Introduction
Antibiotic-resistant infections, particularly those caused by multidrug-resistant (MDR) organisms in healthcare settings, pose a serious threat to global public health. In 2019, antimicrobial resistance (AMR) was estimated to have directly caused 1.27 million (95% CI 0.91–1.71) deaths worldwide [1] which reflects the serious impact of AMR on the efficiency of antibiotic therapy [Reference Karakonstantis, Kritsotakis and Gikas2], and the lack of new compounds active against such organisms, specifically, Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species – (ESKAPE) [Reference De Oliveira3]. The latter group was highlighted as being of critical or high priority by the World Health Organization [Reference Tacconelli4] which called for urgent research and antibiotic development for these and other pathogens. ESKAPE organisms together with Escherichia coli (hereafter referred to as ESKAPEE) were responsible for more than 80% of global deaths attributable to AMR organisms in 2019 [1].
Greece is among the European countries with a high prevalence of antibiotic-resistant ESKAPEE infections in acute care hospitals and an estimated annual incidence of > 25 000 patients with nosocomial infections caused by difficult-to-treat pathogens [Reference Kritsotakis5]. Although several national and international investigations have made important contributions on the burden of AMR, they are less informative of local situations where the highest priority pathogens may differ in various locations and settings [1, Reference Fulchini6]. Additionally, existing national AMR surveillance programmes are mainly based on phenotypic data from clinical routine without detailed information on patient characteristics, and consequently may over-represent high-risk patients in tertiary care hospitals [7]. In contrast, surveillance is minimal, and data are sparse from provincial hospitals providing secondary level care to local communities and small geographical regions.
Examining bloodstream isolates is important for monitoring AMR and its disease burden, because bacteraemia is almost universally associated with clinical infection, whereas recovery of pathogenic organisms from non-sterile sites is less likely to represent true infection [Reference Cohen8]. Moreover, there is an ongoing important discussion on the use of hospital-onset bacteraemia as an outcome measure reflecting quality of care, and potentially a publicly reported quality metric [Reference Rock9, Reference Dantes10].
The purpose of this study was to estimate the incidence of ESKAPEE-associated bacteraemia, characterise affected patients, examine AMR patterns and trends, and assess their impact on mortality and length of hospital stay (LOS) in a setting typical of public secondary care provision in Greece.
Methods
Study design
This retrospective cohort study was conducted at the General Hospital of Agios Nikolaos in Crete, Greece, based on all consecutive admissions from July 2016 to December 2021 reporting positive blood cultures and antimicrobial susceptibility results in the database of the hospital's microbiology laboratory. Included were all patients who had a blood culture isolate belonging to one of the seven species of the ESKAPEE group. The number of eligible patients over the study period determined the study size and no a priori statistical calculation of sample size was performed.
Setting
The study site is a 140-bed city hospital that serves a population of ca. 75 500 citizens and admits ca. 6000 patients annually (average LOS of 4 days, all-cause inpatient mortality of 3%). The hospital offers highly differentiated acute-care services in 11 medical and surgical specialities and takes referrals from other district hospitals and primary care centres in the island of Crete and nearby islands. A dedicated three-member committee comprising a clinical microbiologist, an infectious disease physician and a dedicated nurse oversee infection surveillance and control activities in the hospital.
Data collection
For each patient, we recorded age, sex, ICD-10 codes for conditions present on admission, history of hospitalisation in the previous year, dates of admission and discharge, department of admission, department at time of blood culture, date of culture, microorganism(s) isolated, antibiotic susceptibility test results and patient outcome (classified as discharge alive, transfer to another hospital or inpatient death). In repeat confirmatory cultures, only the first blood culture results were recorded. Severity of comorbid conditions was measured by the Charlson comorbidity index (CCI), which was calculated based on the ICD-10 codes of present-on-admission comorbidities using methods previously described [Reference Glasheen11].
