Introduction
Gastrointestinal illness (GII) is a major public health concern globally [Reference Murray1]. There are however regional differences in the GII incidence and ethology [Reference Fletcher, McLaws and Ellis2, Reference Scallan3]. While the GII-related mortality is low in high-income countries, the morbidity is still high, resulting in high societal costs due to sick leave or staying home with children [Reference Roberts4]. Nowadays national GII surveillance systems are commonly used to monitor the GII incidence; however, additional knowledge on the true burden of GII is needed for priority setting and implementation of control and management strategies. Several methods for estimating the GII incidence have been used, each method facing different challenges and limitations [Reference Roy, Scallan and Beach5]. Declining response rates in epidemiological surveys [Reference Galea and Tracy6] have increased the need to identify new innovative ways for data collection. The near-ubiquitous use of mobile phone in most parts of the world offers alternative methods for collecting epidemiological data, via Short Message System (SMS) and mobile apps. SMS has been shown to be a promising tool for epidemiological research, having low cost and high response rate, and not compromising the quality of the data [Reference Kew7, Reference Johansen and Wedderkopp8].
The aim of the present study was to estimate the burden of GII in Sweden and to evaluate SMS as a tool for collecting repeated estimates of self-reported GII in a 1-year prospective cohort. For validation, additional GII estimates were collected by telephone among the cohort participants and nationwide respondents. To our knowledge, this is the first time SMS has been used to collect repeated GII estimates.
Material and methods
Study population
The present study is the pilot study of a project assessing drinking-water-related GII by using SMS as a data collection tool. Part of the data collection method has been described previously [Reference Save-Soderbergh9]. The study population comprised adults (age 18–80 years) living in Ale, a suburban municipality in the South West Sweden. The municipality had a population of ~28 000 inhabitants, an average sized municipality by Swedish standards. Two-thirds of the inhabitants were living in urban areas. The demographic distribution of Ale resembles that of Sweden in general. Prior to the study, information was advertised in the local newspaper and on municipal homepages. Oral informed consent was obtained from all respondents. The Swedish language was exclusively used in all parts of the study (0.3% were unable to participate due to language barriers). The regional ethical review board in Uppsala, Sweden, approved the study.
Initial telephone interviews and recruitment to SMS cohort
In early 2012, computer-assisted telephone interviews were carried out in Ale by professional interviewers (Fig. 1). The national consumer register (PAR, including 87% of the Swedish population >16 years) was used for obtaining a representative selection of respondents in the adult population, based on age and gender. Respondents residing on average <5 days a week in Ale or having chronic/recurring GII (due to illness, medication or pregnancy) were excluded at an early stage of the interviews. Of the 9142 persons included in the target population that was contacted, the response rate was 44%, and of these, 3865 respondents were included in the analyses (excluding respondents having chronic/reoccurring GII or living in Ale <5 days/week). About 60% of non-participation was due to non-response and the remaining was due to refusal. Respondents were asked to report if they have had GII during the last 28 days, and in case of illness, the clinical profile of the last episode was collected: (i) any combination of the symptoms: diarrhoea, bloody diarrhoea, vomiting, nausea, stomach ache and fever; (ii) number of loose stools during 24 h; and (iii) number of days of illness. Background information of respondents and their households was collected as well. To assess the nationwide generalisability, nationwide data among a representative selection of participants were collected in parallel (1000 participants).
