INTRODUCTION
Hantaviruses are rodent-borne, enveloped RNA viruses of the family Bunyaviridae and each hantavirus is carried by a chronically infected, specific rodent (or insectivore) host species. When transmitted to humans, Old World hantaviruses cause haemorrhagic fever with renal syndrome (HFRS), and New World hantaviruses cause hantavirus pulmonary syndrome (HPS), while many hantaviruses seem to be apathogenic to humans. In Finland, an average of 1000–1700 cases of usually mild HFRS known as nephropathia epidemica caused by Puumala virus (PUUV) have occurred annually [Reference Brummer-Korvenkontio1]. Typical symptoms include headache, fever, nausea, vomiting, myalgia, back pain, visual disturbances and signs of renal failure. The diagnosis is based on detection of IgM antibodies to PUUV, and after infection, IgG antibodies prevail. PUUV, and its carrier rodent, the bank vole [Myodes (previously Clethrionomys) glareolus], are found in most of Europe excluding the Mediterranean region. The population densities of bank voles have a 3-year cycle in Finland, driven by predator–prey dynamics. The population densities are high during two consecutive autumns and winters and in parallel, human epidemics occur. Recently, the cycles have been synchronous over most of Finland resulting in high numbers of human cases every three calendar years, with, e.g. 2300, 774, 2603, 2526 and 3259 cases in 1999, 2000, 2002, 2005 and 2008, respectively. Approximately 5% of the Finnish population are IgG-seropositive but in endemic areas the figure rises to 20% [Reference Vapalahti2].
Although the obvious source of human infection is the carrier rodent, the possible modes of transmission of hantaviruses to humans have not been thoroughly investigated and risk factors for contracting the disease beyond contact with rodents are not clear. Previous epidemiological studies of risk factors associated with hantavirus infection have shown significant odds ratios (ORs), e.g. in a Franco-Belgian study (PUUV) for exposure to dust or working with wood in a forest and entering buildings that might have rodents [Reference Crowcroft3], in an American study (Sin Nombre virus) for entering closed buildings [Reference Zeitz4], and in Chinese studies (Hantaan virus) for cat ownership and sleeping in huts [Reference Xu5, Reference Xu6].
Our aim was to study risk factors for PUUV transmission with a case-control approach in the Finnish setting during a winter epidemic peak with a large number of cases and controls, where the latter were verified to be susceptible to PUUV infection on the basis of negative serological results.
METHODS
Research material
We designed a non-matched case-control study; cases and controls were persons suspected and tested for acute PUUV infection in the diagnostic laboratory of Department of Virology, HUCH Laboratory Diagnostics (currently HUSLAB), Helsinki from 29 November 1999 to 28 February 2000. There was an epidemic peak of PUUV infection in Finland at that time. The data were collected by an extensive questionnaire (see next section) sent to all patients with a confirmed PUUV infection (cases: PUUV IgM- and IgG-positive), and to all patients suspected of PUUV infection, but who did not have markers of acute PUUV infection or of old immunity (controls: PUUV IgM- and IgG-negative). The diagnostic laboratory is an accredited (FINAS T200/M07/2008) university hospital laboratory and a WHO collaborating centre for hantavirus diagnostics and research; the PUUV μ-capture-IgM-EIA test [Reference Vapalahti7] and IgG-IFA test [Reference Hedman, Vaheri and Brummer-Korvenkontio8, Reference Vapalahti9] have been evaluated thoroughly [Reference Sjolander10, Reference Kallio-Kokko11] to have specificity and sensitivity of 100% and 100%, respectively, for the IgM test and 100% and 95%, respectively (compared to neutralization) for the IgG test, and are under external quality control. The questionnaire was sent together with the letter stating the result of the PUUV serology testing and was delivered to the patients (who signed a paper of agreement and informed consent) via the responsible physician. An ethical permit for the study was obtained from the coordinating ethical committee, HUCH. The research material consisted of returned questionnaires of 282 (response rate 80%) PUUV IgM- and IgG-positive (cases) and 204 (response rate 52%) PUUV IgM- and IgG-negative persons (controls).
Questionnaires
The questionnaires (English translation downloadable at www.hi.helsinki.fi/zoonoosivirukset/questionnaire) contained 66 questions divided into eight sections: (1) basic information (name, age, gender, address, education, occupation); (2–4) clinical signs, onset of illness and previous chronic diseases; (5) potential contacts with rodents, cats, dogs, and horses; (6) habits, such as smoking, outdoor activities, visiting outbuildings (most questions referring to 1–5 weeks before onset of illness); (7) characteristics of the respondent's house and its environment; (8) information on potential summer cottage (if visited 1–5 weeks before outbreak of illness). On average, 4% and 6% of cases and controls, respectively, used ‘I don't know’ each question and 6% of both left a question unanswered.
