INTRODUCTION
The aetiology of acute appendicitis, a common cause of surgical intervention in children and young adults, is largely unknown [Reference Walker and Segal1]. Dietary fibre intake [Reference Rendle Short2, Reference Arnbjörnsson3], luminal obstruction [Reference Wangensteen and Bowers4, Reference Arnbjörnsson and Bengmark5] and genetic factors [Reference Basta6, Reference Ergul7] have all been implicated in the development of the disease, while a number of infectious and parasitic organisms are known to involve the appendix and may occasionally result in appendiceal inflammation [Reference Lamps8]. More generally, it would seem that acute appendicitis is attributable to multiple aetiological factors that vary by patient [Reference Lamps8].
Interest in the possible involvement of common acute childhood infections in the aetiology of appendicitis can be traced to the early twentieth century [Reference Kelly9]. Since that time, case reports have described the occurrence of appendicitis in the clinical course of such diseases as chickenpox [Reference Camens10, Reference Goodman and Silverman11], measles [Reference Goodman and Silverman11–Reference Paik16], mumps [Reference Goodman and Silverman11, Reference Sandler and Finne17–Reference Lucas and Dias19], rubella [Reference Goodman and Silverman11, Reference Rabau, Avigad and Wolfstein20], scarlet fever [Reference Kelly9, Reference Goodman and Silverman11, Reference Brandman21] and whooping cough [Reference Goodman and Silverman11, Reference Brandman21]. While these and related reports have prompted speculation over the direct or indirect role of common childhood infections in the aetiology of acute appendicitis [Reference Lamps8, Reference Lucas and Dias19, Reference Rabau, Avigad and Wolfstein20, Reference Jackson22], and models of the pathological process in relation to viral infections have been proposed [Reference Jackson22, Reference Carr23], systematic population-based analyses of the hypothesized comorbid associations are lacking in the literature.
Drawing on a classic epidemiological dataset, assembled by the School Epidemics Committee of the United Kingdom's Medical Research Council (MRC) in the inter-war period, this paper presents a historical analysis of the association between outbreaks of each of six common acute childhood infections (chickenpox, measles, mumps, rubella, scarlet fever and whooping cough) and the recorded incidence of acute appendicitis in 27 English public boarding schools, 1930–1934. Our analysis demonstrates, apparently for the first time, evidence of a significant and positive association between the level of appendicitis activity and the recorded incidence of one infectious disease (mumps). Framed by perspectives on the infectious aetiology of acute appendicitis, further studies are required to determine whether the comorbid association described in this paper has a causal underpinning.
MATERIALS AND METHODS
Background to the data source
The history and operation of the MRC School Epidemics Committee, and its particular association with the work of Professor Major Greenwood and the MRC Statistical Unit at the London School of Hygiene and Tropical Medicine, is summarized elsewhere [24–27]. Originally conceived by Greenwood and colleagues as an extension of the principles of experimental epidemiology to ‘human controls’ [Reference Greenwood and Topley28, Reference Greenwood29], the Committee had been appointed in March 1929 to undertake the first comprehensive multi-centre study of common acute childhood infections and other ailments in the ‘semi-isolated communities’ of England's residential schools [24]. With the compliance of a core sample of 27 single-sex public boarding schools, supplemented by two naval schools and two public day schools, a card system for the continuous daily monitoring of disease activity in the entire pupil population of the 31 participating institutions was implemented in January 1930. Surveillance continued, albeit in a much-reduced sample of schools from the mid-1930s, until the sudden suspension of the Committee's activities with the outbreak of World War II in September 1939 [27].
During the decade-long period of surveillance, 1930–1939, the Committee amassed records of some 92 000 episodes of common infectious diseases (including chickenpox, diphtheria, influenza, measles, mumps, nasopharyngeal infections, poliomyelitis, rubella, scarlet fever and whooping cough) and other illnesses (including appendicitis, tinea and trauma) that resulted in ⩾1 day of absence from lessons [27]. Although we have been unable to trace the current whereabouts of the original data archive, at least part of which was lost in the war years [Reference Cheeseman25, Reference Cheeseman26], the Committee's 5-year interim report [24] includes termly counts and rates of recorded disease activity in the anonymized set of participating schools for the truncated period, 1930–1934, and we draw on this published data source in the present study. We supplement the published evidence with additional information on the participating schools as contained in the unpublished files of the MRC School Epidemics Committee [27].
