INTRODUCTION
Hepatitis A virus (HAV) may cause hepatitis 2–6 weeks after exposure. Infection and vaccination generally result in long-term immunity. In children, HAV infection is often asymptomatic. Symptoms of infection are more severe in the elderly, with a case-fatality rate of 0·8% in people aged >40 years of age [Reference Brown and Persley1]. Transmission follows the faecal–oral route, and mainly occurs through contact with symptomatic or asymptomatic infected persons, i.e. person-to-person transmission. HAV has recently been recognized as a sexually transmitted infection, especially in men who have sex with men (MSM) [Reference Urbanus2]. Infection may also be foodborne, i.e. after ingestion of contaminated food. Underreporting of outbreaks due to foodborne sources is likely, as patients need to recall their exposures of 2–6 weeks preceding their illness. HAV is endemic in most countries in Africa, Asia, South America and Central America [Reference Jacobsen and Koopman3]. For most Western countries such as the USA, Australia and countries in Europe, the risk of HAV outbreaks is changing because endemic circulation has become less common with improving sanitary conditions. Consequently, the non-vaccinated population has become more susceptible [Reference Koopmans4].
In 1995–1996, a first population-based serosurveillance study was performed in The Netherlands [Reference De Melker and Conyn-van Spaendonck5], in which the prevalence of HAV antibodies was estimated to be 34% [Reference Termorshuizen6]. Since that time many factors associated with HAV exposure may have changed. Since 1994, a vaccine inducing long-term immunity [Reference Nothdurft7] has become increasingly available for Dutch travellers to endemic countries. More people from endemic countries have immigrated into The Netherlands [8], and since 1998 Municipal Health Services in The Netherlands have carried out HAV vaccination programmes focused on immigrants' children to reduce import and secondary HAV infections [Reference Suijkerbuijk9]. The travelling behaviour of the Dutch population has changed considerably [8], coinciding with increased risk of outbreaks due to importation of the virus from an endemic country [Reference Perevoscikovs10]. Apart from travel, the virus can also be introduced through a foodborne source [Reference Wheeler11–Reference Petrignani13]. Globalization of the food industry with consequential international distribution of products has increased the risk of such outbreaks [Reference Romalde14].
A second population-based serosurveillance study was performed in the general population of The Netherlands in 2006–2007 [Reference van der Klis15]. This study offered the opportunity of investigating the current status of HAV serology in The Netherlands, to get insight in changes of the immunity in the population over time and in changes in infection pressure. Our objectives were to estimate the current seroprevalence of the population at risk for HAV infection, and compare this to the seroprevalence of the preceding study, and to determine current risk factors for hepatitis A infection in order to identify target groups for vaccination strategies in The Netherlands.
METHODS
Study population and questionnaire
To ensure comparability, the study design for the second serum bank in 2006–2007 (second study) was kept similar to that of the first serum bank in 1995–1996 (first study). Details on the study design and data collection have been published elsewhere [Reference De Melker and Conyn-van Spaendonck5, Reference van der Klis15]. In short, eight municipalities were sampled within each of five geographical Dutch regions with a probability according to their size [Reference van der Klis15]. Nine municipalities that were sampled in 1995–1996 were by chance again sampled in 2006–2007. An age-stratified sample (age groups: 0, 1–4, 5–9, …, 75–79 years) was randomly taken from the population register of each municipality. Overall, 19 781 persons were invited to participate in this second study in 2006–2007, including over-sampling of 2574 non-Western immigrants from 12 municipalities. Subjects were requested to give a blood sample, to complete a questionnaire and to bring their vaccination certificates. From adults a maximum of 22 ml blood was taken and depending on their age and the degree of discomfort, less blood (0·1–8 ml) was taken from children. The questionnaire addressed demographic characteristics, vaccination history, health perception and diseases, activities possibly related to infectious diseases (e.g. travelling, profession, food habits, gardening, pets). Information related to sexually transmittable diseases was obtained for participants aged between 15 and 80 years. For all invitees, information on age, gender residence, country of birth and country of birth of both parents was available from the population register. Non-responders were sent a non-response questionnaire containing a limited set of questions on demographic characteristics, vaccination history and health perception. Samples and data of the 2006–2007 study were collected during the period from February 2006 to June 2007.