Subsets
In order to confirm the relevance of the results derived from the entire dataset, we further studied two subsets of the data: (1) those who lived in the most endemic region consisting of central and eastern parts of Finland (referred to as ‘Endemic area’; see Fig. 1 a); and (2) matched pairs selected from cases and controls (matched case-control study) (Fig. 1 b). The pairs were matched on the basis of the following criteria: maximum age difference 10 years, gender, type of housing, living environment in terms of urbanity including distance from forest or fields [evaluated also from maps available on the internet (http://020300200.com)], geographical distance <130 km. PUUV infection is rare in children, and the study was further restricted to persons who were at least 15 years old, resulting finally in 275 cases and 188 controls in the entire dataset, 154 cases and 104 controls in the ‘Endemic area’ and 96 matched pairs. Basic characteristics of cases and controls in all three study groups are given in Table 1.

Fig. 1. Map of geographical distribution of the two separately studied subsets: (a) the endemic area and (b) the matched pairs, for which the municipality where each case and control lived is indicated with grey and white dots, respectively. (Inset: location of Finland in northern Europe.)
Table 1. Basic characteristic of the Puumala virus risk-factor study material
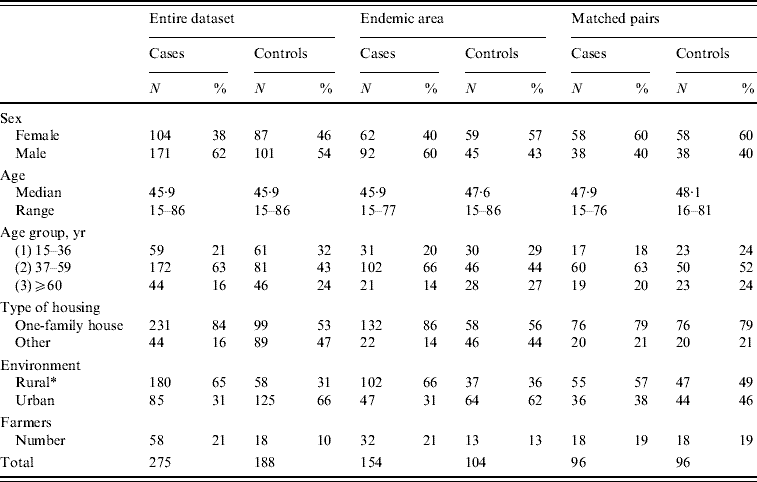
* Rural=person who reported not living in a town or suburban area; if an answer was not available, information was deduced from internet maps (http://020300200.com). Notably, the matching of pairs was not based on the reported answer, but on similar community structure and distance from forest and fields in internet maps.
Statistical methods
Statistical analysis was carried out as described below.
(1) Potential confounding factors were considered thoroughly for the whole research material. According to published studies age, sex, living environment, type of housing and being a farmer were previously known risk factors and their role as potential confounders was checked. In addition to these, chronic diseases and visiting a summer cottage were presumed to be potential confounding factors because of uneven distribution among cases and controls and suspected association with acquiring PUUV infection. However, chronic diseases, being a farmer and visiting a summer cottage had no significant or meaningful confounding effect. This was tested by calculating ORs for all individual exposure variables with and without adjustment by these factors and modelling (by logistic regression model) with and without them. ‘Type of housing’ and ‘living environment’ had a strong correlation. Sex, age group and living environment (urban vs. rural/remote) remained as confounding factors. They were used in analyses for the entire dataset and the endemic area. These three confounding factors were also the criterion for matching pairs.
(2) Crude and adjusted ORs with 95% confidence intervals (CIs) for all possible individual exposure variables were calculated for the whole research material and both subgroups (Table 2).
(3) Every significant (P<0·05) exposure variable (adjusted OR>1 and lower 95% CI>1 or OR<1 and upper 95% CI<1) in the entire dataset or either of the subgroups was introduced to multivariate analysis which was carried out for the whole research material and both subgroups. Multivariate analysis was done using logistic regression modelling for the entire dataset and the endemic area, and by conditional logistic regression modelling of the matched pairs. Interactions up to the second order were taken into account.
