INTRODUCTION
Staphylococcus aureus infections are widespread in the USA, and commonly manifest as minor skin and soft tissue infections such as boils, abscesses, furuncles, folliculitis and cellulitis. Complications of infection include bloodstream infections, surgical wound infections, urinary tract infections, and pneumonia. An increasing proportion of S. aureus infections have become resistant to antibiotics such as methicillin, penicillin, and cephalexin; these infections, known as methicillin-resistant S. aureus (MRSA), are often referred to as a ‘superbug’ by the media. The Centers for Disease Control (CDC) estimate that about 25–30% of the US population is colonized with S. aureus, while about 1–5% harbour MRSA; however, carriage rates can vary by geographic location and the specific population being sampled [Reference Rim and Bacon1, Reference Salgado, Farr and Calfee2]. MRSA infections occur most frequently in persons with weakened immune systems who undergo surgery or are admitted to a hospital or long-term care facility; these infections are referred to as hospital-associated MRSA (HA-MRSA). However, MRSA is becoming more prevalent in healthy individuals within the general community.
Community-associated MRSA (CA-MRSA) infections were first reported in the early 1980s and were often associated with spread of strains from hospitals into the community [Reference Crum3]. Studies and published reports indicate that CA-MRSA is on the increase, accounting for 8–20% of MRSA, depending on the population tested [Reference Aiello4]. CA-MRSA strains are distinguishable from HA-MRSA in genotypic characterization and clinical presentation [Reference Crum3, Reference Klevins5, Reference Webber6]. Genetic characteristics commonly attributed to CA-MRSA strains include: staphylococcal cassette chromosome mec (SCCmec) type IV elements, the Panton–Valentine leukocidin gene (PVL), and sequence type (ST)8 by multilocus sequence typing (MLST) [Reference David7]. These characteristics are believed to contribute to the observed variation in antibiotic resistance between community- and hospital-associated infections. CA-MRSA strains are generally resistant to penicillin and semi-synthetic penicillin-class antibiotics (i.e. oxacillin and cephalosporins) whereas multidrug resistance in hospital-acquired strains is relatively common [Reference Aiello4, Reference Webber6, Reference Baum8–Reference Kallen12]. A study conducted by Naimi et al. revealed that CA-MRSA isolates are more likely than HA-MRSA to be susceptible to ciprofloxacin (79% vs. 16%), clindamycin (83% vs. 21%), gentamicin (94% vs. 80%), and trimethoprim–sulfamethoxazole (95% vs. 90%) [Reference Naimi13]. Antibiotic selective pressure is much lower in the community than in hospitals, and the survival advantage of multidrug resistance is much lower [Reference Bothwell, Shvidler and Cable14].
CA-MRSA infections are more likely to present as skin and soft tissue infections as opposed to invasive infection [Reference Crum3, Reference Webber6, Reference Baum8–Reference Kallen12, Reference Chambers15–Reference Grundmann18]. Although CA-MRSA infections are less likely to be invasive than their HA-MRSA counterpart, an estimated 6% of CA-MRSA infections are classified as invasive [Reference Salgado, Farr and Calfee2–Reference Aiello4, Reference Chambers15, Reference Farley17, Reference Grundmann18]. CA-MRSA cases also lack traditional risk factors associated with HA-MRSA such as recent hospitalization, history of medical procedures, residence in long-term care facility, or frequent antibiotic use. Instead, persons with CA-MRSA infections tend to be young and healthy; populations shown to be at increased risk for CA-MRSA include children in daycare centres, athletes, prison inmates, and military trainees [Reference Salgado, Farr and Calfee2–Reference Aiello4, Reference Webber6, Reference Baum8–Reference Kallen12, Reference Bothwell, Shvidler and Cable14–Reference Como-Sabetti16, Reference Kenner19–Reference Zinderman21].
Rates of up to 5% MRSA colonization or carriage in high-risk groups such as military trainees have been reported [22]. Outbreak investigations and prevalence studies in military populations have identified a number of probable risk factors associated with the spread of CA-MRSA skin infections such as sharing crowded barracks or equipment, inadequate hygiene, and physical trauma associated with trainee training [Reference Salgado, Farr and Calfee2–Reference Aiello4, Reference Baum8, Reference Kenner19–Reference Zinderman21]. An additional concern in military trainee populations which may increase risk is under-utilization of health services for fear of being held back and forced to repeat the entirety of basic military training (BMT).