Serology
After collection, the sera were stored at −80°C. HAV specific antibodies were determined using the HAVAB 2.0 (AxSYM, Abbott, USA) between November 2008 and March 2009. The individual data of the 2006–2007 study were transformed into international units per litre (IU/l) using HAVAB 2.0 quantitative standard calibrators. To ensure comparability of the laboratory results in the first and second studies, the same assay was used and the cut-off value for reactivity in the HAVAB 2.0 assay was validated by testing five replicates (on different days) of serially diluted standard HAV antibody-positive serum (0, 2·5, 5, 10, 20 IU/l, data not shown). This validation showed that the 50% turning point of reactivity in the HAVAB 2.0 assay was in the 10 IU/l samples. All sera with HAV antibody levels >10 IU/l were regarded as seropositive for HAV.
Statistical analysis
Seroprevalence analysis
The overall seroprevalence was first estimated for both nationwide samples. For the estimation of the seroprevalence of naturally acquired HAV infections, analyses were limited to non-HAV-vaccinated persons. Because migrants were also included in the seroprevalence estimation, seroprevalence estimates were weighted not only for age and gender, but also for ethnicity and degree of urbanization, based on the total population in The Netherlands on 1 January 1997 for the first study and on 1 January 2007 for the second study [8]. Seroprevalences were adjusted for the two-stage cluster sampling by taking into account the strata (five regions) and clusters (40 municipalities) [Reference van der Klis15]. Prevalence rates per year of age and 90% confidence intervals (90% CI) were estimated for the first and second studies using spline functions for smoothing [Reference Rothman, Greenland and Lash16]. Weighted seroprevalences for the 2006–2007 sample were separately estimated for groups based on following countries of birth: (1) The Netherlands, (2) Surinam and Netherlands Antilles and Aruba, (3) Morocco and Turkey, (4) other non-Western countries, and (5) other Western countries.
Identification of risk factors for a past HAV infection
In the 2006–2007 sample, logistic regression analysis was performed to determine which variables could be identified as univariate predictors of naturally acquired seropositivity for HAV, after adjustment for age, gender and ethnicity. To identify risk factors for naturally acquired HAV infection, certificate-confirmed HAV-vaccinated persons were excluded, because their seropositivity probably reflects vaccination-induced antibodies; and infants aged <1 year were excluded since their seropositivity is probably maternally derived. Endemic regions were defined as the continents Africa, Asia, and the South- and Mid-Americas, as these were the levels of detail inquired in the questionnaire. Variables were included in a multivariate model if their P value was <0·05 in adjusted univariate analysis or if the risk factor is borderline-significant with 0·05<P<0·10 and described in the literature. The variables remained in the multivariate model if the P values were <0·05 while the backward selection procedure was used. Missing values were classified as ‘unknowns’, in order to be able to include the maximum number of participants in the multivariate logistic regression. Analysed variables were included as continuous where possible. Data were analysed using SAS v. 9.2 for Windows (SAS Institute Inc., USA).
Characteristics of HAV-vaccinated persons
To gain insight into the HAV-vaccinated population in 2006–2007 and the effect of the vaccination strategy, the confirmed HAV-vaccinated subjects were compared to non-HAV-vaccinated subjects using univariate and bivariate logistic regression for the following characteristics: gender, ethnic origin, travel history, sexual behaviour, and living in urbanized or rural area.
RESULTS
Study population
Of 19 781 invited subjects, a total of 6386 (32%) provided a serum sample. Non-responders were more often males, of non-Western descent and living in highly urbanized areas, and for these factors correction was made in the seroprevalence estimates based on the population register. Non-responders were similar with respect to region, educational level and health status. Of 6386 serum samples provided, 6229 (98%) were of a sufficient volume for HAV testing. Persons with insufficient volumes did not differ from the participants with sufficient volumes with respect to gender, urbanization, and ethnicity; however, this group more frequently contained children aged <1 year.
To show the comparability of the first and second studies to each other and to the Dutch population, frequencies of factors possibly associated with seroprevalence in general, and with seroprevalence of HAV antibodies specifically, are presented in Table 1. The differences between the two study groups for which no correction was made during analysis, i.e. educational level and travelling behaviour, are differences that are also seen in the general population in The Netherlands.
