Introduction
Influenza is an acute respiratory illness caused by influenza viruses and is responsible for outbreaks and epidemics associated with significant morbidity and mortality worldwide. Influenza A, B and C are the three types of viruses known to infect humans. Of these, types A and B are the major human pathogens. The most extensive and severe outbreaks of influenza are caused by the influenza A virus, in part due to its remarkable ability to undergo periodic antigenic variations (antigenic drift) and antigenic shifts, which can be associated with pandemics [Reference Dolin, Longo, Fauci, Kasper, Hauser, Jameson and Loscalzo1]. It is estimated that each year, about 3–5 million cases of severe illness and about 290 000–650 000 of respiratory deaths are caused by influenza epidemics [2].
Influenza types and subtypes may have different impacts on morbidity and mortality, with yearly variations. Influenza-related morbidity is highly dependent on the age of the patient and the contracted strain; novel antigenic variants were linked in the past with an impact on all age groups [Reference Olson3]. Adults over the age of 65 and young children are among the high-risk populations associated with disease complications [Reference Thompson4].
Symptoms of influenza virus infection usually appear after an incubation period of 1–2 days and include abrupt onset of fever, headache, myalgia and malaise, accompanied by manifestations of respiratory-tract illness, such as cough, sore throat and rhinitis; manifestation may vary between paediatric patients and adults [Reference Silvennoinen5–Reference Paules and Subbarao7]. The primary antiviral agents recommended for the prevention and treatment of influenza are oseltamivir or zanamivir, to which most strains of influenza A and B are susceptible [8]. For prevention, it is also recommended that an annual influenza vaccination be given for everyone 6 months of age or older [Reference Uyeki9]. The viral strains in each year's vaccine are based on predictions and calculations in order to try and match the circulating viruses.
In late March 2009, a novel influenza A/H1N1 outbreak was reported in Mexico, later spreading rapidly and leading to a worldwide pandemic [10]. Following its occurrence in 2009, several reports were published, comparing characteristics of seasonal influenza and 2009 pandemic influenza A/H1N1. Some of these reports found pandemic influenza A/H1N1 to have increased morbidity and mortality while others found it to have similar or less severe outcomes [Reference Esposito11–Reference Stein17]. Since then, the 2009 pandemic influenza A/H1N1 strain became a seasonal strain circulating in successive winters.
The major influenza strains recently responsible for morbidity in Israel, according to the Israel Center for Disease Control (ICDC) are seasonal influenza A/H3, 2009 A/H1N1 and influenza B, with significant yearly variations [18–22]. In recent years, a few reports were published describing influenza epidemiology and clinical manifestations while considering the possible clinical and epidemiological differences between the common strains. Most of these reports focused on the 2009 pandemic and included either adults or children. Mixed results regarding the severity of the 2009 H1N1 strain were evident: some reports found worse outcomes in H1N1-infected children, while others reported a mild disease in the majority of cases [Reference Tasher23–Reference Koliou25]. In some studies conducted after 2009, significant increase in proportions of 2009 H1N1 patients with a severe disease in the first post-pandemic season, mostly in adults, was evident in Germany, while evidence from Denmark from first, second and third waves of pandemic influenza 2009 H1N1 showed that hospitalised patients were mostly children and younger adults [Reference Lehners26, Reference Ørsted27]. One study that reviewed clinical and epidemiological characteristics of severe cases of influenza in hospitalised patients from the 2013/2014 season in Canada found that most 2009 H1N1 patients were younger than 65 years [Reference Mcneil28]. In terms of influenza strain-related morbidity, there was evidence that 2009 H1N1 strain caused more severe disease than H3N2 or B in hospitalised patients in the USA in the 2010/2011 season [Reference Chaves29].
The purpose of this study was to characterise influenza manifestations in both children and adults who were admitted to three medical centres in northern Israel and were diagnosed with influenza in the winter of 2015–2016. This season was characterised by a relatively high number of A/H1N1 patients, alongside non-H1N1 influenza A and B patients. Particular emphasis was placed on the comparison between the clinical and laboratory characteristics of the major influenza strains circulating in Israel. An attempt was made to find distinguishing clinical factors for influenza A/H1N1. To the best of our knowledge, this is the first study in Israel of a post-pandemic influenza season to compare clinical manifestations of the three most common influenza types in both children and adults.