Microbiology
Blood cultures were processed using the BACTEC 9050 automated instrument (Becton Dickinson, Barelas SA, Greece). Isolates were identified to species level by phenotypic tests and antimicrobial susceptibility was determined with the MicroScan autoSCAN-4 System (Beckman Coulter, Leriva SA, Greece). E tests were used for susceptibility testing of relevant species isolates to colistin, acinetobacter to tigecycline (breakpoint 0.55 mg/l), and staphylococci and enterococci to vancomycin (breakpoint 4 mg/l).
Definitions
The following terms were defined prior to data analysis. Bacteraemia was classified as healthcare-associated if onset occurred on or after the third hospital day [Reference Dantes10, Reference Glasheen11], or earlier in patients receiving haemodialysis, and/or, admitted in a healthcare facility in the previous 90 days; otherwise, it was considered as community-onset. Onset of bacteraemia was defined as the date of collection of the blood sample for culture and polymicrobial bacteraemia was classed as isolation of two or more species isolates from the same blood culture.
Isolates with intermediate antibiotic susceptibility, or resistance, were grouped together to form the non-susceptible group. Each isolate was classified as follows: (i) non-MDR if non-susceptible to up to two antimicrobial groups; (ii) MDR if non-susceptible to three or more, but susceptible to at least two groups; (iii) extensively drug-resistant (XDR) if susceptible to only one or two groups; and (iv) pandrug-resistant (PDR) if non-susceptible to all agents in all antimicrobial groups. An isolate was reported as non-susceptible to an antimicrobial group if it was non-susceptible to at least one agent within the group. Antimicrobial groups and agents were defined according to the lists compiled by the ECDC and the US Centers for Disease Control and Prevention [Reference Magiorakos12]. As not all required antimicrobials were routinely tested (see Supplementary Table S1), the PDR category was interpreted as ‘possible’ in this study.
Statistical analysis
Multivariable Poisson regression with log-link function and robust variance estimation [Reference Knol13] was utilised to estimate adjusted relative risks (aRR) and 95% CI values for factors associated with the MDR/XDR phenotypes compared with non-MDR isolates. Investigated factors included patient sex, age, CCI, ICU stay, pre-culture LOS, healthcare-associated episode, polymicrobial growth and pathogen species. Age (years) and LOS (days) were modelled as continuous covariates.
To describe crude inpatient mortality over time and compare between pathogens and their AMR levels, cumulative incidence function plots based on competing risks analysis of survival times were utilised [Reference Brock14]. Differential effects between early and delayed fatalities were examined by assessing deaths in 14 and 30 days after the onset of bacteraemia and upon discharge from the hospital. Survival times were administratively right censored for patients who remained hospitalised after 14 or 30 days, respectively. Censoring of survival times was assumed non-informative for patients transferred to other hospitals before end of follow-up. Discharge alive was analysed as a competing event to in-hospital death [Reference Brock14]. Cause-specific hazard ratios (csHR) and their CIs were estimated with the Cox proportional hazards model to describe the direct effect of bacteraemia caused by each pathogen on the two competing events of interest (i.e. discharge alive and inpatient death). A low csHR for discharge alive reflected a low daily rate of discharge resulting in a prolonged LOS. We adjusted for confounding effects by age, ICU stay and CCI as common baseline risk factors for both prolonged LOS and increased mortality.
Full antibiogram data could not be retrieved for four (1.7%) isolates recovered from three (1.3%) patients, and these were excluded from related analyses. Data management and statistical analyses were performed using Stata version 17 (Stata Corp., College Station, TX, USA); statistical significance was indicated at the P < 0.05 threshold.
Ethics and reporting
The study was approved by the hospital's Review Board and the 7th Regional Health Authority of Greece (approval no. 34392) and is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [Reference von Elm15].
Results
ESKAPEE bacteraemia incidence and affected patients
Over the 5.5-year study period, the hospital admitted 32 996 patients for 131 654 patient-days (mean LOS, 4 days; 1009 inpatient deaths; crude mortality 3%). We recovered 239 ESKAPEE bloodstream isolates from 226 patients (0.7% of all admissions); the pooled rate was 1.8 cases (95% CI 1.6–2.1) per 1000 patient-days.