The data
Of the 31 schools that were originally enrolled by the MRC School Epidemics Committee, we select the core set of 27 single-sex (18 boys' and 9 girls') public boarding schools for examination in the present paper. Figure 1 shows the location of the 27 schools in southern and central England, while Table 1 provides summary statistics of the pupils under surveillance at each institution. In total, the study cohort included 24 203 pupils (17 512 boys and 6691 girls), with an average termly school size of 422 pupils (range 73–815 pupils) and with about 90% of the cohort in the age range 13–17 years (average 14·8 years). For additional details of the study population, see [24].

Fig. 1. Location map of 27 English public boarding schools surveyed by the MRC School Epidemics Committee, 1930–1934. Boys' schools (shaded): (1) Bradfield College; (2) Cheltenham College; (3) Christ's Hospital; (4) Downside School; (5) Gresham's School; (6) Haileybury College; (7) Lancing College; (8) Leys School Sanatorium; (9) Malvern College; (10) Marlborough College; (11) Mill Hill School; (12) Oundle School; (13) Rugby School; (14) Sherborne School; (15) Stowe School; (16) Wellington College; (17) Winchester College; (18) Worth Preparatory School. Girls' schools (unshaded): (19) Cheltenham Ladies' College; (20) Godolphin School; (21) Malvern Girls' College; (22) Queen Anne's School; (23) Roedean; (24) Royal School; (25) St Felix School; (26) Sherborne School for Girls; (27) Wycombe Abbey School. Additional details of the schools are given in Table 1.
Table 1. Boarding schools included in the study of the MRC School Epidemics Committee, 1930–1934 [24, 27]

* See Figure 1 for locations of schools.
† Range excludes a small number of pupils aged >18 years.
‡ Range excludes a small number of pupils aged <10 years.
Data matrix formation
In the absence of the MRC School Epidemics Committee's original data archive, the selection of time unit for the present analysis (school term) is dictated by the data recording interval used in the Committee's surviving unpublished (annual) and published (5-yearly) reports. For each of the three academic terms of the traditional English school year, namely Lent (January–March), Summer (April–July) and Christmas (September–December), epidemiological information included in the Committee's 5-year interim report (1930–1934) was abstracted to form two related sets of [(3 terms×5 years×27 schools)−1=] 404-term matrices of disease activity. Here, the correction factor of −1 allows for the belated enrolment of one girls' school (Roedean) in the second term of the 15-term study period [24, 27]. The two sets of data matrices were:
(1) Data matrices 1: Common acute childhood infections. These matrices consisted of disease counts and disease rates per 100 population for a sample of six common acute childhood infections (chickenpox, measles, mumps, rubella, scarlet fever, whooping cough) for which a possible association with acute appendicitis has been described in the literature [Reference Camens10–Reference Jackson22];
(2) Data matrices 2: Appendicitis. These matrices consisted of counts and rates per 100 population for operated cases of appendicitis. The rationale for restricting the analysis to operated, rather than (operated+unoperated =) total, cases of appendicitis is outlined in the Data issues section, below.
Reported morbidity for the diseases included in data matrices 1 and 2 is summarized in Table 2.
Table 2. Twenty-seven English public boarding schools, 1930–1934: reported morbidity for six infectious diseases and appendicitis in data matrices 1 and 2 [24]
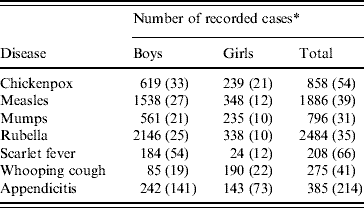
* The number of terms in the feasible set of 404 with recorded outbreaks of each of the six infectious diseases, and with recorded cases of appendicitis, is given in parentheses. For chickenpox, measles, mumps and rubella, all counts are based on terms with attack rates of ⩾1·0% in a given school. For appendicitis, scarlet fever and whooping cough, all counts are based on terms with recorded cases.