Table 1. Percentages of relevant factors for seroprevalence in general and of HAV antibodies in particular, for HAV-tested participants in the first nationwide sample in 1995–1996 and the second in 2006–2007, and compared to these proportions in the Dutch population at 1 January 1997 and 1 January 2007 [8]. Infants (⩽1 year) were excluded (first study: n=7191; second study: n=6038)
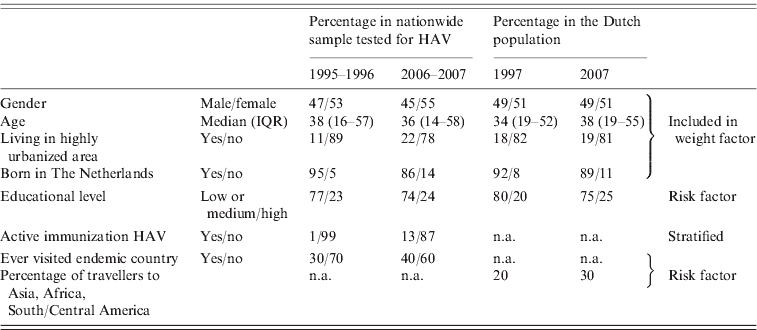
IQR, Interquartile range; n.a., not available.
Seroprevalence
The overall weighted seroprevalence was 39·3% (95% CI 37·0–41·6) in the second study, which was significantly higher than the 33·9% (95% CI 31·8–36·0) in the first study. The seroprevalence did not significantly differ between men (37·3%, 95% CI 34·4–40·3) and women (41·3%, 95% CI 38·6–43·9). Of the study population in the second study, 87·4% was not vaccinated and the seroprevalence for this group was 30·6% (95% CI 28·4–32·8). In the first study 99·2% of the study population was not vaccinated, and the seroprevalence for this group was 30·3% (24·4–36·1), which did not significantly differ from that found in the second study. The age-dependent seroprevalence for non-HAV-vaccinated persons in the first and second studies are plotted per year of age in Figure 1, showing a cohort effect, i.e. persons born after the Second World War that were susceptible in 1995–1996 were still susceptible in 2006–2007. A relatively high seroprevalence was seen in infants aged <1 year (18%, 95% CI 12–23), which is a reflection of maternally derived antibodies. The seroprevalence increased from 4% (95% CI 0–8) for 1-year-olds to 70% (95% CI 56–84) for 79-year-olds.

Fig. 1. Age prevalence of hepatitis A antibodies presented per year in age including 90% confidence intervals in non-HAV-vaccinated persons in two nationwide samples of the Dutch population in 1995–1996 (first study, – – – , n=7287, excluding 59 vaccinated participants) and 2006–2007 (–––, n=5442, excluding 786 vaccinated participants).
Differences were seen in naturally acquired seroprevalence between ethnic origins of the Dutch population in 2006–2007 (Table 2). The study population was broken down into three categories in order to be comparable to the analysis of the first study sample: children (aged 1–14 years), young and adult persons born after the Second World War (15–61 years) and older participants (⩾62 years). Seroprevalence for the indigenous Dutch population was higher in persons born during or before the Second World War, in comparison to those born afterwards. People from Turkish and Moroccan origin as well as from Surinam, The Netherlands Antilles or Aruba and other non-Western countries, were more often seropositive compared to the native-Dutch population in all three age groups. Seroprevalence in people from other Western countries was not statistically different from that of the indigenous Dutch.
Table 2. Weighted seroprevalence of HAV antibodies in non-HAV-vaccinated persons aged >1 year in the national sample of the second study, 2006–2007, by gender and ethnic origin and age (n=5175)
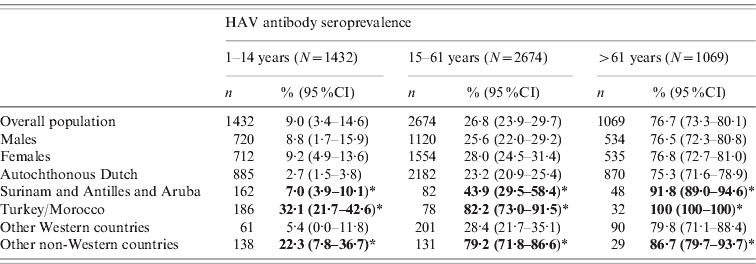
* Percentages presented in bold are significantly higher than the autochthonous Dutch population.
Risk factors for acquired HAV infection
In logistic regression analysis of the 2006–2007 sample for the identification of risk factors, 22/42 investigated factors appeared to be associated with presence of HAV antibodies in univariate analysis after adjustment for the possible confounding effect of age, gender and ethnic origin (see Table 3 note). MSM was borderline-significantly associated and previously described as a risk factor for HAV in The Netherlands. We therefore included this risk factor in multivariate analysis.