While multiple studies regarding outbreak investigations exist, data regarding long-term trends in CA-MRSA infection, clinical presentation/management and antibiotic susceptibility patterns in both the general population and high-risk groups are lacking. This study was initiated in order to assess these particular outcomes within one high-risk setting, a military training site. Basic trainees at Fort Benning, one of the U.S. Army's five BMT sites, served as the study population for this evaluation based on an identified need and the existence of historical data collected on site.
METHODS
The study population consisted of military service members (active duty, reserve, and guard) and trainees stationed at Fort Benning with a culture-confirmed MRSA infection. This was based on review of medical and laboratory data collected at Fort Benning's Martin Army Community Hospital (MACH) for all MRSA-confirmed infections in this military cohort. CA-MRSA infections were defined using the following criteria: (1) clinically recognized skin or soft tissue infection; (2) S. aureus-positive wound culture with identified resistance to oxacillin and confirmatory resistance to cefotaxime as defined by CLSI guidelines; and (3) no history of surgery or hospitalization within 30 days prior to infection.
For identified CA-MRSA infections, information regarding patient demographics, clinical presentation/management, attributable burden of infection (e.g. number of days admitted, number of days assigned to limited duty, and total number of patient visits related to the infection prior to the date of positive culture), and antibiotic susceptibility patterns of the isolates were retrieved and entered into a CA-MRSA surveillance database designed and maintained by the MACH infection control nurse. Data obtained were collected from January 2002 to December 2007. Aggregated population summary data extracted from the Defense Enrollment Eligibility Reporting System (DEERS) was used to determine the monthly population of service members eligible for medical care and residing within a 20-mile radius of the MACH during the surveillance period. The data served as denominators for monthly incidence rate calculations. The Defense Manpower Data Center (DMDC) was also consulted to determine the proportion of active duty members stationed at Fort Benning who were trainees for each month evaluated; similar estimates were not available for Reserve and Guard members. Additionally, aggregated laboratory summaries for all S. aureus-positive cultures collected at the MACH were reviewed to determine the proportion attributable to MRSA and, in turn, the burden attributable to CA-MRSA in the study population.
Descriptive analyses of data gathered were performed; case demographics were restricted to those recorded at the time of the initial or incident infection. Monthly CA-MRSA rates were calculated. Trends in provider antibiotic prescription practices were evaluated based on published efficacy of antibiotic therapies; antibiotics active against >60% of isolates were considered effective for the purposes of the analysis [Reference Gilbert23]. Antibiotic susceptibility patterns of the CA-MRSA isolates collected were examined for antibiotics with efficacy averaging >60% during the study period.
RESULTS
More than 27 000 trainees pass through Fort Benning for BMT each year with a training cycle of about 9 weeks. Advanced individual training (AIT) and one-stop unit training (OSUT) which combines BMT and AIT requirements also occurs at Fort Benning and typically last 14 weeks. About 17 000–24 000 medically eligible service members and trainees were determined to reside within the designated 20-mile catchment area of Fort Benning's MACH in a typical calendar month and trainees comprised about one quarter of this population. Service members and trainees were overwhelmingly Army active-duty soldiers as opposed to service members from other branches of the U.S. military.
From 2002 to 2007, a total of 6560 S. aureus isolates was collected from the target population and 4309 (65%) of these were identified as MRSA. This proportion peaked at about 73% in 2005, and declined to 67% in 2007. A total of 3531 MRSA isolates (82%) met the case definition of CA-MRSA (Fig. 1). Monthly rates of CA-MRSA reached 7·2 cases/1000 soldiers in October 2005 as annual rates peaked at around 41·4 cases/1000 in 2005 (Fig. 2). Rates declined thereafter, but remained relatively high with more than 35 cases/1000 soldiers each subsequent calendar year. Seasonal CA-MRSA infection patterns were observed with increases beginning around July and decreasing around September with the exception of 2005 which began in August and diminished after October.