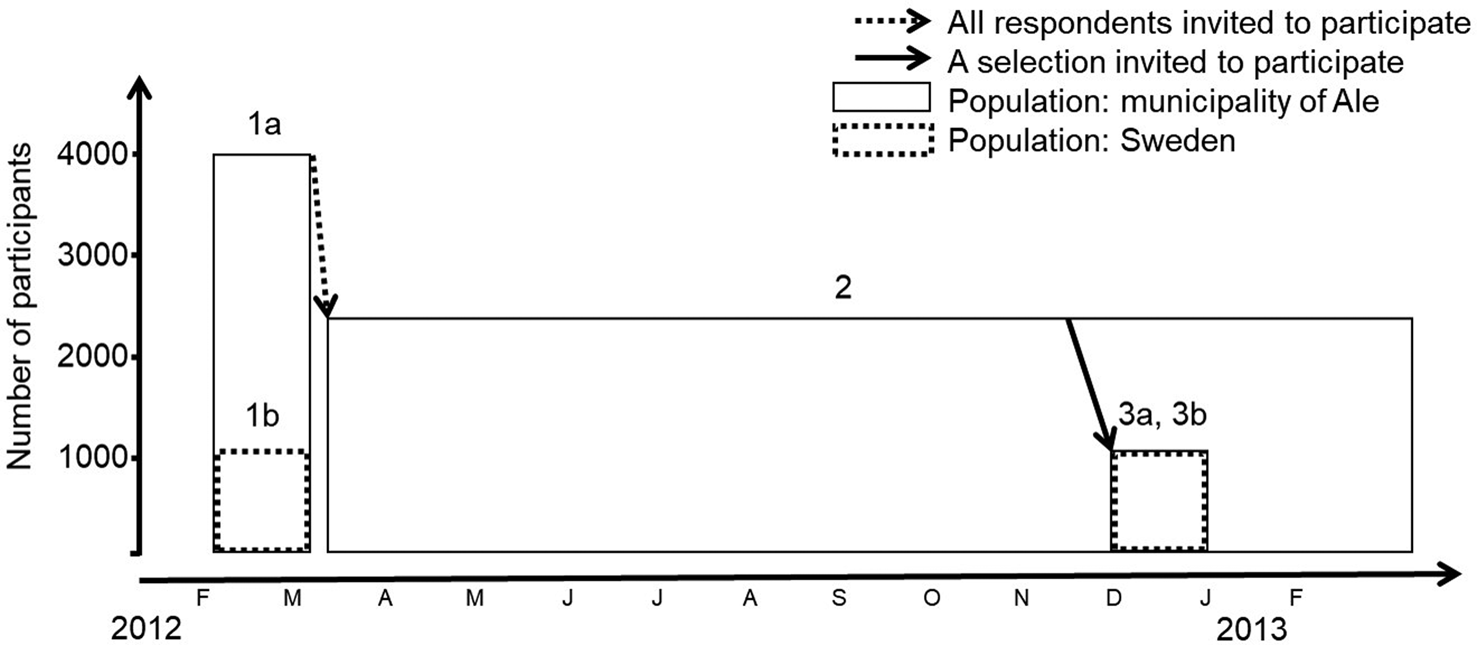
Fig. 1. Schematic view of the data collection 2012–2013. Telephones interviews (1a) in the municipality of Ale were carried out to recruit participants to the 1-year SMS study (2). Additional telephone interviews were conducted for a representative selection of participants in the 1-year SMS study (3a). Two nationwide telephone interview surveys (1b and 3b, new nationwide selection contacted for each survey) were conducted in parallel with the telephone interviews in Ale.
Twelve-month SMS cohort
The respondents of the telephone interviews were given the opportunity to continue their participation in the subsequent 12-month SMS cohort (Fig. 1). The 67% (2668 respondents) that accepted, received additional instructions by mail. The participants of the SMS cohort were divided into two sub-panels. The sub-panels were similar in composition with regard to gender, age, type of water source and daily water consumption. Each of the panels received monthly questionnaires around either the turn or the middle of each month, from March 2012 to February 2013 (Fig. 2). In total, each participant received 12 SMS questionnaires. SMS questionnaires were sent out according to a pre-set schedule, ensuring an even distribution between days of the week over the entire year. The first question was always sent at 10 AM and 24 h prior to the SMS questionnaire, a pre-reminder SMS was sent out. A questionnaire was composed of several questions, each question distributed as a separate SMS. The respondents were asked how many episodes of GII they have had during the last 28 days (the maximum allowed number of episodes of GII during 28 days was three, as seven disease free days should have passed between two episodes). In case of illness, respondents received additional questions on GII during the last 14 days and were asked to define the clinical profile of the last occurring episode: (i) symptoms: diarrhoea/vomiting/nausea/stomach ache/fever, (ii) number of loose stools during 24 h and (iii) number of days of illness. Data on bloody diarrhoea were not collected during the SMS cohort. Respondents were informed not to reply if they had been away from home for a continuous period of 7 days during the last 28 days, to reduce travel-related GII. In case of non-response during 24 h, a reminder was sent out. The questionnaires were closed after 48 h. The average response time to complete the SMS questionnaires after send-out was 4 h. As a reimbursement, the respondents received a lottery scratch card (value ~€1) each month.