(4) In order to compare smoking habits of cases and controls to population data on smoking habits in Finland we used the 1999 smoking statistics provided by the Finnish National Public Health institute (www.ktl.fi).
Statistical analyses were performed using SAS version 9.1 proc logistic program (SAS Institute Inc., USA).
Table 2. Odds ratios of relevant exposure variables for PUUV infection
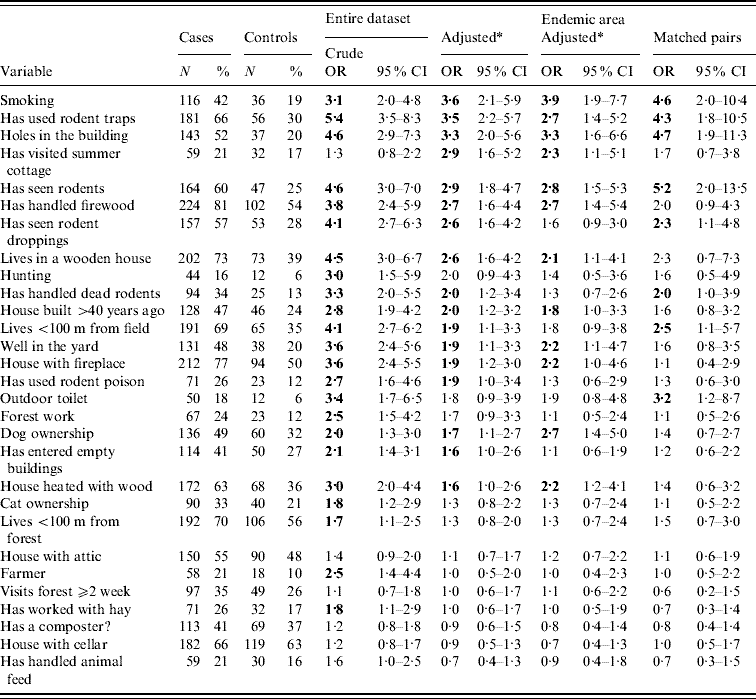
OR, Odds ratio; CI, confidence interval. The ORs that are statistically significant (P>0·05 when 95% CI lower limit >1) are in bold. The exposure variables are listed in (descending) order of adjusted ORs for the whole research material.
* Adjusted by age, gender and living environment (rural vs. suburban/urban).
RESULTS
ORs for exposure variables
The most important exposure variables and their ORs with 95% CIs (adjusted for age, gender, and living environment), counted for the whole study material and both subgroups (the endemic region and matched pairs), are given in Table 2. The predisposing factors under study could be categorized into either physical living environment (properties of the building, etc.) or personal activities and habits, and if relevant, the latter were restricted in the questionnaire to include only the period of 1–5 weeks before onset of illness.
Significant risk factors according to adjusted ORs in the whole study material and both subgroups were smoking, use of rodent traps for control, presence of holes in the building through which rodents may enter, and seeing rodents (Table 2).
Multivariate logistic regression models
We attempted to build the model in various ways and combinations of risk factors, e.g. adding or removing individual factors one by one or doing backwards or forwards modelling for selected combinations. When we included smoking, holes in the building, use of rodent traps, handling firewood and confounding factors in the model, the remaining factors had no significant effect. Apparently, this was because of considerable correlations between the above-mentioned and other factors. Only smoking, holes in the building, use of rodent traps, handling firewood were therefore selected in the model. However, ‘holes in the building’ reduced markedly the effect of the use of rodent traps and handling firewood (due to correlations), but not the effect of smoking. We finally built the model in two ways: either including all subjects in the study or excluding from the model those who had reported ‘holes in the building’ (models 1 and 2, respectively, in Table 3). This was done for all three groups (entire dataset, endemic area, matched pairs). The best Hosmer and Lemeshow goodness-of-fit test value was obtained in model 2 for the entire dataset (χ2=1·542, P=0·98)
Table 3. Multivariate analysis of significant risk factors for PUUV

OR, Odds ratio; CI, confidence interval.
* Model 2 was not applicable to matched pairs because, e.g. for smoking none of the remaining 16 controls living in houses with ‘no holes in the building’ were smokers (compared to 7/16 of cases).
† See Figure 1 for definitions of age groups.