Data issues
The quality of the information included in the data matrices is reviewed elsewhere [24, Reference Cheeseman25]. While standardization of disease reporting across the set of schools was promoted by the implementation of an alpha-numeric system of disease notification, and school medical officers were paid a modest stipend for their assistance, spatial and temporal variations in the recognition, diagnosis and recording of the diseases are suspected. In all instances, disease recording was limited to clinical cases of illnesses that resulted in ⩾1 day of absence from lessons while, for four diseases included in the present study (chickenpox, measles, mumps, rubella), available information was restricted to school terms with recorded attack rates of ⩾1·0% in the population exposed to risk. As regards the appendicitis data, special concern attaches to the possible misdiagnosis of acute attacks of abdominal pain that mimic appendicitis [Reference Lau30]. While the MRC School Epidemics Committee recommended that epidemiological analysis should be limited to appendicitis cases that were considered sufficiently severe to require surgical intervention [24], and we focus on operated cases of appendicitis in the present paper, the inclusion of an unknown proportion of negative appendectomies cannot be ruled out [Reference Lau30, Reference Hale31]. Under these circumstances, all results are subject to the caveat of data quality.
Data analysis
Two analytical approaches to the identification of associations between the occurrence of a given infectious disease (data matrices 1) and operated appendicitis (data matrices 2) are adopted in the present paper: (i) difference of means tests and (ii) multivariate negative binomial (NB) regression. We outline each approach in turn.
Difference of means tests
This approach compares the recorded levels of appendicitis activity in school terms with and without recorded outbreaks of the infectious disease of interest. Taken relative to terms without outbreaks of the infectious disease, raised levels of appendicitis activity in outbreak terms would be consistent with a positive comorbid association. Conversely, lowered levels of appendicitis activity in outbreak terms would be consistent with a negative comorbid association.
For a given school i, we denote terms with recorded cases of infectious disease d in data matrices 1 as T id (‘outbreak terms’) and terms with no recorded cases as T id/ (‘no outbreak terms’). For each group of terms {Tid} and {T id/}, corresponding groups {A id} and {A id/} of appendicitis case counts and case rates were formed from data matrices 2. Differences in the {A id} and {A id/} for a given d were then examined for the entire set of 27 schools using the t test for unequal variances [Reference Ruxton32]. Here, our selection of the unequal variance t test was determined by the identification of non-homogeneous sample variances in preliminary analysis. All tests were two-tailed.
For reference, Table 2 gives the total number of outbreak terms for each of the six sample infectious diseases in the set of 27 schools and in the subsets of boys' and girls' schools. The equivalent information for terms with recorded cases of appendicitis is also given.
NB regression
One important limitation of the foregoing approach is that it takes no account of potential sources of confounding that may arise from the systematic effect of such factors as school, year and season on reported levels of disease activity. This class of problem is handled in epidemiological cohort studies by the use of a generalized linear model (GLM) with appropriate (e.g. Poisson or NB) response and offset for rate data [Reference Hardin and Hilbe33].
Model selection
Of the several models that were initially considered for the evaluation of the appendicitis data (Poisson, zero-inflated Poisson, NB and zero-inflated negative binomial (ZINB); see [Reference Hardin and Hilbe33]), the NB was chosen for the current analysis. Here, model selection was based on evidence of extra-Poisson variation in the data, thereby necessitating a relaxation of the assumption of equidispersion in the Poisson modelling procedure. Given the relatively large number of observation units (school terms) with zero appendicitis counts, parallel analysis with a ZINB model was undertaken as a statistical check on the appropriateness of the NB model.
Model specification
For the set of school terms t (t=1, …, 15) in each of the participating schools i (i=1, …, 27), let Y it represent the count of reported appendicitis cases, P it the count of the student population and D it the duration of the term (in weeks). Then we specify the model
where log(P it×D it) is the offset variable used in the calculation of the appendicitis rate, X 1it−X 6it are the log-transformed rates for each of the six infectious diseases (chickenpox, measles, mumps, rubella, scarlet fever, whooping cough) and X 7it−X 9it are categorical variables that control for the potential confounding effects of school (X 7it, 27-level factor), year (X 8it, 5-level factor) and calendar term (Lent, Summer, Christmas) as an index of seasonality (X 9it, 3-level factor). Finally, β0−β9 are the regression coefficients to be estimated.