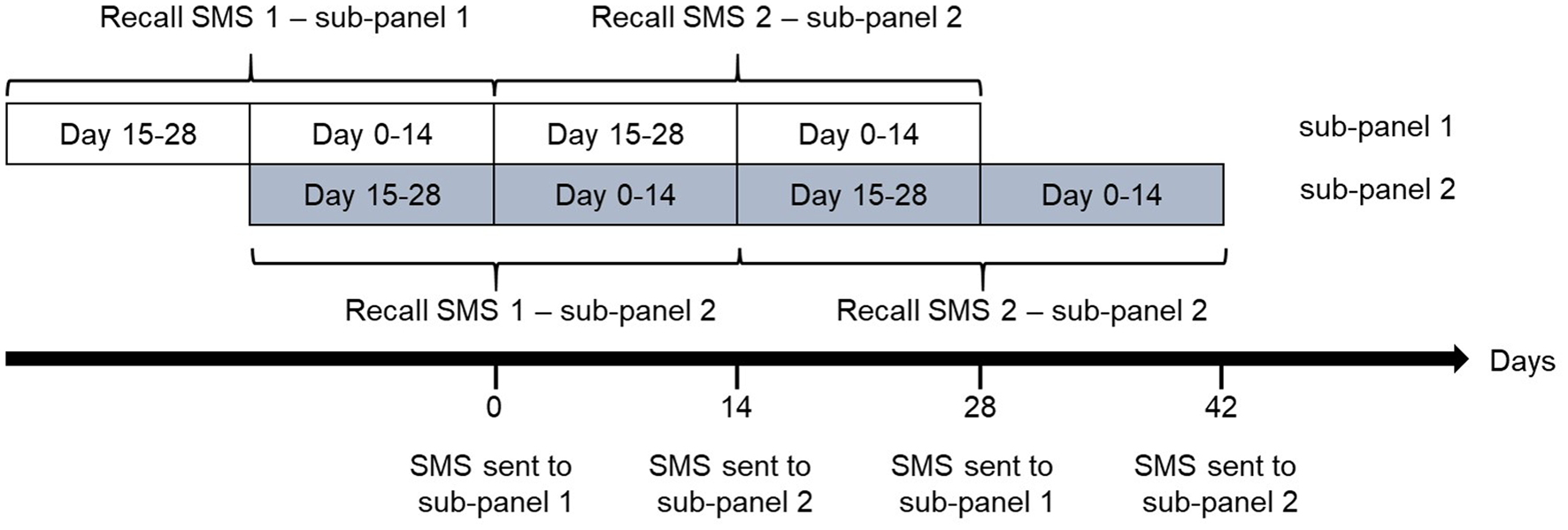
Fig. 2. The participants of the SMS cohort were divided into two sub-panels. Each of the panels received monthly SMS-questionnaires around either the turn or the middle of each month. In total, 12 waves of questionnaires were sent to each of the sub-panels during the course of a year. Upon receiving an SMS questionnaire, each participant reported whether he or she had experienced gastrointestinal illness (GII) in the last 28 days, and if yes during the last 14 days, yielding estimates of both 28- and 14-day recall of GII.