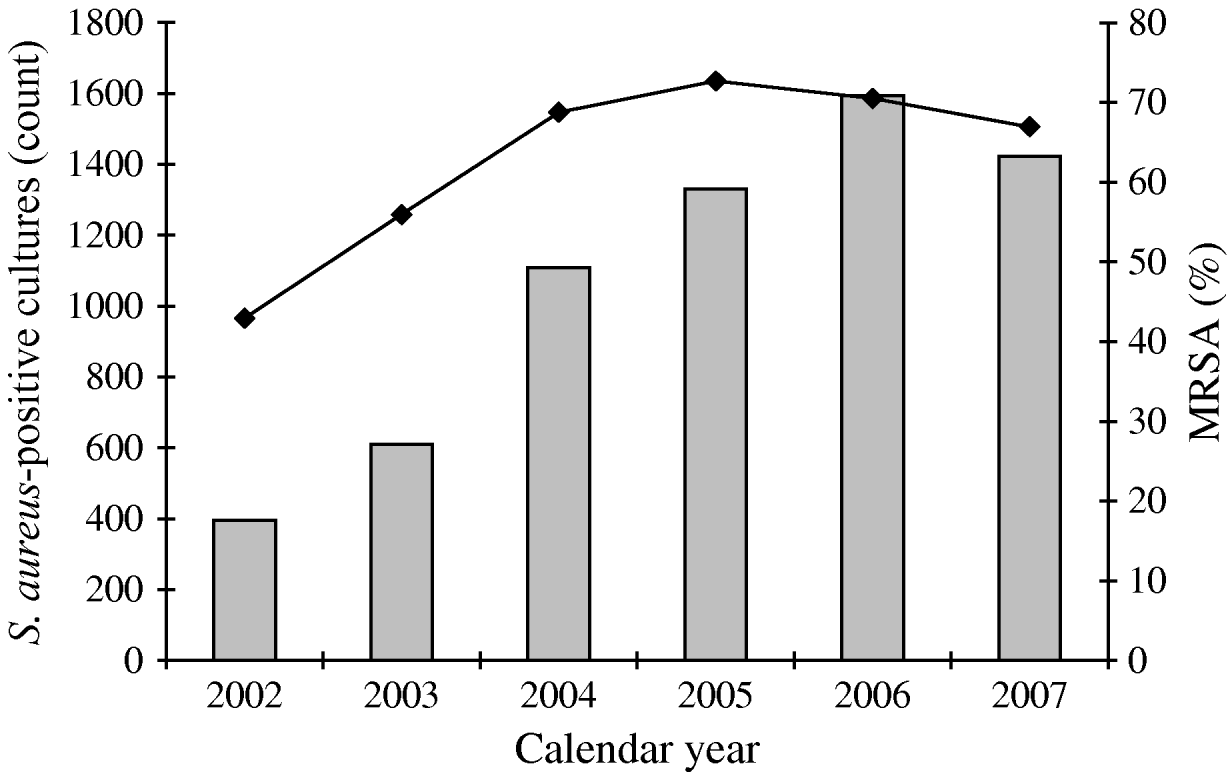
Fig. 1. Proportion of positive S. aureus isolates identified as MRSA, Fort Benning, January 2002 to December 2007 (unknowns excluded from percentiles). A total of 6560 S. aureus-positive isolates (![]() , military and non-military beneficiaries); 4309 MRSA (–◆–, hospital and community acquired) isolates identified of which 3531 were determined to be CA-MRSA in military service members/trainees.
, military and non-military beneficiaries); 4309 MRSA (–◆–, hospital and community acquired) isolates identified of which 3531 were determined to be CA-MRSA in military service members/trainees.
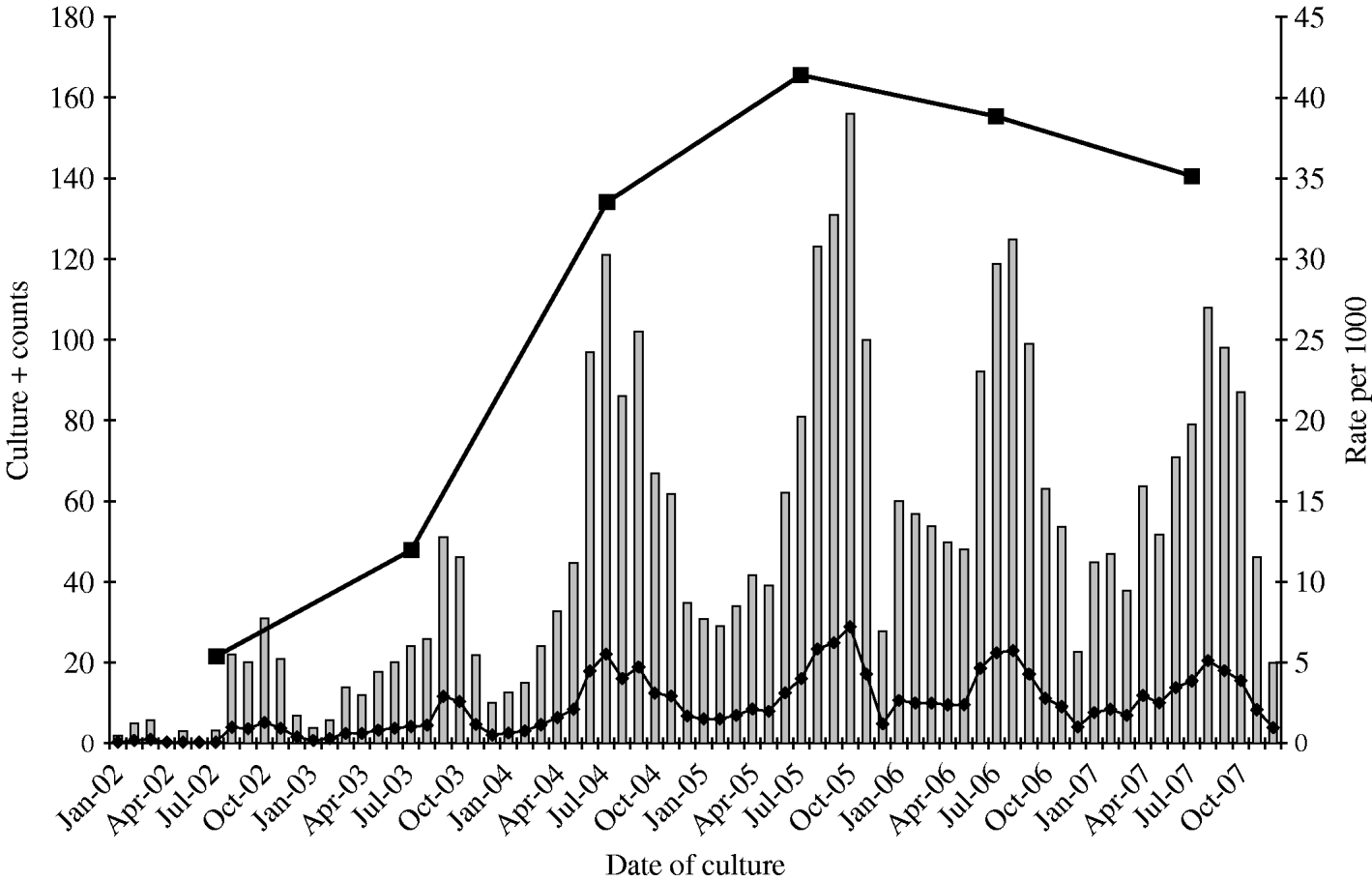
Fig. 2. Monthly CA-MRSA rates in Fort Benning service members and trainees, January 2002 to December 2007. ![]() , Frequency; –◆–, monthly rate/1000; –▪–, annual rate/1000 (annual rate displayed at mid-year).
, Frequency; –◆–, monthly rate/1000; –▪–, annual rate/1000 (annual rate displayed at mid-year).
Table 1 shows that a total of 3175 individual CA-MRSA cases was identified over the 6-year period. Some cases had multiple CA-MRSA infections (1–5 infections), equating to the yield of 3531 isolates. Cases were predominantly men (97·7%; women are not trained at this