‡ OR values counted without interactions. When counted with interactions the ORs and CIs were for living environment (rural) (OR 0·5, 95% CI 0·2–1·5), using rodent traps (OR 0·9, 95% CI 0·4–2·2) and handling firewood (OR 0·7, 95% CI 0·3–2·1). Interactions did not affect other variables or other models.
Smoking remained a significant risk factor independent of the model. In Model 1, ‘holes in the building’ was another clear risk factor in all the three groups. The significance of the two other factors, ‘handling firewood’ and ‘using rodent traps’ varied.
Smoking
Smoking was a clear risk factor according to adjusted ORs (Table 2) and in the multivariate logistic regression models (Table 3) in all groups studied (in different models the significance of smoking varied from P<0·0001 to P=0·0015). Unlike for most other exposure variables shown in Table 2, adjusting did not reduce the significance of the ORs for smoking, but actually increased it. To confirm that smoking was a true risk factor, we further compared the smoking habits of the controls and cases with those of the Finnish general population based on statistics available for 1999 (Fig. 2). In each age group, the cases smoked considerably more often than either controls or the general population, e.g. 11/14 cases in males in the youngest age group (15–24 years old) were smokers (Fig. 2 a); the findings were similar for females (Fig. 2 b). We also evaluated smoking by counting the OR of smoking using simulated controls (same number as in our study) whose smoking habits were replaced with the national ‘average smoking’ data. Especially for females, the smoking habits of our controls were very similar to those of the general population (Fig. 2 b). The OR for smoking adjusted by age for the comparable age groups was with our ‘real’ controls 2·8 (95% CI 1·7–4·6) and with our ‘simulated’ controls 2·5 (95% CI 1·6–4·0).
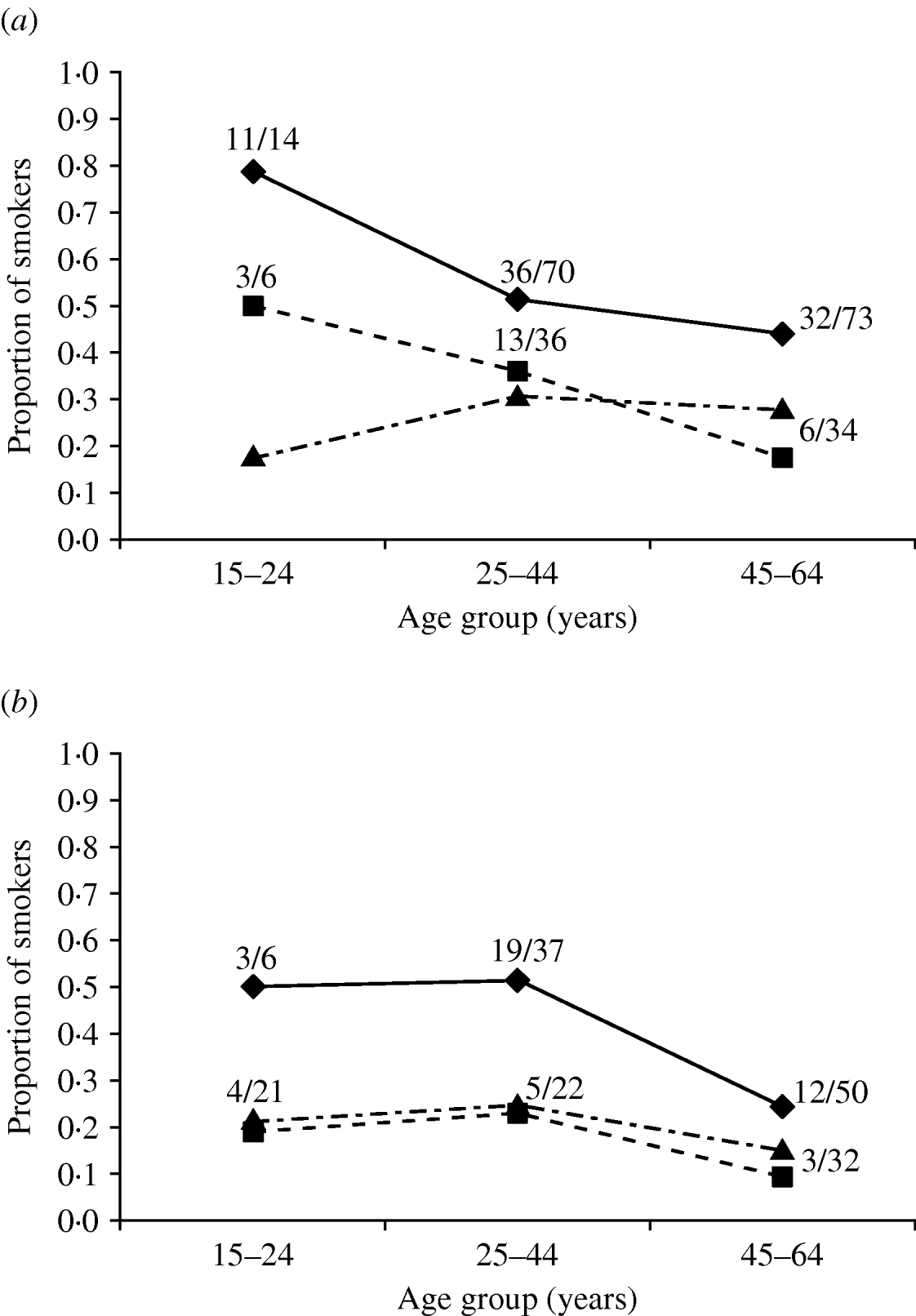
Fig. 2. Age-group-specific proportion of smokers in (a) male and (b) female acute Puumala virus infection cases (–◆–, PUUV IgM- and IgG-positive), controls (- -▪- -, PUUV IgM- and IgG-negative), and in the general population (· -▴- ·) in 1999 in Finland. The fraction numbers refer to total number of smokers per number of cases or controls in each age group.
DISCUSSION
PUUV infection is a common disease in Finland with 5% seroprevalence and an average 1700 reported cases annually (www.thl.fi). Although the excreta of the chronically infected specific host rodent are the source of human hantavirus infection, it has remained unclear how the infection is actually acquired and which risk factors are involved.
The approach was to recruit as many PUUV cases [PUUV IgM(+)] and susceptible [PUUV IgG(–)] controls studied by our diagnostic laboratory as possible for a questionnaire study during the peak of an epidemic season (from December to February). The epidemic peak under study turned out to be exceptionally high resulting in a high case:control ratio with 275 cases and 188 controls, aged at least 15 years, accepted in the study. Because the possibility of contracting the infection is