Affected patients had a median age of 75 years, 57% were male, 28% had a CCI ≥ 1, 67% had history of previous hospitalisation and 44% were treated in the ICU. The median LOS before onset of bacteraemia was 2 days, but most episodes (80%) were healthcare-associated. After bacteraemia onset, 14-day, 30-day and overall, in-hospital case-fatality rates were 20%, 27% and 30%, respectively (Table 1).
Table 1. Demographic and clinical characteristics and outcomes of patients (n = 226) with bacteraemia due to an ESKAPEE pathogen

IQR, interquartile range; LOS, length of hospital stay.
a Data are presented as n (%) for categorical variables and median (IQR) for continuous variables. The sum of reported percentages for organisms exceeds 100% due to polymicrobial infections.
b Enterococcus spp. includes E. faecium (n = 9), E. faecalis (n = 21) and other or not-specified enterococci (n = 14).
c Enterobacter spp. includes E. cloacae (n = 10) and E. aerogenes (n = 1).
d From the time of bacteraemia onset.
Pathogen and AMR distributions and trends
E. coli (33%) was the most frequent isolate, followed by Enterococcus spp. (20%), S. aureus (19%) and A. baumannii (16%). The distributions of multi-resistance levels among isolates are shown in Figure 1. Distributions of non-susceptibility rates for commonly used antibiotics per pathogen are given in Supplementary Table S2. A. baumannii was highly non-susceptible (>80% to 100%) to all antimicrobial agents tested, expect colistin (33%), and was almost exclusively classified as MDR or higher (97%), including four each of XDR and ‘possibly PDR’ phenotypes. None of the other pathogens were classed as XDR. MDR was most frequent in Enterobacter spp. (91%) and K. pneumoniae (60%), and less so in E. coli (33%) and P. aeruginosa (30%). A significant trend of increasing MDR incidence was observed in ESKAPEE isolates over the study period (mean annual increase of 0.1 isolates per 1000 patient-days; P = 0.036). S. aureus showed a statistically significant trend of increasing incidence of the MDR phenotype over time (Supplementary Table S3).

Fig. 1. Resistance levels of ESKAPEE bloodstream isolates (n = 235). Four isolates were excluded as full antibiogram data were missing (one A. baumannii, two E. coli and one K. pneumoniae).
Risk factors for MDR or XDR (as opposed to non-MDR) ESKAPEE bacteraemia are presented in Table 2. When evaluated using a multivariable model, MDR/XDR bacteraemia was more likely to be healthcare-associated than community-onset (aRR 1.67, 95% CI 1.04–2.65). The risk of these resistance phenotypes was also significantly more associated for patients with A. baumannii (aRR 1.90, 95% CI 1.23–2.92) or Enterobacter spp. bacteraemia (aRR 1.92, 95% CI 1.26–2.94) relative to those with E. coli. No significant association was found between the risk of MDR/XDR bacteraemia and patient sex, age, comorbidity, pre-culture LOS or prior ICU stay.
Table 2. Multivariable analysis of factors associated with the MDR or XDR as opposed to non-MDR phenotypes in patients with bacteraemia (n = 223)

non-MDR, non-multidrug resistant; MDR, multidrug resistant; XDR, extensively drug resistant; aRR, adjusted risk ratio; CI, confidence interval; ICU, intensive care unit; LOS, length of stay; IQR, interquartile range.
a Patients with polymicrobial cultures were assigned the highest level of resistance among the pathogens isolated.
Clinical impact
In total, 67 of the study patients (30%) died in the hospital; these were generally older, and significantly more likely to have a CCI ≥ 2, an ICU stay prior to the onset of the bacteraemia and isolation of A. baumannii of an MDR or XDR phenotype (Supplementary Table S4). A trend of increasing in-hospital mortality with increasing AMR level was also evident (Supplementary Fig. S1). Case fatality rates were 22%, 35% and 63% for non-MDR, MDR and XDR episodes, respectively (P = 0.004 for trend).