Table 3. Odds ratios (OR) and 95% confidence intervals (95% CI) for independent associations between different variables and the prevalence of HAV antibodies in the Dutch population in 2006–2007, as found in a multivariate logistic regression modelFootnote *. HAV-vaccinated subjects and infants (aged <1 year) were excluded (n=5175)
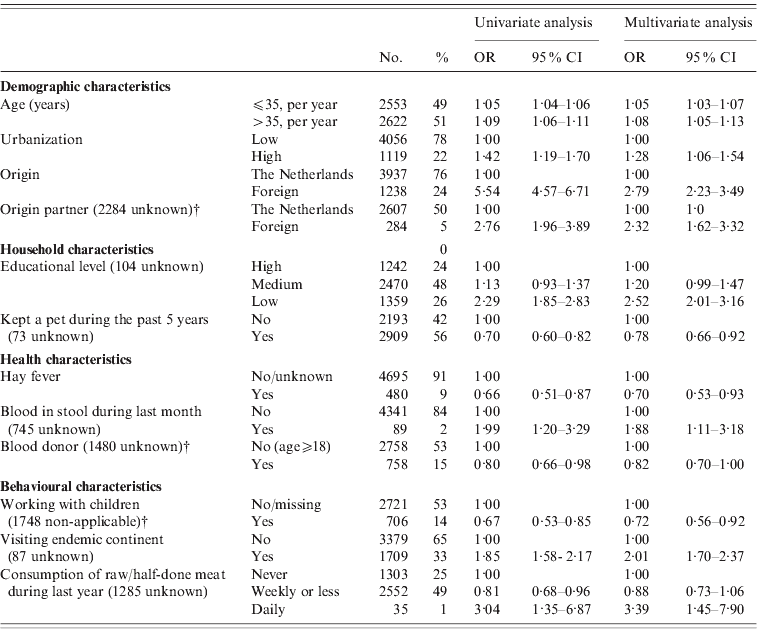
* The following factors were significant or borderline-significant in univariate analysis after correction for age, gender and ethnicity, and entered in a multivariate backward selection model: living in an highly urbanized area; having a foreign partner; low income or education level; self-reported bad health conditions, blood in stool, fever or visiting the physician during the past month; first sexual contact at age <18 years; visited family or holidays abroad, travelling duration >3 weeks, visiting a HAV-endemic continent; or eating raw or half-done meat during the past 12 months, youngest person in household 5–17 years of age (compared to 1–4 years), having kept a pet during the past 5 years, critical opinion towards vaccination, eczema, hay fever, having been a blood donor, gardening without gloves in the past 12 months, working with children, and medical job necessitating vaccination (other than HAV), male-to-male sex. The following factors were not significantly associated in univariate analyses after correction for age, gender and ethnicity, and not entered in a multivariate selection model: the number of persons in household, the number of persons spoken to yesterday (categorical or continuous), presence of children in household attending day care, risk per extra day attending day care, food allergy, vomiting in the previous month, health complaints resulting in sick leave in the previous month, ever having received a blood transfusion, the number of sexual partners, age of first sexual experience (continuous), duration of gardening, meat consumption, consumption of unwashed raw vegetables (yes/no; frequency).
† These items were only requested from people >15 years of age.
In multivariate analysis (Table 3), the following factors were independently positively associated with a past HAV infection: age, high urbanization level, non-Dutch origin participant, non-Dutch origin partner, lower educational level, blood in stool during the past month, having visited a HAV endemic continent, and eating raw meat on a daily basis during the past 12 months. Factors identified to be negatively associated in multivariate analysis were: hay fever, working with children, having kept a pet, and having donated blood.
Characteristics of HAV-vaccinated persons
In the second study 786/6229 (12·6%) participants were actively HAV-vaccinated, according to their vaccination certificates. A larger proportion of the HAV-vaccinated subjects (87%) had travelled to endemic countries, compared to the non-HAV-vaccinated subjects (33%), and of these travellers women were more frequently vaccinated than men (OR 1·3, 95% CI 1·1–1·5). People living in rural areas were less frequently vaccinated compared to people living in urbanized areas (OR 0·7, 95% CI 0·6–0·9). People from Turkey and Morocco were less frequently vaccinated (OR 0·6, 95% CI 0·4–0·9). However, when limiting the comparison to the target group of the Dutch vaccination campaign since 1998 (i.e. Turkish and Moroccan children aged ⩽15 years) [Reference Suijkerbuijk9], Turkish and Moroccan children were more often vaccinated (OR 1·9, 95% CI 1·0–3·4). Still, only 7% of Turkish and Moroccan children aged ⩽15 years were HAV-vaccinated, compared to 4% of children of Dutch ethnicity aged ⩽15 years.