strictly temporally linked to the presence of virus in carrier rodents and their population densities, recruiting more controls afterwards was not possible, because they would no longer have had the opportunity to have been exposed to the infection. To control for possible geographical differences in rodent population densities of living environments of cases and controls, in addition to the entire dataset we studied separately a highly endemic region with 154 cases and 104 controls, and 96 matched case-control pairs. Exclusion of people with pre-existing PUUV immunity was especially important in the endemic area since, e.g. in the commune of Ilomantsi in Northern Karelia >50% of males aged >60 years are PUUV-seropositive compared to an average of 5% of Finns in general [Reference Brummer-Korvenkontio1].
The most striking and clear finding was that smoking was an obvious risk factor for acquiring hantavirus infection. The relationship was stronger after adjusting for confounding factors and in the models if the data were restricted to those who did not have ‘holes in the building’; the variable had no significant interactions with other risk factors or confounding factors. Moreover, comparison with population-based control data showed that our cases were more often smokers in all age groups (Fig. 2). This finding is consistent with hantaviruses being primarily transmitted to man by inhalation of aerosols; it could be hypothesized that the condition of airways, e.g. the ciliary movement, influences whether the infectious aerosol enters the alveoli and remains there long enough for the infection to occur. One possibility is that the infection could occur by ingestion of hantavirus particles by macrophages or immature dendritic cells in the alveoli; the latter were recently shown to be induced to mature by hantavirus infection [Reference Raftery12] which could lead them to be recruited to lymph nodes for further systemic spread of the infection. Interestingly, cigarette smoke extract has been shown to cause immunomodulatory effects in dendritic cell function [Reference Vassallo13]. Moreover, chronic inflammation could contribute to the initial spread of the infection. It has been shown that smoking is also a risk factor for some other, at least partially aerosol-transmitted or airway pathogen diseases such as common cold, influenza, and invasive pneumococcal pneumonia [Reference Nuorti14]. Our result could further help to explain why children rarely acquire PUUV infection, although they are probably exposed to the virus: both PUUV antibody positivity and the disease are almost non-existent in children aged <10 years [Reference Brummer-Korvenkontio1]. On the other hand, as an alternative explanation smoking may lead to more frequent contact between hand and mouth. In any event, our results suggest that cigarette smoking can be a risk factor, not only for infections arising from the flora of the respiratory tract, but also for microbial infections acquired from the environment via the respiratory route. More detailed studies on the impact of history and quantity of smoking are still needed.