Cumulative incidence function plots, which account for competing risks and LOS censoring, showed that the disparity between the rates of in-hospital death in patients with non-MDR, MDR and XDR isolates was rapid in the first few days following bacteraemia onset and significantly increased with time (Fig. 2). In addition, the upper right panel in this figure shows a significant trend of decreasing discharge-alive rates, thereby increasing duration of hospital stay, with higher AMR levels. Stratification by pathogen genus (lower panels) revealed differential clinical impacts; A. baumannii was associated with the highest mortality rate and S. aureus the lowest. By contrast, E. coli and S. aureus bacteraemia had the shortest LOS (highest discharge rates) while A. baumannii and Enterococcus spp. episodes had the highest LOS.
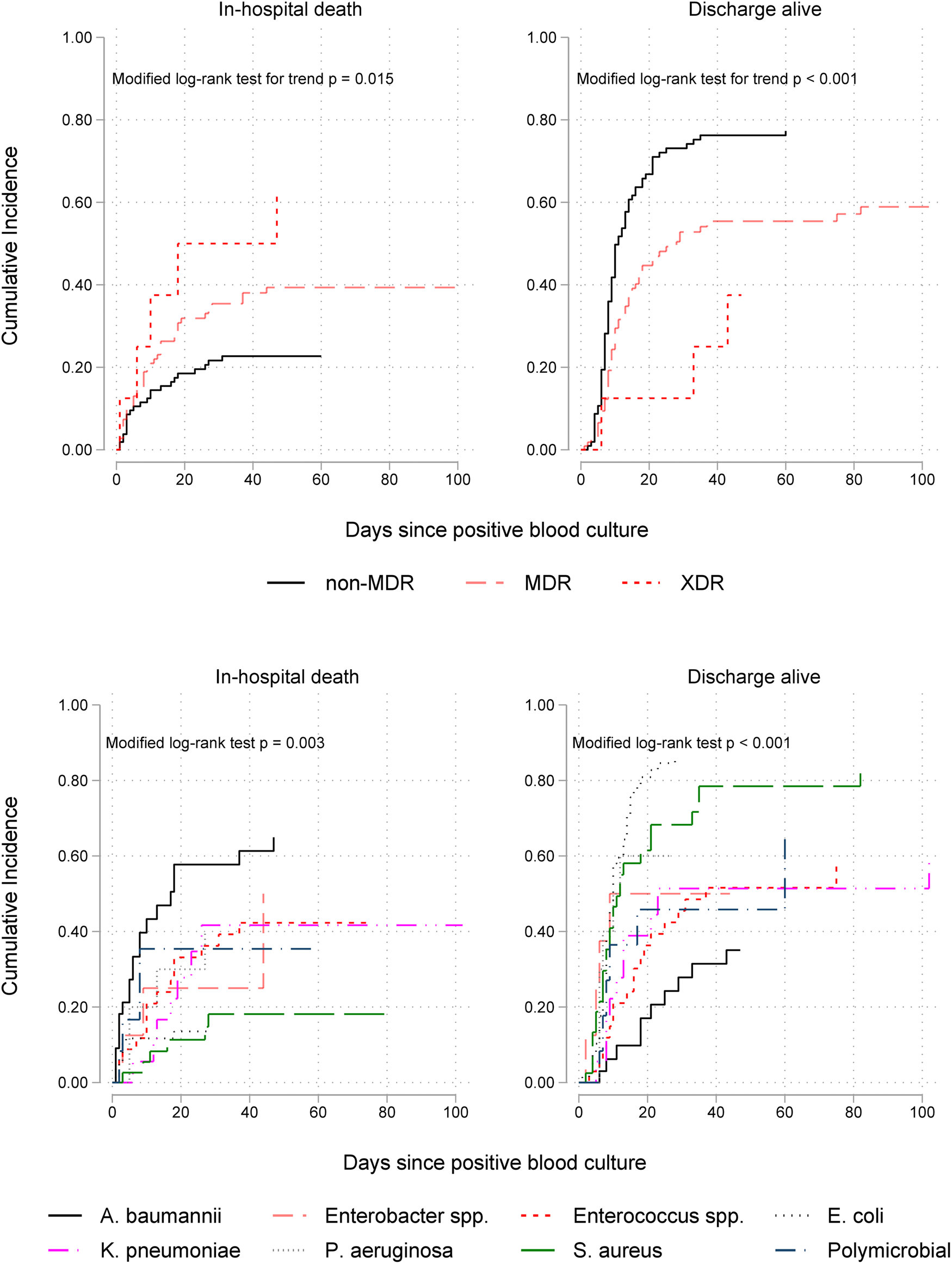
Fig. 2. Cumulative incidence functions for in-hospital mortality (on the left) and discharge alive (on the right) by antimicrobial resistance level (upper panel) and ESKAPEE organism (lower panel) isolated from blood in 226 patients. Lower incidence of hospital discharge alive indicates longer hospitalisation after bacteraemia onset. MDR, multidrug resistant; XDR extensively drug resistant.