Materials and methods
Data collection and patient characteristics
Clinical, epidemiological and laboratory data were collected from the charts of hospitalised patients diagnosed with influenza, from December 2015 until March 2016, in three medical centres in northern Israel: Galilee Medical Center in Nahariya (GMC, 700 hospital beds, serving a population of 600 000), Ziv Medical Center in Safed (ZMC, 330 hospital beds, serving a population of 250 000) and Baruch Padeh Medical Center in Poriya (BPMC, 340 hospital beds, serving a population of 300 000). No official guidelines exist for the clinical diagnosis of influenza in these hospitals; physicians rely on compatible clinical symptoms with confirmation based on rapid real-time polymerase chain reaction (RT-PCR) testing performed several times a day. The diagnosis of influenza and subtype determination (A/non-H1N1, B and A/H1N1) was made based on a positive RT-PCR test in the GeneExpert™ system using the Xpert® Flu kit (Cepheid, Sunnyvale, California, USA) of nasal or nasopharyngeal swabs. It should be noted that all patients included in the study from GMC were under the age of 18, as opposed to ZMC and BPMC where patients from all age groups were included. For all participating subjects, data were collected from medical records and computerised laboratory software, and included both demographic data, such as age, gender and pregnancy status, and clinical data: signs and symptoms, possible complications, type of treatment, hospitalisation duration and in-hospital mortality. Laboratory data included confirmed virus type, and the leucocyte, lymphocyte, platelet and haemoglobin counts. The study was approved by the local ethics committees of GMC, ZMC and BPMC.
Statistical analysis
Microsoft Excel 2016 (Microsoft, Redwood, WA, USA) was used for data collection. SAS version 9.3 (SAS Institute, Cary, NC, USA) was used for data analysis. Differences in qualitative parameters were analysed by Analysis of Variance (ANOVA) model. Differences in the distribution of categorical parameters were analysed by χ 2 test. A P value < 0.05 was considered significant.
Results
Demographic data
The rates of various influenza types by age group, gender and in pregnant women are shown in Figure 1. During the study period, a total of 251 patients were diagnosed with influenza, of whom 128 (51%) were males, and 123 (49%) were females. The average age for ZMC and BPMC patients was 45.1 years (range: 0–94). Of the study population, 116 (46%) were under the age of 18 (children group), and 135 (54%) were 18 years old and above (adult group). In the adult group, 16 were pregnant women.
