symptoms, the presence of cough or sore throat was negatively associated with COVID-19 test positivity (P < 0.001). Diarrhoea increased by 42% the odds of testing positive (aOR 1.42, 95% CI 1.22–1.65). Running the same model only with multiple adjustment for symptoms, without including age, sex, health region and presence of comorbidities (possible cofounders), led to the same conclusions within groups of symptoms (Supplementary Table S1).
Discussion
Based on the results presented, symptoms most prevalent in SARS-CoV-2-positive cases were cough and fever, demonstrating testing criteria previously described in case definition. Nevertheless, we acknowledge also that myalgia, fatigue and headache, classified as general symptoms, were presented each in more than one-third of COVID-19 cases. Indeed, ECDC last updated case definition criteria on 29 May 2020, acknowledging additional less specific symptoms to be considered in clinical criteria. Headache, chills, myalgia, fatigue, vomiting and/or diarrhoea were described as to be considered [14].
In our study, non-respiratory symptoms, including general and gastrointestinal symptoms (diarrhoea), were strongly associated with a positive test for SARS-CoV-2. Based on 12 symptoms analysed, the most parsimonious model retained nine symptoms with the strongest association. Presence of fever, myalgia, headache, fatigue or diarrhoea was associated with a COVID-19 laboratory confirmation. Compared with other respiratory viruses, fatigue, headache and myalgia were more common among human coronavirus-infected patients [Reference Friedman15]. This study highlights the predictive value of general non-respiratory symptoms as a differential tool to distinguish COVID-19 cases in the universe of respiratory symptoms patients.
This is not the first study where respiratory symptoms in COVID-19 cases played a minor role in predicting laboratory test results for SARS-CoV-2 [Reference Nobel5, Reference Tostmann8]. Although respiratory symptoms, such as cough, are frequent among COVID-19 cases and other human coronavirus infections, in our study, COVID-19 cases report less cough comparing to other respiratory viruses and less shortness of breath than other human coronaviruses [Reference Friedman15]. Furthermore, some symptoms initially not diagnosed or not associated with this virus are being reported, such as smell and taste disorders [Reference Beltrán-Corbellini6]. In consequence, the addition of general and gastrointestinal symptoms proven to be strongly associated with a positive test for SARS-CoV-2 is an asset on cases identification and resource-saving. In fact, this study contributes to the evidence that supports last updates on COVID-19 case definitions, highlighting reported less specific symptoms [14].
This study has some limitations, as it reflects the clinical presentation of suspected and confirmed cases of COVID-19 at the time of notification. This means that we were assessing early symptoms in some cases, and several days of symptoms in others. Although methods exist to deal with missing data, completeness of data was a major issue in this analysis: due to poor completeness, we opted to analyse complete case reports to improve confidence in our results, though we cannot exclude the presence of a selection bias. In this sense, we compared our sample with all COVID-19 suspected cases regarding age, sex, presence of comorbidities and health region. Our sample was younger, had higher female proportion and reported less comorbidities at the time of notification (P < 0.001) (further details in Supplementary material S2). Although there is evidence on sex differences regarding COVID-19 mortality, the effect of sex on disease presentation and diagnosis is still not clear [Reference Gebhard16, Reference Palaiodimos17]. There is also evidence on older age groups having a higher risk of atypical disease presentation (asthenia, delirium, fall), which is likely related to the higher prevalence of comorbidities, being one of the variables that we adjusted for in our analysis [Reference Godaert18]. Furthermore, due to the younger mean age in this sample, it is expected that the occurrence of SARS-CoV-2-positive test is higher in this group due to higher probability of typical clinical presentation, possibly overestimating the reported magnitude of the association. Individuals in our sample had a lower prevalence of comorbidities, leading to a likely underestimation of the association between having a prior medical precondition and a positive test result, since having the first increases the risk for SARS-CoV-2 infection [Reference Jain and Yuan19]. In this comparison, variables presented small statistically significant differences, in both directions, and not surprisingly due to correspondent population and sample sizes. Due to the study design, identification of symptoms was dependent on accurate clinical documentation on SINAVE predefined questionnaire. Moreover, we acknowledge that some symptom misclassification likely exists, but expect that it is non-differential, as test outcomes were unknown at the time of their documentation.
Our results can be compared with the settings using the same case definition and similar surveillance system, due to comprehensiveness of detection and reporting of patients.
Conclusion
These results highlight that general and gastrointestinal symptoms, at the time of notification, are strongly associated with a positive test for SARS-CoV-2. In contrast, the presence of respiratory symptoms was less likely to lead to a positive test for SARS-CoV-2. Based on our findings, respiratory symptoms, such as cough, although frequent among cases, are negatively associated with COVID-19 case status. In this sense, the inclusion of general symptoms such as myalgia, headache and fatigue, as well as diarrhoea, together with actual clinical criteria for suspected cases, already updated and included in COVID-19 case definition, can lead to increased identification of cases and represent an effective strength for transmission control.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S095026882100042X
Data availability statement
The data that support the findings of this study are available from the Portuguese Directorate-General of Health (DGS). Restrictions apply to the availability of these data, which were used under licence for this study. Data are available from the authors with the permission of DGS.
Acknowledgements
As this is a collaborative work, we would like to thank all public health teams performing case and contact investigation at local and regional levels, the Directorate-General of Health COVID-19 Task-Force members. We also acknowledge the Porto Public Health Institute (ISPUP) COVID-19 Task-Force for criticizing study design and data analysis. This study relies on secondary data from the National Epidemiological Surveillance System and so, we would like to thank all medical notifiers. We also would like to acknowledge Dr Neil Saad for all inputs regarding overall study concept and data analysis.
Author contributions
MPD, RSM, HL, CC and RM conceived the study. MPD wrote the manuscript. RSM supervised the overall study. RM was responsible for data acquisition. MPD and CC did data cleaning and MPD analysed the data. RSM, DA and LH critically revised the manuscript.
Disclosures
The contents of this article are solely the responsibility of the authors and do not necessarily represent the views of the affiliated institutions. We declare that we received no funding.
Conflict of interest
None.