DISCUSSION
A cohort effect was seen for HAV antibody prevalence on the basis of two cross-sectional studies among the Dutch population. People born after the Second World War show a statistically significantly lower seroprevalence compared to people born before or during this war. The Second World War was a turning point in hygiene standards in The Netherlands. With a seroprevalence of 30·6% in the 87·4% non-HAV-vaccinated people, over 60% of the Dutch population will be at risk in the event of a HAV outbreak in The Netherlands, and this susceptible population is increasing in age. This is a point of concern which should be the future focus for public health action, for several reasons. First, HAV infection is more severe for the elderly, with a case-fatality rate of 0·8% [Reference Brown and Persley1]. Second, there is increasing risk of exposure through imported foods or travellers from endemic countries. Outbreaks of HAV may very well occur within susceptible populations after incidental introduction of the virus through food or persons. This is illustrated with several recent large international outbreaks reported in Latvia [Reference Perevoscikovs10], USA [Reference Wheeler11] and Australia [12], and with severe outcome as recently seen in a small outbreak in The Netherlands [Reference Petrignani13].
Overall, an increase was seen in the prevalence of HAV antibodies from 34% to 39%, which can be attributed to increased vaccination, and an increased immigrant group. However, despite the fact that the study design in the two studies ensures random selection, a response rate of 32% in 2006–2007 and 55% in 1995–1996 makes non-response bias possible. A thorough non-response analysis for the first study showed that for some characteristics (age, gender) an association with seroprevalence was considered likely. For other characteristics (marital status, degree of urbanization) the effects were not clear but assumed to be small [Reference De Melker, Nagelkerde and Conyn-van Spaendonck17]. Further, for the estimation of seroprevalences and the identification of risk factors in the second study, we weighted for several factors which may be associated with seroprevalence [Reference De Melker, Nagelkerde and Conyn-van Spaendonck17]. Moreover, comparison between the two studies shows differences that are due to real changes in the total population in The Netherlands, i.e. an increase in immigrants and an increase in travelling [8]. The changes in vaccination strategies can explain the large increase in the proportion of vaccinated persons in the second survey.
As was found in the first study in 1995–1996 [Reference Termorshuizen6], age, ethnic origin, educational level and living in an urbanized area were independent risk factors for seroprevalence of HAV antibodies. The risk of travelling to endemic regions, or having a partner from one of these regions, was logically found to be an independent risk factor. Hay fever was surprisingly identified to be negatively associated with HAV antibodies, although comparable results were previously found in the USA [Reference Matricardi18, Reference Matricardi19]. A potential causal association may be attributed to the general improvement of hygienic circumstances, i.e. the ‘hygiene hypothesis’ [Reference Strachan20, Reference Wills-Karp, Santeliz and Karp21]. Further research is needed to confirm this finding. The protective association for blood donors may be explained by the fact that persons having recently travelled to endemic countries are excluded from donating blood. For other risk factors, the meaning remains largely unexplained, and these factors may be indirectly linked to some unknown factor. Close contact with children attending day-care facilities was previously described as a risk factor [Reference Lemon22] and does not correspond with our protective association of working with children. Sexual behaviour has been described to be associated with HAV infection in The Netherlands [Reference Urbanus2, Reference Reintjes23] and elsewhere [Reference Lemon22]. In accordance, we found that first sexual contact before age 18 years, as well as MSM may be risk factors for naturally acquired HAV infection in The Netherlands. However, effects did not remain after correction for other risk factors, which may have been caused by the low proportion of known MSM participants in our study (n=12, 0·2%). Further investigation is warranted to determine whether MSM significantly attributes to acquiring HAV infection in The Netherlands.