Validation
In order to validate the study, additional telephone interviews were conducted at the end of the SMS cohort among a subset of the respondents (1007 participants, a total response rate 56%) (Fig. 1). Respondents were asked to report how many episodes of GII they have had during the last 28, 14 and 7 days. In case of illness, the clinical profile of all reported episodes was collected: (i) any combination of the symptoms: diarrhoea, bloody diarrhoea, vomiting, nausea, stomach ache and fever; (ii) number of loose stools during 24 h; and (iii) number of days of illness. To assess the nationwide generalisability, telephone interviews were also carried out in parallel among a representative selection of respondents in Sweden (1000 participants).
Case definition
Based on the self-reported GII, the following case definitions were used: self-defined GII (sdGII, reported by participant, any symptom or symptoms unaccounted for), acute gastrointestinal illness (AGI, vomiting and/or at least three loose stools during a 24 h period), severe acute gastrointestinal illness (sAGI, vomiting and at least five loose stools during a 24 h period) and mild gastrointestinal illness (mGII, defined as sdGII minus AGI) (Fig. 3). In addition, specific symptoms, individually or in combination, were collected in the validation study: vomiting, diarrhoea and bloody diarrhoea.
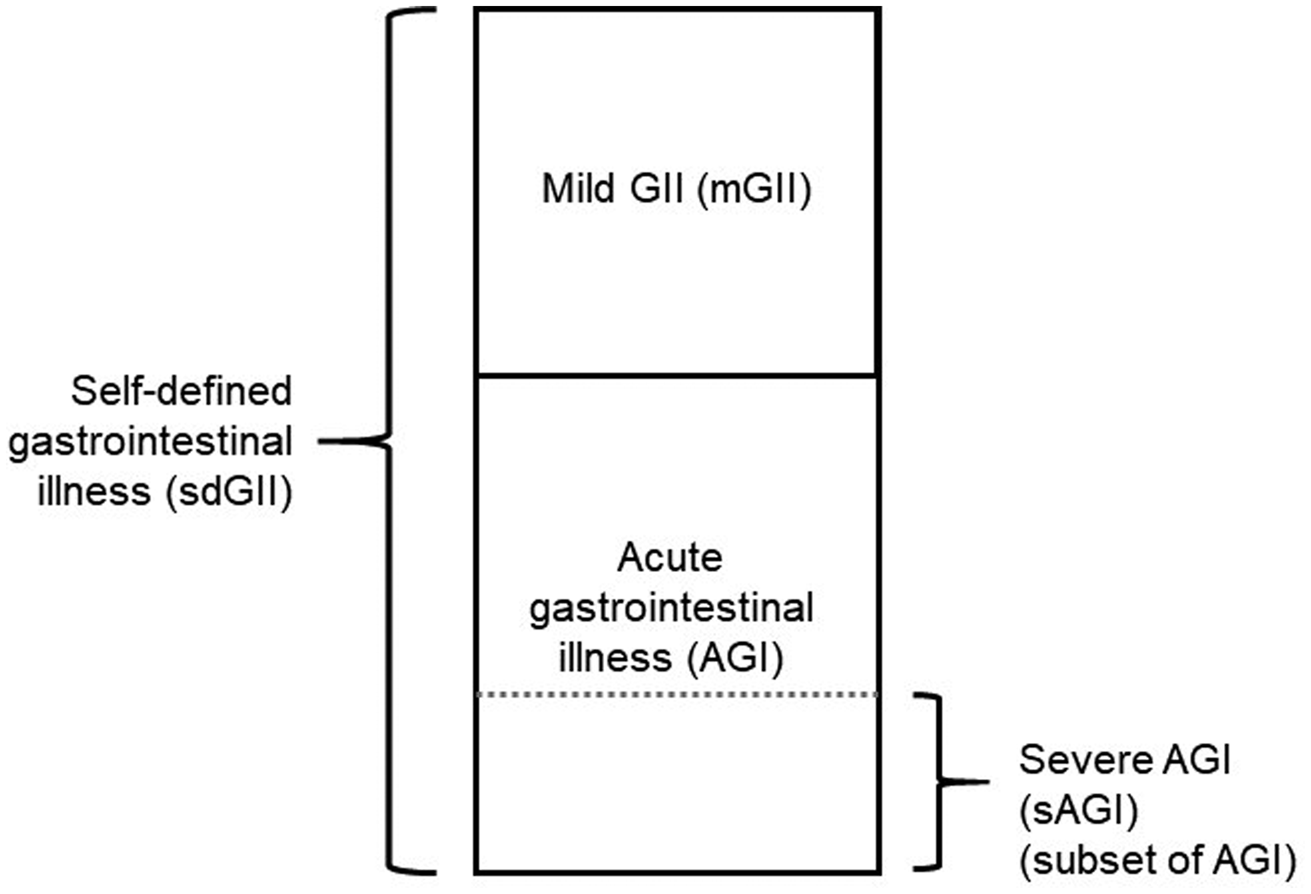
Fig. 3. Case definitions of gastrointestinal illness (GII) used in this study: self-defined GII (sdGII, reported by participant, any symptom or symptoms unaccounted for), acute gastrointestinal illness (AGI, vomiting and/or at least three loose stools during a 24 h period), severe acute gastrointestinal illness (sAGI, a subset of AGI, vomiting and at least five loose stools during a 24 h period) and mild gastrointestinal illness (mGII, defined as sdGII minus AGI).
Statistical analyses
Cases of GII were defined according to the different case definitions and all non-responses were excluded from the analyses. Prevalence was calculated as: (number of cases)/(total number at risk), while the annual incidence rate, episodes per person-year, was calculated as: (number of cases)/((total number at risk)×(interval of time)), by using binary case data for 14-day recall. For multiple episodes of GII reported in SMS questionnaires, the case definition