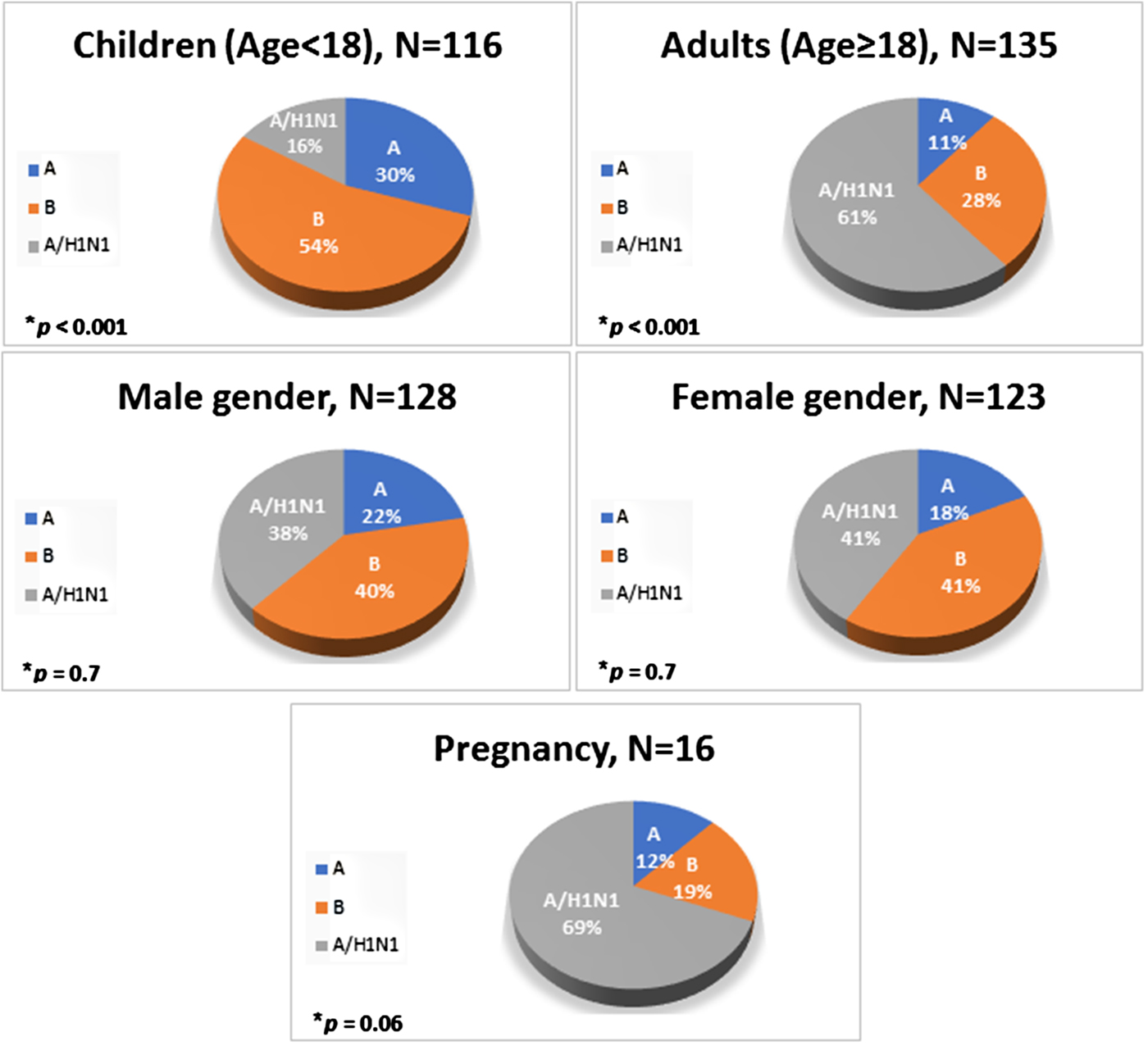
Fig. 1. Distribution of various influenza strains by age group, gender and in pregnant women.
Clinical and laboratory data
Influenza A/non-H1N1 was detected in 50 patients (20%), influenza B in 101 (40%) and influenza A/H1N1 in 100 (40%) (Fig. 1). Influenza B was the most prevalent type of influenza in children whereas A/H1N1 was the most common type in adults, including pregnant women (P < 0.001, Fig. 1).
The rates of various signs and symptoms in different influenza types and in different age groups are listed in Tables 1–4. The most prominent sign was cough, occurring in 70% of all patients, and more common in A/H1N1 patients, although the difference was not statistically significant (P = 0.08, Table 1). Cough and shortness of breath were more common in adults compared with children whereas gastrointestinal symptoms were more prominent in children (Table 2). Fever occurred in only 66% of patients (Table 1), and even though fever was more common in children compared with adults, this difference was not significant (P = 0.08).
Table 1. Signs, symptoms and clinical manifestations in different influenza types

MC, medical centre.
a Influenza A = A/non-H1N1.
b Duration in days.
Table 2. Signs, symptoms and clinical manifestations in different age groups

Table 3. Signs, symptoms and clinical manifestations in different influenza types for the adult group

a Influenza A = A/non-H1N1.
Table 4. Signs, symptoms and clinical manifestations in different influenza types for the children group

a Influenza A = A/non-H1N1.
Laboratory values in different influenza types and in different age groups are listed in Tables 1–4. The mean leucocyte count was significantly lower in patients infected with influenza B compared with influenza A/non-H1N1 (P = 0.005, Table 1); this finding was significant in children but not in adults (Tables 3 and 4).
Complications
The rates of various complications as well as hospitalisation data in different influenza types and in different age groups are listed in Tables 1–4. Pneumonia was found to be the most prominent complication (Table 1); it was more common in the A/H1N1 group (P = 0.002, Table 1) and in adults (P < 0.001, Table 2). In the children group, intensive care unit admission was more prevalent in A/H1N1 patients (P = 0.02, Table 4).
Mortality rates were 2% and 3% in children and adults, respectively (overall six patients, Table 2) with no significant differences in age groups or virus type. Patient mortality could not be linked with delayed antiviral therapy.