site), aged <25 years (76·6%), and trainees with 14-week infantry training brigade (ITB) requirements (58·2%) at the time of initial infection. Wounds occurred predominantly on lower and upper extremities (40·9% and 29·4%, respectively). Skin at joints (e.g. knees and elbows) was the main area affected within each respective extremity, accounting for 15·7% and 9·9% of infections, respectively (Table 2). An average of three patient visits (±2 visits, range 1–30 visits) were associated with infection during the 6-year period, representing a total of 10 854 patient visits. Military disposition data, which was collected from 2005 to 2007, indicated that about 39% of infections resulted in limited duty profile lasting for an average of 5·3 days (±3·8 days, range 1–36 days), with a cumulative total of 5046 limited duty days for the 3-year period. A total of 354 (10%) infections required hospitalization during the 6-year study, with a mean duration of 4·6 days (±3·3 days, range 1–33 days) (Table 3).
Table 1. CA-MRSA case demographics (n=3175)Footnote *
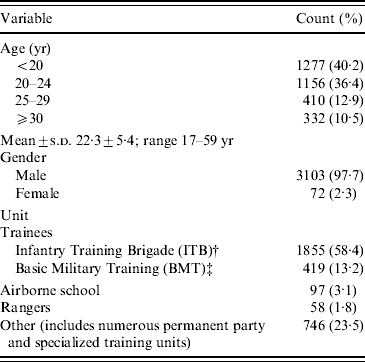
* Demographics based on incident infection; multiple infections per case were observed with 1–5 infections per case for a total of 3531 infections.