Another clear risk factor was the presence of holes in the building through which rodents could enter. Together with cigarette smoking, this risk further suggests that transmission occurs by inhalation indoors; the possibility of contracting high amounts of infectious aerosols for inhalation is evidently higher when rodents can enter the building.
The association with rodent control was interesting, because instead of being protective, using rodent traps seemed to be associated with some risk (Tables 2 and 3) whereas in the two subsets, using rodent poison for control was not a risk factor at all. It is likely that when traps are used, one needs to remove the dead rodent and therefore be exposed to their excreta. On the contrary, when poison is used, rodents usually leave human dwellings before they die, without further human contact. For example, in our dataset, 50% vs. 5% of the diseased using vs. not using traps had handled a dead rodent, respectively.
Handling firewood is believed to be a risk factor for acquiring PUUV infection and Swedish patients have reported that this was their most likely exposure [Reference Olsson15]. Our study findings support this view, although the ORs were not uniformly significant (Tables 2 and 3). Peridomestic woodsheds are a probable contact place for rodents and humans.
Other potential risk factors were also found but they were not uniformly detected in all subsets of the data. Unlike for Hantaan virus in China [Reference Xu6] we could not find compelling evidence for cats being a vehicle of transmission nor to have a protective effect, whereas some risk could be attributed to dog ownership. Observation of rodents was a risk factor, as in previously published studies, indicating that our case and control groups were adequate. In this study we analysed the risk factors beyond this phenomenon (observation of rodents), which is dependent on the rodent densities, and it did not remain as an independent risk factor in the regression analysis.
Previous epidemiological studies on PUUV infection have shown occupational risk attributed to farming in Finland [Reference Vapalahti16] and Sweden [Reference Ahlm17]. In a case-control study conducted in Belgium and France, the only statistically significant risks were working in the forest and entering potentially rodent-infested buildings; e.g. rodent control had a non-significant protective effect [Reference Crowcroft3]. A Belgian study suggested that woodcutting, reopening of a non-aerated room, and strenuous physical effort were risk activities [Reference Van Loock18]. Studies in China have suggested that, e.g. camping or living in huts in the fields, living in a house on the periphery of a village, and cat ownership are risk factors [Reference Xu5, Reference Xu6, Reference Ruo19]. In a study of American soldiers in Korea, sleeping in tents was a risk [Reference Pon20]. The risk of acquiring Sin Nombre virus infection has been attributed, in addition to high rodent contact [Reference Childs21], to entering peridomestic buildings that have been unused for long periods [Reference Zeitz4].
CONCLUSIONS
In conclusion, our study has shown that cigarette smoking and living in buildings which have holes allowing rodents to enter were the most important risk factors for PUUV infection. These findings are compatible with hantavirus infection being an aerosol-transmitted disease dependent on the condition of airways and infectious virus concentration in the aerosol, which is highest indoors. In addition, considerable risk may be attributed to use of rodent traps (as opposed to poison) and handling firewood, but this finding was not as uniformly clear as for the two main risk factors. The findings could have direct public health impact on recommendations for prevention of the disease, emphasizing the role of blocking the entry of rodents to human dwellings and avoiding or minimizing inhalation of potentially contaminated dust indoors, suggesting that poison could be better than traps for rodent control and adding yet another health threat attributable to cigarette smoking.
Our study represents one of the largest epidemiological studies of risk factors for acquiring hantavirus infection. While more studies are needed and these results cannot perhaps be fully extrapolated outside the endemic regions for PUUV infection in wintertime, the findings should be helpful in designing future studies and in planning for preventive measures for hantavirus infections.
ACKNOWLEDGEMENTS
We thank Professor Ian Gardner (University of California) for critical review of the manuscript and Professor Markus Brummer-Korvenkontio for advice during the planning stage. Financial support for this work was obtained from the University of Helsinki and EU grants (K2-QLRT-1999-01119 and QLK2-2002-01358) and HUS/EVO grant TYH 4211 and the EU grant GOCE-2003-010284 EDEN (Emerging Diseases in Changing European environment), catalogued by the EDEN Steering Committee as EDEN0026 (www.eden-fp6project.net). We also thank Raija Leveelahti, Irja Luoto, Hilppa Nykyri, Johanna Myllynen and Maija Mäkinen for professional technical and secretarial assistance.
DECLARATION OF INTEREST
None.