As seen in Table 3, the multivariable competing risks analysis of 14-day outcome controlling for patient age, prior ICU stay and CCI, confirmed that of all ESKAPEE pathogens A. baumannii bacteraemia was the most fatal (csHR 3.39, 95% CI 1.74–6.63) with S. aureus the least fatal (csHR 0.27, 95% CI 0.08–0.87). Bacteraemia caused by Enterococcus spp. was independently associated with a prolonged LOS (csHR 0.44, 95% CI 0.23–0.85). Pathogen-specific effects on mortality and LOS diminished but remained significantly high when the analysis time was extended to 30 days after the onset of bacteraemia (Table 3) and at the time of hospital discharge (Supplementary Table S5).
Table 3. Multivariable competing risks survival analysis of in-hospital mortality up to 14 and 30 days after the onset of ESKAPEE-associated bacteraemia

csHR, cause-specific hazard ratio; CI, confidence interval; P, P-value; ICU, intensive care unit; MDR, multi-drug resistance; XDR, extensive drug resistance.
a The fitted model includes age, ICU stay, Charlson comorbidity index and organism.
b Each organism was entered separately into the model as a binary indicator variable to estimate its effect compared to any other organism and adjusting for age, ICU stay and Charlson index.
c Due to high correlation between resistance level and organism, a separate model was fitted to estimate the effect of resistance level adjusting for age, ICU stay and Charlson index.
Regarding the independent prognostic effects of multi-resistance patterns, our results were consistent with an increased risk of 14-day death associated with the presence of MDR (csHR 1.32, 95% CI 0.68–2.57) and XDR (csHR 1.61, 95% CI 0.45–5.78) phenotypes, independently of the patient's underlying condition, but these associations were not statistically significant.
Discussion
In this study, bacteraemia due to sentinel ESKAPEE pathogens occurred in about seven of every 1000 hospitalisations. The episodes were primarily healthcare-associated and affected patients were characterised by advanced age, severe comorbidity and/or underlying illness requiring intensive care, previous exposure to healthcare facilities and high inpatient mortality. An increasing trend of the MDR phenotype was evident over time, mostly related to healthcare-associated bacteraemia due to A. baumannii or Enterobacter spp. An increasing level of AMR was associated not only with increasing mortality, but also with simultaneously lengthier LOS. This is important because prolonged hospitalisation increases healthcare costs, raises the risk of acquiring other nosocomial infections, and the spread of AMR, to other vulnerable patients. In addition, our analysis demonstrated a differential clinical impact depending on the pathogen. Bacteraemia caused by A. baumannii (almost exclusively MDR, including some XDR strains) was the most fatal while S. aureus infection was the least fatal. Moreover, Enterococcus spp. bacteraemia was independently associated with prolonged LOS.