† ITB lasts 14 weeks; includes the units of the 198th and the 2/54 OSUT unit.
‡ BMT lasts 9 weeks; includes the units of the 192nd and the reception station.
Table 2. CA-MRSA wound locations (n=3531)Footnote *

* A total of 3175 cases – multiple wound sites per case were observed.
† Partial listing of common wound sites identified.
‡ Percentages based on exclusion of 101 unknowns.
§ Upper vs. lower arm regions could not be quantified.
Table 3. CA-MRSA clinical management (n=3531)
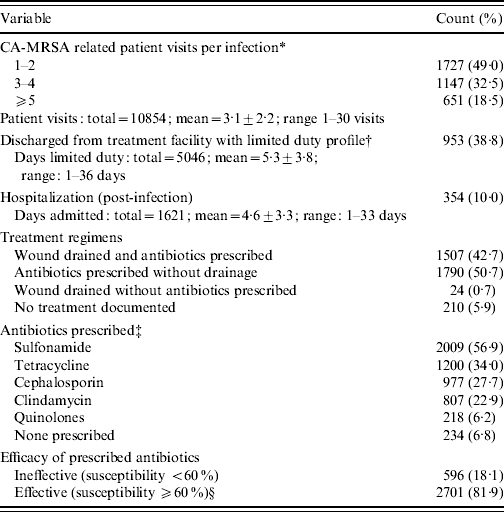
* Represents all visits completed for care related to the CA-MRSA infection prior to the date of laboratory confirmation.
† Profile data collection began on 1 January 2005; 2456 infections documented from 2005 to 2007, all other data from 2002 to 2007.
‡ Partial listing of antibiotics prescribed; multiple antibiotics may have been prescribed per case.
§ Anyone given at least one effective antibiotic was assigned to this group.
Antibiotics, alone or in combination with wound drainage, were the treatment options of choice. Approximately half of the cases were prescribed antibiotics alone, 43% received antibiotics plus wound drainage, and 1% had wound drainage without antibiotics; 6% of the cases had no documented treatment. Of patients prescribed antibiotics, 82% received antibiotics classified as effective for the purposes of this study (Table 3). The use of effective antibiotics improved over time, with a significant shift in prescription of clinically effective antibiotics beginning in 2005 (Fig. 3).
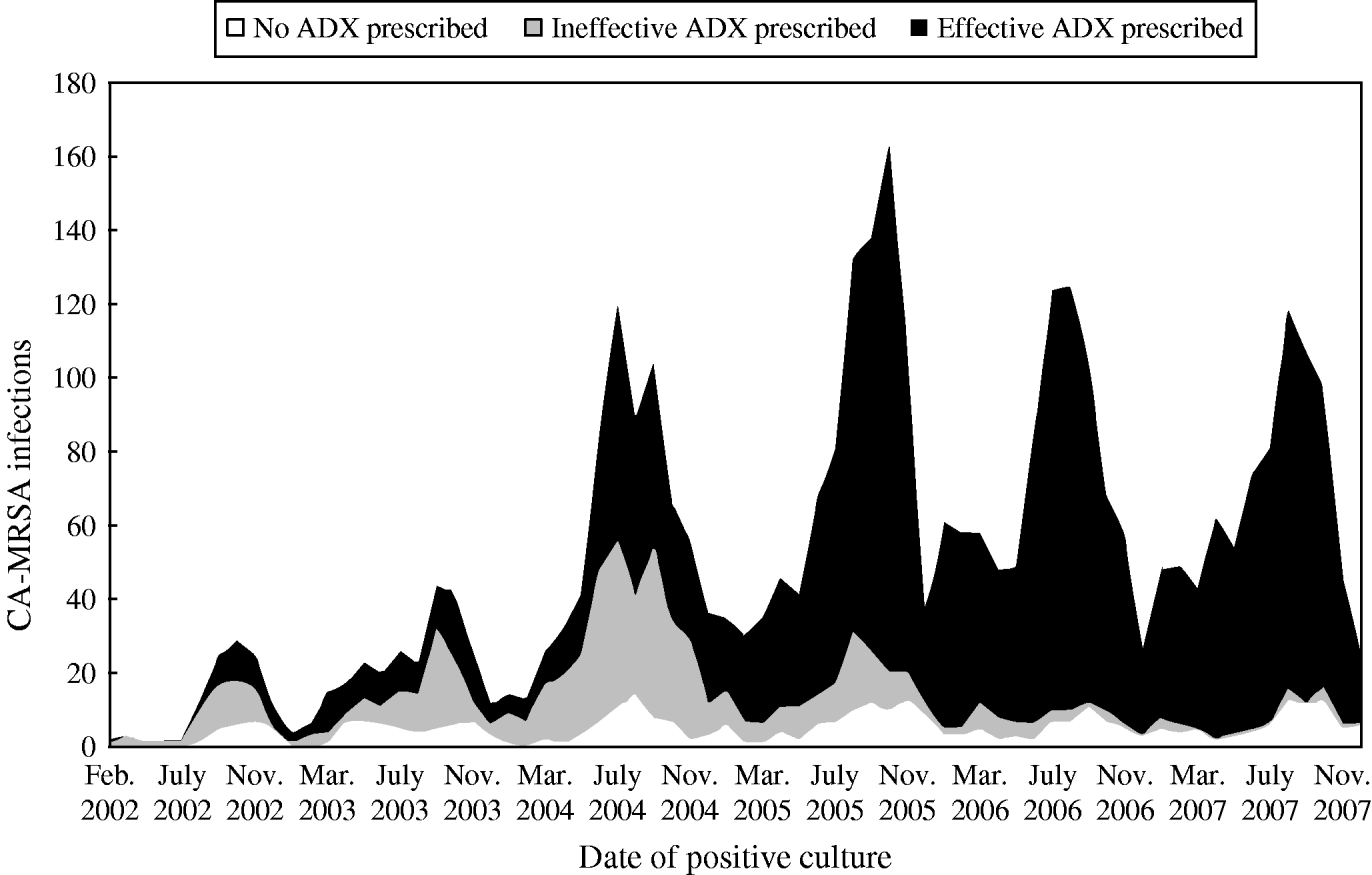
Fig. 3. Antibiotic prescription patterns for CA-MRSA infections. □, No antibiotics; ![]() , ineffective antibiotics prescribed; ▪, effective antibiotics prescribed.