Equation (1) was evaluated for the standard (NB) and zero-inflated (ZINB) negative binomial models with robust standard errors. Model fitting was undertaken using SPSS Release 17.0 (SPSS Inc., USA) and Stata Release 10.1 (StataCorp, USA).
RESULTS
Difference of means tests
Interval plots of appendicitis activity in the groups of outbreak and no outbreak terms for each infectious disease are shown for the set of 27 schools in Figure 2a (appendicitis cases) and Figure 2b (appendicitis case rates). The results of the unequal variance t test for each outbreak/no outbreak pairing are summarized in Table 3. The table gives the number of outbreak {T id} and no outbreak {T id/} terms and the corresponding mean levels of recorded appendicitis activity, the estimated difference of means, the 95% confidence intervals (95% CI), as well as the computed t statistics and their associated P values.

Fig. 2. Interval plots of appendicitis activity in 27 English public boarding schools. Open symbols (○) give the mean level of appendicitis activity in school terms with outbreaks/no outbreaks of each of six infectious diseases. Bars attached to the open symbols give the 95% confidence intervals for the mean appendicitis rate. (a) Appendicitis cases; (b) appendicitis case rates per 100 population.
Table 3. Twenty-seven English public boarding schools, 1930–1934: results of t tests for levels of appendicitis activity in relation to outbreaks of sample infectious diseases

* Tid, Outbreak terms for infectious disease d in school i; Tid /, no outbreak terms for infectious disease d in school i.
† Appendicitis case rates are expressed per 100 population.
Figure 2 and Table 3 identify a significantly raised level of appendicitis activity, as judged by both appendicitis cases (t=3·57, p<0·001) and case rates (t=2·81, P=0·005), in school terms with recorded outbreaks of mumps. With the exception of statistically marginal increments in appendicitis case counts in relation to outbreaks of whooping cough (t=1·85, P=0·066) and appendicitis case rates in relation to outbreaks of rubella (t=1·79, P=0·074), no significant differences in recorded levels of appendicitis activity were identified for the remaining outbreak/no outbreak pairings.
NB regression
Notwithstanding the relatively large number of observation terms (190) with zero appendicitis counts, the ZINB model did not offer a significantly better fit than the standard NB model (Vuong test: z=0·00, P=0·500) and we summarize the results of the standard NB modelling procedure here. The estimated regression coefficients β, 95% CIs and associated tests of variable effects (Wald χ2 and P values), along with a measure of the overall goodness-of-fit of the model (deviance), are given for the full model in Table 4. The model identifies a positive association between the termly appendicitis count and the mumps rate (β=0·15, 95% CI 0·07–0·24, P<0·001) when adjusted for school, year and season. No significant association is identified between the termly appendicitis count and the remaining five infectious diseases (chickenpox, measles, rubella, scarlet fever, whooping cough) in Table 4.
Table 4. Variables, coefficient estimates and tests of variable significance (Wald χ2 and associated P values) for a negative binomial model of the termly rate of appendicitis in 27 English public boarding schools, 1930–1934

* Control variables excluded. Goodness-of-fit of model: deviance=308·54, d.f.=397, deviance/d.f.=0·78.
† Control variables included. Goodness-of-fit of model: deviance=264·14, d.f.=365, deviance/d.f.=0·72.
Notwithstanding the inclusion of the school factor to control for individual school effects (e.g. population size and reporting completeness) in the NB modelling procedure, a statistical check for a putative size of school effect on reported levels of disease activity was undertaken by computing the correlation matrix between the offset variable and the appendicitis and infectious disease rates. No significant associations between school size and disease rates were identified. This finding was maintained when one apparent outlier (Worth Preparatory School) was omitted from the analysis. The coefficient estimates for the NB model with the controls excluded (diseases-only model) are also shown in Table 4; the results confirm that the inclusion of controls does not have a substantive affect on the overall findings of the analysis.