Treatment
Data regarding treatment in different influenza types and in different age groups are shown in Tables 1 and 2. The antiviral treatment was oseltamivir, given to 226 patients (90%), 96% of adults and 84% of children (P = 0.002, Table 2). Of patients treated with oseltamivir, the highest rate of treatment was given to patients infected with A/H1N1 (P = 0.04, Table 1). No differences were noted between adults and children or between viral types in the duration of symptoms until admission or until antiviral therapy was given. However, treatment was given relatively late during the course of influenza (mean = 3.6 days of symptoms until treatment). Only two patients (0.9%) experienced adverse events attributed to antiviral treatment.
The mean hospitalisation duration in this study was 4.2 days (range: 1–39). The duration was significantly higher in the A/H1N1 group (P = 0.04, Table 1) and in adults (P = 0.03, Table 2).
Discussion
This multicentre study described influenza manifestations in 251 patients, including children and adults who were admitted to three medical centres in northern Israel and were diagnosed with influenza in the 2015–2016 influenza season.
We found influenza B and A/H1N1 to be the major strains and equally frequent in patients, and influenza A/non-H1N1 to be the third, less common strain. This finding is different from the results of influenza surveillance done in Israel by the ICDC, which reported influenza B to be the most common subtype during the same season (56%), followed by A/H1N1 (43%) and influenza A/H3 (1%) [18]. In assuming the Xpert® Flu kit result of A/non-H1N1 correlates with the A/H3 strain detected by the ICDC, one possible explanation for this difference may be the fact that ICDC included all laboratory-confirmed influenza patients seen at the centre's sentinel clinics in various areas in Israel, while our study included only hospitalised patients in northern Israel, who may represent more severe disease in some of the influenza types. It is also possible that our study had a relatively higher percentage of children, who had higher rates of influenza B. Furthermore, it may be that the deployment of the sentinel clinics does not represent the country uniformly, which may give different results in some areas. It should be noted that the possibility of detection flaws due to factors related to the PCR kit is highly unlikely. The Xpert® Flu kit, which was used in all participating centres has a sensitivity of 100% for all three strains and a specificity of 98.6%, 95.7% and 99.6% for influenza A/non-H1N1, B and H1N1, respectively [30].
The most prevalent type of influenza in children was influenza B (54%); it was significantly more common than influenza A/non-H1N1 (30%) and influenza A/H1N1 (16%). Contrary to this, A/H1N1 was the most prevalent type in adults (61%), followed by influenza B (28%). During the 2009 pandemic, 45% of the 272 patients hospitalised with H1N1, in a study from the USA, were children under the age of 18 years [Reference Jain31]. Other studies that described the 2009 influenza A/H1N1 epidemiology found it to be more common in children and young adults; A/H1N1 infections in the older age groups were not common [Reference Jhung32, Reference Miller33]. It was speculated that a pre-existing immunity protected older age groups from A/H1N1 infection [Reference Miller33]. The constant exposure of children to the A/H1N1 virus in the 6 years that have passed since the 2009 pandemic may have led to an acquired immunity, which may explain the differences between our results and the 2009 studies. Alternatively, the virus may have changed since 2009 in a way that rendered the adult immunity to it ineffective. It could also be that the sample size of our study was not big enough to get to the same results.
In pregnant women, influenza subtypes overall were comparable to the adult subtypes with influenza A/H1N1 as the most prevalent type. Previous studies reported that pregnant women are more susceptible to influenza complications and have worse disease outcomes compared with the general population. This is attributed to changes in immunosuppression and respiratory and cardiovascular system physiology during pregnancy [Reference Jhung32, Reference Meijer34]. Worse outcomes for pregnant women infected with influenza A/H1N1 compared with a non-H1N1 influenza were reported but we found no differences in outcome in our study, possibly due to the small sample size of pregnant women [Reference Siston35].