, ineffective antibiotics prescribed; ▪, effective antibiotics prescribed.
Antibiotic susceptibility of CA-MRSA wound isolates was evaluated for 24 antimicrobials from 2002 to 2007. Overall, CA-MRSA were susceptible to 11 of the agents including vancomycin (100%), trimethoprim–sulfamethoxazole (TMP–SMX) (99·8%), rifampin (99·3%), chloramphenicol (98·9%), gentamicin (96·3%), tetracycline (91·8%), clindamycin (90·5%), gatifloxacin (78·2%), levofloxacin (66·3%), and ciprofloxacin (62·3%). Isolate susceptibility trends remained fairly stable over the 6-year period for vancomycin, rifampin, chloramphenicol, and TMP–SMX; however, variations in susceptibility to the remaining 11 antibiotics were observed (Fig. 4). Of note, overall susceptibility to clindamycin, ciprofloxacin, and levofloxacin decreased from 2002 to 2007 by 6%, 17%, and 14%, respectively.
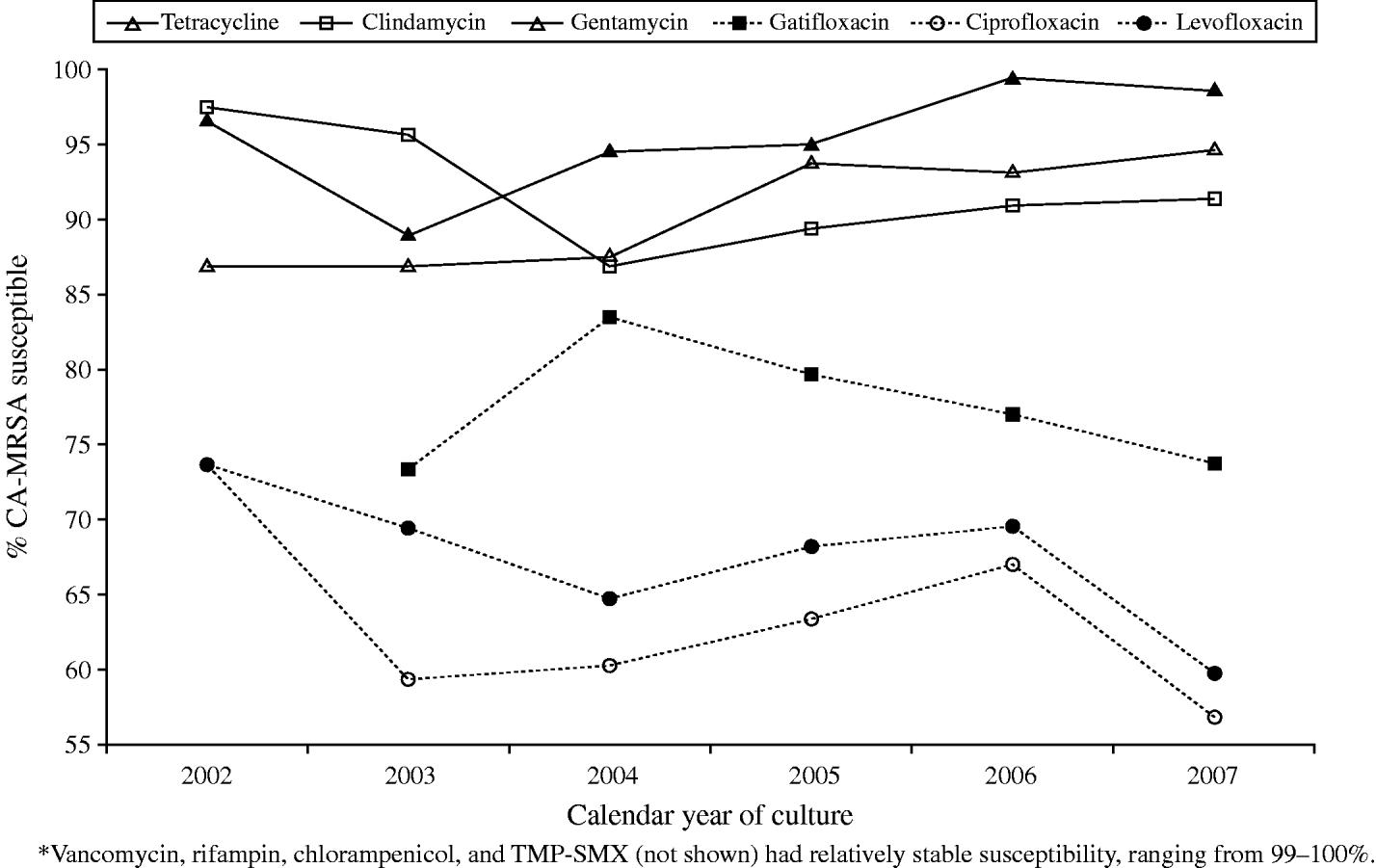
Fig. 4. Variations in CA-MRSA antibiotic susceptibility, 2002–2007. Vancomycin, rifampin, chloramphenicol, and TMP–SMX (not shown) had relatively stable susceptibility, ranging from 99% to 100%.
DISCUSSION
This study documents the persistent burden of CA-MRSA in medically eligible service members and trainees stationed at Fort Benning, Georgia. Estimates of the CA-MRSA disease burden in the general population and in high-risk settings such as this are lacking so it is difficult to generalize the results of the study; however, the sustained high levels of CA-MRSA observed in this population approximate those reported during outbreaks at other U.S. military bases [Reference Zinderman21]. The burden associated with CA-MRSA infection was considerable as measured by number of clinic