DISCUSSION
Historical studies of the disease records of institutions can provide valuable perspectives on the epidemiology of medical conditions of unknown or uncertain aetiology [Reference Lancaster34, Reference Lancaster35]. The present analysis has drawn on a classic epidemiological data-set, originally assembled by Major Greenwood and colleagues of the MRC School Epidemics Committee in the 1930s, to examine the association of sample infectious diseases and acute appendicitis in the institutional setting of 27 English public boarding schools. While epidemiological aspects of the school environment are considered elsewhere [24, Reference Joseph36], we note here that our selection of institution was predicated on the existence of large and well-defined aggregates of children and adolescents for whom term-time population mixing was largely restricted to the close confines of the school. The MRC Committee deemed the pupils to be drawn from broadly comparable (‘middle’ and ‘upper-middle’ class) socio-economic backgrounds while, by virtue of their age and medical histories, they were regarded as highly susceptible to common acute childhood infections and other afflictions (including appendicitis) at the time of school entry [24, Reference Cheeseman25].
The 5-year period encompassed by the present study (1930–1934) corresponds with the high plateau of a national ‘epidemic’ of acute appendicitis in children and young adults that had first manifested in England and Wales during the late nineteenth century, and which fell away steeply from the late 1930s [Reference Young and Russell37, Reference Barker38]. Our selection of a period of high appendicitis incidence, several decades prior to the introduction of mass vaccination against many common childhood diseases, has provided us with an opportunity to search for statistical associations that may have been masked by lower levels of disease activity in later time periods.
While an aetiological link with dietary fibre intake has been hypothesized to account for the epidemic rise of appendicitis [Reference Rendle Short2, Reference Burkitt39], early speculation over an infectious aetiology of the disease [Reference Kelly9] has been revived in recent decades [Reference Walker and Segal1, Reference Barker38, Reference Martin and Gustafson40–Reference Guo42]. In addition to a range of viruses (e.g. adenovirus, Epstein–Barr virus), bacteria (e.g. Salmonella spp., Yersinia spp.), fungi (e.g. Histoplasma capsulatum) and parasites (e.g. schistosomes) that are known to involve the appendix and which may occasionally cause appendiceal inflammation [Reference Lamps8], case reports have described the occurrence of appendicitis in the clinical course of such classic childhood infections as chickenpox, measles, mumps, rubella, scarlet fever and whooping cough [Reference Goodman and Silverman11–Reference Brandman21]. With the notable exception of measles [Reference Whalen13–Reference Paik16]; however, evidence for the involvement of the latter infections in the causation of appendicitis has received little attention in the post-war literature.
Subject to the increased risk of type I experimental errors in multiple hypothesis testing [Reference Ottenbacher43], the present study has identified a strong and positive association between the recorded level of appendicitis activity and mumps in the 27 boarding schools under analysis (Table 3). The association is maintained when NB regression is used to control for the potential confounding effects of school, year and season (Table 4). While certain complications of mumps (e.g. right-sided orchitis and oophoritis) can mimic appendicitis, and this may have resulted in the inclusion of an unknown number of negative appendectomies in the present analysis, our findings are consistent with numerous confirmed reports of acute appendicitis in the clinical course of mumps [Reference Sandler and Finne17–Reference Lucas and Dias19, Reference Finch44]. Our findings are also consistent with the raised incidence of confirmed appendicitis in patients with mumps vis-à-vis other common childhood infections in the historical records of one US contagious diseases hospital [Reference Goodman and Silverman11] and with the ‘bizarre and difficult to explain’ observation of raised antibody titres to mumps virus in children with appendiceal disease [22, p. 713]. The direct or indirect role of mumps virus in the causation of appendicitis, including the possibility of a metastatic involvement akin to mumps-related pancreatitis and orchitis, was postulated by Sandler & Finne [Reference Sandler and Finne17] and Donnelly & Oldham [Reference Donnelly and Oldham18] in the early 1930s and, more recently, by Jackson and colleagues [Reference Jackson22] in the 1960s. We note, however, that the putative relationship between mumps and appendicitis is still little known [Reference Lucas and Dias19] and, on the basis of the present findings, merits further investigation.
Our analysis has failed to identify an association between recorded levels of appendicitis activity and the occurrence of three viral (chickenpox, measles, rubella) and two bacterial (scarlet fever, whooping cough) diseases (Tables 3 and 4). The findings for measles are especially surprising given the known participation of the appendix in measles virus infection [Reference Lamps8] and the recognition of acute appendicitis as a gastrointestinal complication of measles [Reference Whalen13–Reference Paik16, Reference Perry and Halsey45]. In contrast, our findings for chickenpox, rubella, scarlet fever and whooping cough are consistent with the relatively few published reports of acute appendicitis in the clinical course of these diseases [Reference Kelly9, Reference Camens10, Reference Goodman and Silverman11, Reference Brandman21].