Cough and fever were the most prominent signs of influenza in our population. However, we found cough to be more prominent in A/H1N1 patients. Past studies found a similar spectrum of clinical illness in both seasonal and pandemic influenza, with modest differences. However, gastrointestinal symptoms were found to be more common in A/H1N1 influenza, especially in children [Reference Carcione16, Reference Jhung32]. This observation did not correlate with our findings. Indeed, children presented with higher rates of gastrointestinal symptoms, but no significant association to a certain influenza strain was found in this age group. We found significantly lower leucocyte counts, but not lymphocytes, and lower platelet counts in children infected with influenza B compared with influenza A/non-H1N1. Indeed, a study conducted before the 2009 pandemic linked lower leucocyte counts in paediatric patients with influenza B compared with influenza A/non-H1N1 [Reference Shen36]. In addition, higher platelet counts were found among children compared with adults. These differences may be attributed to either a distribution difference of influenza types between the age groups or physiologic differences between children and adults, a possible result of the immaturity of the innate and/or adaptive immunity in children.
In terms of disease complications, we found that H1N1-infected patients had higher rates of pneumonia. In general, pneumonia and pleural effusion were more common in adults compared with children, but the increased rate of complications in adults cannot be attributed to a certain influenza type. In children, however, we found a correlation between H1N1 and increased rates of pneumonia and intensive-care admission. Indeed, in a 2009 study from Argentina, 2009 H1N1 influenza strain was found to have worse outcomes in children compared with seasonal influenza [Reference Libster12]. In a study from Israel, a significant part of children hospitalised for pneumonia during the 2009 pandemic were infected with 2009 H1N1, and these children displayed increased disease severity [Reference Tasher23]. In a study from the USA, of hospitalised H1N1 patients, 249 of 272 patients underwent chest radiography, of whom 40% had findings consistent with pneumonia [Reference Jain31]. Considering the fact that in our study, A/H1N1 infection was less common in children, a possible explanation for increased disease severity in A/H1N1-positive children could be that these children may have lacked pre-existing immunity to the A/H1N1 strain due to lack of prior exposure, and thus were more inclined to its complications.
Regarding antiviral treatment, the Israeli Ministry of Health recommended since 2009 universal treatment with oseltamivir to all hospitalised patients with influenza, preferably within the first 48 h of illness [37]. Our study demonstrated that a significantly smaller percentage of children were treated with antiviral therapy compared with adults. The time elapsed between first symptoms of influenza and the diagnosis was similar in adults and children, but children were hospitalised for a significantly shorter duration than adults, implying that by the time the diagnosis was made, some of the children have already improved, rendering antivirals unnecessary. In addition, we found that A/H1N1 patients were more likely to be treated compared with other influenza types. Although the reason for this is not completely clear, since the 2009 pandemic, the medical community has displayed higher concerns about A/H1N1 infections and the complications associated with them. It may be that these concerns and the higher rates of complications seen in our patients were driving doctors to pay particular attention to the treatment of patients infected with H1N1 compared with other influenza types.
This study provides new findings about type-specific predilection of influenza for various ages as well as type and age-specific complications, and highlights important differences in doctors' decisions on who to treat with antivirals, in a unique influenza season with three circulating types. Since we included all hospitalised patients with laboratory-confirmed influenza, regardless of clinical manifestations, we avoided the related sampling bias. Laboratory influenza confirmation was done using the same kit in all participating medical centres, which provides more reliable and uniform results. However, our study has several limitations. We evaluated a relatively small number of patients, which may have limited the strength of our findings. In one of the participating centres, we evaluated only children. This may have resulted in a selection bias when considering the differences found in influenza strains reported nationally in Israel for the same season. Finally, we evaluated only patients with confirmed influenza infection, and this group may not be representative of hospitalised patients with influenza who have not been tested.
Conclusions
In this study of hospitalised patients with influenza in the 2015–2016 season, we found, in contrast to the 2009 pandemic, that influenza A/H1N1 infected mainly adults while influenza B was the common type in children. Pneumonia was more common in A/H1N1 group and in adults. Antiviral treatment with oseltamivir was prescribed to most patients but was given relatively late in the course of the disease. A significantly smaller percentage of children, compared with adults, was treated with antiviral therapy; overall, patients infected with A/H1N1 had the highest rates of treatment. Influenza A/H1N1 is still an important strain responsible for significant morbidity. Strategies to improve early diagnosis of influenza and timely treatment with antivirals of all circulating strains are needed.
Acknowledgement
The authors wish to thank Mr Basem Hijazi for help with statistical analysis. Tomer Kalish: The article was written as part of the requirements of the Azrieli Faculty of Medicine, Bar Ilan University, for an MD degree.
Financial support
None.
Conflict of interest
None.