Directly comparing the observed incidence and associated mortality of bacteraemia in this study to other studies is problematic. Most research has focused on the burden of hospital-onset (or nosocomial) bloodstream infections and a systematic review reported incidence rates per 1000 patient-days of 0.5 in France, 0.6 in England, 0.8 in Finland, 1.3 in Spain and 0.8 in the USA [Reference Goto and Al-Hasan16]. Our study included only sentinel ESKAPEE bacteria, but based on results of the multinational SENTRY surveillance programme, these organisms constituted at least 70% of all bloodstream infections [Reference Diekema17]. Considering that 80% of our patient cohort were healthcare-associated, the observed rate of 1.8 cases per 1000 patient-days is largely comparable to the aforementioned studies and probably lies at the upper end of the range of reported rates in other countries. Inpatient mortality observed here (20% at 14 days, 30% overall) is within the spectrum of case fatality for bloodstream infections typically reported from studies in Europe (12–32%) and the USA (15–30%) [Reference Goto and Al-Hasan16]. However, given the observed differential epidemiology within the ESKAPEE group it is more appropriate to discuss their health burden in a pathogen-specific context.
In many studies, E. coli and S. aureus are numerically the most frequent, and with P. aeruginosa, constitute the most prevalent reported organisms causing mortality [Reference Goto and Al-Hasan16, Reference Kern and Rieg18]. P. aeruginosa, K. pneumoniae and staphylococci are typically associated with healthcare acquisition, while E. coli is more frequent among community-acquired cases [Reference Kern and Rieg18]. Our data are consistent with these observations. Nevertheless, the unique burden of A. baumannii found in this study is noteworthy and possibly reflects its greater incidence in the Greek hospital system as a whole, both in terms of its hospital-wide frequency and detrimental impact on patient outcome [Reference Kritsotakis5].
According to the results of SENTRY [Reference Diekema17], A. baumannii first appeared in the worldwide top-10 list of pathogens causing hospital-onset bacteraemia after 2005. It is more frequent in Europe than North America, and even more prevalent in the Asia-Pacific and Latin America regions [Reference Diekema17]. Once considered of low virulence, the species is now recognised as a significant healthcare pathogen [Reference Mohd Sazlly Lim19]. In Greece, a national-level study found A. baumannii to be the second most prevalent pathogen causing various hospital-acquired infections in 37 hospitals in 2012, and attributed more than 10 000 inpatient deaths annually in the country to carbapenem-resistant A. baumannii infections [Reference Kritsotakis5]. In this study, A. baumannii was an important cause of bacteraemia (>16% of cases) and the leading cause of inpatient mortality. Reasons behind the widespread persistence of A. baumannii in Greek hospitals have not been fully investigated, but it is likely related to the intrinsic resistance of the organism to many antibiotics and its abilities to survive in inanimate environments for long periods along with rapid development of new resistances, and clonal spread. Moreover, a robust global seasonal (temperature-associated) pattern of Acinetobacter infections in hospitalised patients has been recognised [Reference Kritsotakis and Groves-Kozhageldiyeva20], and thereby environmental or climatic factors may impact on the local epidemiology of this organism.
AMR in Acinetobacter spp. is a widespread problem in Europe where combined resistance to fluoroquinolones, aminoglycosides and carbapenems was noted for more than half of invasive isolates reported to EARS-Net from 29 countries in 2020 (Europe-wide mean 34.1%) [7]. In this study, A. baumannii bloodstream isolates were highly non-susceptible (>80% to 100%) to all agents tested except colistin, and about one in five isolates were XDR including four strains classified as possible PDR. One-third of A. baumannii isolates were colistin non-susceptible and this proportion reached 50% during 2020 and 2021, constituting an alarming finding for our region.
We noted a significant trend of increasing MDR S. aureus (24% MRSA) over time, but associated bacteraemia was the least fatal outcome for our patients compared to other pathogens. We should note, however, that S. aureus bloodstream infection continues to carry a serious prognosis, as inpatient mortality after acquisition of the organism was 7.5% and 15% at 14 and 30 days, respectively, which is in line with large multinational cohorts at tertiary care centres reporting corresponding rates of 11–15% and 21–22%, respectively (including post-discharge deaths) [Reference Kaasch21, Reference Nambiar22]. Similar to our results, MRSA was not independently associated with survival in those studies [Reference Nambiar22]. Likewise, no adverse association between MRSA and patient survival was reported in a large cohort of Australian patients with S. aureus bacteraemia after adjustment for important prognostic factors [Reference Yaw, Robinson and Ho23]. This observation that mortality was irrespective of methicillin resistance status perhaps advances a case towards novel interventions such as decolonisation strategies [Reference Samuelson24].