visits, number of days with limited duty, and number of days hospitalized. While anticipated, the clustering of cases in trainees observed is worth noting; more than two-thirds of identified cases occurred in this group which comprised roughly one-quarter of the study population each month.
Results were consistent with the medical literature with regard to CA-MRSA patient characteristics, seasonality, and increasing levels of resistant S. aureus isolates. Cases identified were primarily young and healthy, and most infections were soft tissue skin infections, usually occurring on the extremities [Reference Beilman9]. High levels of β-lactam resistance in S. aureus isolates were also observed, with MRSA rates stabilizing at about 67%. Similar increases within civilian and military settings have been reported [Reference Baum8, Reference Chambers15, Reference Herman24].
Clinical management of the CA-MRSA cases identified primarily involved prescription of antibiotics and/or wound drainage. Substantial improvements in clinician antibiotic choice were documented over time, with increasing use of antibiotics active against these strains. The increase may be partially attributable to heightened awareness in healthcare providers of the predominance of MRSA in S. aureus isolates and/or reports of CA-MRSA. About 6% of cases had no documented treatment as mild cases of infection did not warrant aggressive therapy. In fact, a treatment algorithm of Herman et al. [Reference Herman24] showed education on hand washing and wound drainage was the only treatment needed for most wounds; mild infections only required treatment with a topical ointment or one of three antibiotics (TMP–SMX, clindamycin or tetracycline).