A general shortcoming of serological surveys measuring HAV antibodies is the fact that they reflect an infection in the past, which may have been decades ago. Waning immunity, i.e. decline of antibodies over years, did not result in an amount of HAV antibodies below the protection level within 12 years [Reference Van Herck24], and may cover an even longer period since exposure [Reference Jacobsen and Koopman3, Reference Nothdurft7]. Also, exclusion of HAV-vaccinated persons was based on confirmation with a vaccination certificate, implying that some potentially vaccinated persons may have been included. These factors may have diminished the found effects of risk factors. The questionnaire inquired risk factors related to last month, year or 5 years (blood in stool, kept a pet, consumption of raw meat) or factors that are likely to reflect a longer time span (age, urbanization, origin, origin of partner, educational level, working with children, hay fever, ever having donated blood or visited an endemic continent, first sexual contact aged <18 years). The latter are more likely to reflect true associations, whereas the risk factors based on a shorter history remain largely unexplained or may be indirectly linked to some other risk factor.
However, the occurrence of expected lifelong immunity is the exact reason why vaccination is so successful, and may be advisable for the identified risk groups. The proportion of HAV-vaccinated persons was found to be 12·6%, which is much higher than the reported 0·8% in the previous serosurvey in The Netherlands in 1995. Since we included only registered vaccinations, the actual proportion may be even higher. Part of this difference can be attributed to the successful use of active vaccine, which has been recommended to travellers since 1994 and has gradually become more accepted. Vaccination targeted at population groups at risk of infection has previously found to be cost effective [Reference Jacobsen and Koopman3], and vaccination programmes can result in incidence reduction through herd immunity [Reference Hollinger25]. Nevertheless, although universal vaccination may not be cost-effective, it is more likely to reduce incidence and mortality compared to vaccination targeted at groups at risk of infection [Reference Bauch26]. This is especially the situation for the elderly, which may not be a group with increased risk of infection, but a group at risk of severe illness once infected. Although vaccination of the elderly (>50 years) may be associated with impaired immune response [Reference Genton27], it reaches seroprotection in 98% of people aged >50 years who received a booster [Reference D'Acremont, Herzog and Genton28]. Moreover, HAV vaccine is successful given the persisting memory immunity even after disappearance of HAV antibodies, which might be protecting against a clinically relevant infection [Reference Van Herck24, Reference Cederna, Klinzman and Stapleton29]. Administering vaccination as post-exposure prophylaxis has proved effective in containing an ongoing outbreak [Reference Kohl30, Reference Tricco31]. For these reasons, either prophylactic or post-exposure vaccination may be an effective strategy to prevent large outbreaks from occurring in The Netherlands. Further investigation is needed into the cost-effectiveness of adding the elderly as a target group for prophylactic vaccination. Although the case-fatality ratio increases with age, the exact age of higher risk is not clearly defined. Moreover, vaccination may need to be confined to those with additional risk factors. Although currently <25% of the elderly are susceptible to infection, this proportion is likely to increase in the coming decades. Nevertheless, although the effect of the vaccination strategy targeted at children <15 years with parents of Turkish and Moroccan origin is seen in terms of statistical significance, the relevance of 7% vaccination coverage of these children compared to 4% of other Dutch children is debatable.
In conclusion, the group of indigenous Dutch persons susceptible of HAV infection in The Netherlands is becoming older, with the turning point being born after the Second World War. Risk of infection in the Dutch population is currently mainly travel-related, with an increase in travel frequency seen over the last decade. HAV vaccination is increasingly administered mainly to travellers, and is found to be very effective. Despite the increased vaccine coverage within The Netherlands, risk of HAV transmission in the non-vaccinated population in The Netherlands is realistic. Given the globalization of the food market, initial introduction of the virus may very well occur via food, with risk of outbreaks with international consequences in non-HAV-vaccinated persons [Reference Petrignani13]. Targeted vaccination, either prophylactic or post-exposure, of risk groups will be an effective intervention to contain HAV outbreaks in The Netherlands. Adding post-war elderly as a target group for prophylactic vaccination will reduce morbidity and mortality during HAV outbreaks in the future.
ACKNOWLEDGEMENTS
We thank the Public Health Services, the municipalities involved, the 2006–2007 study Project Team for their contribution to the realization of the 2006–2007 study. We are grateful to all participants of the 2006–2007 study for their contribution. We are grateful to Dr G. Boland (University of Utrecht) and her laboratory for determination of HAV-specific antibodies and for providing the data on the correlation between the HAV results of the 1995- and 2006 studies.
DECLARATION OF INTEREST
None.