It is important to emphasize that our findings in Tables 3 and 4 are based on a spatially and temporally disaggregated analysis of the available data, and the associations that we report may not be evident at the aggregate level of the 27 institutions. So, although the Lent Term of 1931 is noteworthy as a period of pronounced mumps activity across the set of schools (101 reported cases), the corresponding appendicitis count (21 reported cases) approximates the termly average for the 5-year observation period. This apparent lack of temporal association may reflect the limited size of the several institutional outbreaks of mumps in this term, with all seven falling at or below the median rate for the entire observation period. More generally, for the period 1930–1934, the average appendicitis rate was markedly higher in school terms with mumps outbreaks of above median (0·61 appendicitis cases per 100 population) than below median (0·37 appendicitis cases per 100 population) size.
One noteworthy feature of Table 4 is the identification of school as a significant factor in the NB regression model. While inter-school variations in recorded levels of appendicitis activity may reflect a broad range of influences (e.g. size, demographic base, immunity levels and monitoring and reporting practices of individual institutions), we note here that, as all the schools are single-sex, gender is embedded in the school factor. Recognizing that gender is entirely confounded by school, the effect of gender on the association between appendicitis and infectious diseases is not identifiable in the current analysis.
We emphasize that the data derived from the MRC School Epidemics Committee's survey are subject to a number of limitations. First, case data are unlinked across the diseases; the available data do not permit assessment of the infection status of appendicitis cases, and the results presented here relate solely to associations in the recorded patterns of disease activity. Second, the time unit of analysis adopted in the current study (school term) has been dictated by the data recording interval used in the Committee's surviving reports. In the absence of the Committee's original data archive, it is impossible to determine whether the term-based results are replicated on finer temporal intervals (e.g. monthly or half-termly periods). Third, as described in the Materials and Methods section, the quality of diagnosis and the completeness of recording of diseases are likely to have varied in space and time in an unknown manner, while the loss of case data in consequence of the home treatment and convalescence of acutely ill children cannot be excluded. Fourth, to avoid the inclusion of a large number of uncertain or doubtful cases of appendicitis, we have restricted our consideration to reported cases that were considered sufficiently severe to require surgical intervention. Even then, we recognize the considerable difficulties associated with the differential diagnosis of appendicitis; reported case totals may include an unknown number of diagnoses that were not pathologically confirmed and which may serve as potential confounders in the analysis.
Additional data issues also assume importance. With reference to our sample infectious diseases, we note that the exclusion of cases of subclinical infection and milder clinical illness (<1 day of absence from lessons) places limits on the analysis. This factor is especially important for a disease, such as mumps, where a large proportion (~33%) of infections may be inapparent [Reference Heymman46] and where the presence of the infection in a given school term may have gone unnoticed. Finally, notwithstanding the overall size of our study cohort (>24 000 pupils), we note that the identification of associations may have been limited by the unknown, but feasibly considerable, rarity of the comorbid phenomena under examination.
CONCLUSION
To our knowledge, the current paper is the first systematic population-based assessment of the comorbid association between a range of common childhood infections and acute appendicitis in a multi-institutional cohort of high-risk school pupils. In his early twentieth-century appraisal of the epidemic rise of acute appendicitis in Britain, Rendle Short drew attention to the apparent lack of an association between acute appendicitis and the epidemic incidence of measles, mumps and other ‘zymotic diseases’ in a public school in Bristol [Reference Rendle Short2]. Drawing on a much larger set of 27 public schools in the 1930s, we have demonstrated – apparently for the first time – evidence of a significant and positive association between reported levels of appendicitis activity and the occurrence of mumps. Consistent with numerous case reports of acute appendicitis in the clinical course of mumps, the possible role of mumps virus in the aetiology of acute appendicitis would seem to warrant further investigation.
ACKNOWLEDGEMENTS
We are grateful to the anonymous referees for their helpful comments on an earlier version of this paper.
DECLARATION OF INTEREST
None.