The antibiotic susceptibility patterns of the isolates were generally consistent with those reported for CA-MRSA in the literature [Reference Herman24–Reference Gupta26]. The increasing resistance to clindamycin observed here is also consistent with published reports and treatment guidelines. Current military treatment guidelines dictate that clindamycin should not be used alone for life-threatening infections until inducible resistance is ruled out (i.e. after confirmation of clindamycin sensitivity using the double disk diffusion test) [22, Reference Braun27]. Continued monitoring of susceptibilities in concert with treatment outcomes is needed.
There are some caveats to the study that should be considered. First, accurate determination of CA-MRSA as opposed to HA-MRSA was not possible given lack of genotyping of isolates collected. Therefore, CA-MRSA case definitions were epidemiological in nature, relying on documented prior hospitalization. This method is becoming increasingly problematic as HA-MRSA spill over into the community and outbreaks of CA-MRSA in hospitals are increasing. It should also be noted that the increasing rates of S. aureus infections reported in this study may be due in part to enhanced surveillance efforts as well as an increase in performing wound cultures. It was not possible to determine the extent to which surveillance artifact influenced the trends noted. Comparisons with national estimates are also problematic due to differences in surveillance methodology. Despite increased surveillance, rates reported are probably an underestimation due to the fact that mild cases may fail to seek care; clinicians may opt to prescribe treatment without culturing the wound and infected service members may opt for medical care outside of the military health system, although the latter is expected to occur significantly less frequently in a training environment. Additionally, infections may be misdiagnosed as spider or insect bites [Reference Pagac28].
Another limitation of the data collected was that patient identifiers such as social security numbers were not available for the majority of cases identified. This prevented linkage of the data with additional medical and laboratory databases which would have enabled more robust analyses to include classification of invasive cases. Additionally, detailed demographic/denominator data by military units were not available; therefore, unit specific rates could not be ascertained, nor could rates be adjusted based on actual person-days spent at Fort Benning. Exposure and genotypic characterization information was also unavailable. Continued analysis of susceptibility patterns, to include genotyping, would illustrate the optimal therapy for the predominant isolate in this trainee population. Improvements in surveillance methods to include this type of information are needed to facilitate identification of risk factors for infection, identification of potential outbreak sources, and optimal therapy for the predominant CA-MRSA isolate in the trainee population.
Given the sustained high prevalence of CA-MRSA documented, consideration of proven counter-measures is warranted. In addition to effective clinical management, there are a number of critical public health interventions aimed at breaking the chain of transmission and preventing the introduction of new cases. These include educating caregivers, educating troops to identify potential MRSA infections, improved personal hygiene practices, and disinfection of common surfaces, particularly in training areas where increased risk of transmission has been observed. Within the training environment, time constraints limit soldiers' ability to maintain adequate personal hygiene. Command enforcement of rules to improve personal hygiene and increase disinfection of common surfaces could assist in the prevention of further cases of CA-MRSA, although further study is needed to elucidate how effective these interventions may be.
ACKNOWLEDGEMENTS
We thank Dr Darlene Burns, a former preventive medicine physician at the Martin Army Community Hospital (MACH), Fort Benning, GA, for bringing the issue to the attention of the U.S. Army Center for Health Promotion and Preventive Medicine (USACHPPM) Disease Epidemiology Program; Mr Daniel Gillis at the MACH, Fort Benning, GA, for provision of laboratory data regarding trends in proportion of susceptible isolates; Dr Steven Cersovsky, and Mr Paul Pietrusiak of the USACHPPM Directorate of Epidemiology and Disease Surveillance for review of the manuscript; Dr Andrew Plummer, Dr Steven Tobler, Dr Tim Lobner and Dr Samuel Jang, formerly of the USACHPPM, for guidance regarding study methodology and pharmaceutical classification; and Dr Wilkins from the Uniformed Services University of Health Sciences for critical review of the study proposal.
The opinions expressed are those of the authors and should not be construed as representing those of U.S. Army, the Department of Defense (DoD), or any designated DoD or military organization.
DECLARATION OF INTEREST
The study was conducted to assess the burden of CA-MRSA in service members at Fort Benning, GA, to inform leadership, and guide potential public health interventions. The analysis was conducted as public health surveillance under the mission and authority of the U.S. Army Center for Health Promotion and Preventive Medicine. According to 45 CFR 6.101/102, this activity does not constitute research, and institutional review board examination is not required. No external funding was used to conduct this investigation, and contents have been cleared for public release by the U.S. Army Center for Health Promotion and Preventive Medicine.