In general, AMR levels in this study were similarly high as those reported in the Mediterranean, including Italy and Israel [7, Reference Kern and Rieg18]. However, except for A. baumannii, none of the other pathogens presented an XDR phenotype. Major changes and challenges in the epidemiology of E. coli and K. pneumoniae bloodstream infections in Europe have been the emergence of resistance to fluoroquinolones (FQ) and third-generation cephalosporins (3GC), the latter primarily caused by extended spectrum β-lactamase enzymes [7, Reference Kern and Rieg18]. We observed resistance rates in E. coli and K. pneumoniae in our region that were much lower than the national databases reported for Greece, and closer to (but higher than) the European averages. In particular, FQ resistance of E. coli and K. pneumoniae in this study reached 25% and 45%, respectively, as compared to 24% and 34% Europe-wide and 33% and 74% Greece-wide in 2020, respectively [7]. Similarly, E. coli and K. pneumoniae 3GC resistance rates were 12% and 45%, compared respectively to Europe as a whole (15% and 34%) and wider Greece (22% and 75%) [7]. Our overall proportion of difficult-to-treat carbapenem-resistant K. pneumoniae was 25%, which is above the European average (10%) but well below the national average (66%) [7]. These relatively low AMR levels may partially explain the absence of independent adverse associations of E. coli and K. pneumoniae bacteraemia with inpatient mortality observed here after adjustment for indices of the patients' underlying condition.
Our findings should be interpreted in view of several limitations. First, although our local setting is typical of public secondary care provision in Greece, there may be substantial variations within the country such as clinical practice patterns, patient characteristics and microbiological investigation across hospitals in different regions. Second, we reported in-hospital mortality, which possibly underestimates the true case fatality rate, as some patients may die at home or at post-acute-care facilities soon after hospital discharge. Third, despite controlling for key prognostic indices of patients' underlying condition, residual confounding factors cannot be wholly ruled out. We could not account for treatment factors in our analysis and assessing whether worse clinical outcomes were due to intrinsic virulence properties of the offending strains or due to treatment failure, were beyond the scope of this study. Fourth, we were unable to disentangle interactions between AMR level and pathogen genus due to the high correlation between them (e.g. A. baumannii was exclusively MDR/XDR) and because the small sample size per pathogen did not permit pathogen-specific subgroup analyses. Finally, genetic, or molecular testing to examine specific resistance genes or enzymes was not performed; hence, the origin, clonal relationship and nosocomial spread of ESKAPEE isolates were not investigated.
In conclusion, this first comprehensive epidemiological report of bacteraemia caused by sentinel ESKAPEE pathogens in a setting typical of secondary care provision in Greece shows that it remains a rare event for hospitalised patients in this setting, but the overall incidence may lie in the higher end of the range reported from other studies in Europe. Our analysis revealed pathogen-specific differences in incidence, AMR levels, case fatality and prolongation of LOS, and identified the unique role of MDR A. baumannii as a leading cause of inpatient mortality. Moreover, our AMR data showed some marked deviations from national and international benchmarks, and further emphasises the importance of local investigations in healthcare epidemiology.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268822001492.
Author contributions
Study conception: E. I. K. Study design: E. I. K. and D. L. Acquisition and interpretation of data: D. L., E. I. K., E. M., I. G. and A. G. Data management: D. L. and E. I. K. Statistical analysis: E. I. K. Manuscript preparation: E. I. K. All authors critically reviewed and approved the final manuscript.
Financial support
This research received no external grant from any funding agency, commercial or not-for-profit sectors. The HEAL-Link consortium financially supported the publication of the article in open access mode.
Conflict of interest
None.
Data availability statement
All relevant data of this study are available from the corresponding author, EIK, upon reasonable request and subject to full anonymisation of patient data.