of the last occurring GII was applied to all cases. The correlation coefficient (ρ, rho) between 28-day recall SMS responses and telephone responses during corresponding recall period was estimated by using tetrachoric correlations (pairwise correlation of binary variables by maximum likelihood estimator). For descriptive statistics of GII from SMS responses, we used a recall time of 14 days, estimated from the 14- and 28-day estimate (thus estimating last 0–14 and 15–28 days from each SMS send-out) or only the 14-day estimate. For descriptive statistics of the telephone interviews, we used 7-, 14- and 28-day recall. Seasons were defined by months as winter (December–February), spring (March–May), summer (June–August) and autumn (September–November). All statistical analyses were performed using Stata 12.1 (StataCorp, College Station, Texas, USA).
Results
Response rates and population characteristics
The response rate from the participants in the SMS cohort was 88% (participants answering at least one questionnaire) (Table 1). The response rate in the telephone interviews ranged from 38% to 56%, with the highest rate in the validation study, in which participants of the SMS study were contacted. From the 2348 participants in the SMS cohort, 23 961 SMS responses were received, corresponding to 85% of all SMS surveys sent out during the study. We observed no major differences in the population characteristics between the participants in the SMS cohort, telephone interviews in Ale and nationwide telephone interviews (Table 1). Among the participants in the validation telephone study, selected from the SMS cohort, about 81% reported to use SMS at least once on a weekly basis (Table 1). An additional 11% reported to use SMS at least monthly.
Table 1. Population characteristics, expressed as proportion (%) of the total study population

Risk occupation with respect to exposure to pathogens causing gastrointestinal illness: daycare, school, youth recreation centre, medical care, retirement home or sewage-related work.
a Answering at least one SMS questionnaire.
Prevalence, incidence and duration of GII
Among all SMS questionnaires (for 0–14 and 15–28 days recall, based on the 14- and 28-day estimates), the participants reported 1167 2-week periods with at least one sdGII episode (179 2-week periods had two sdGII episodes), corresponding to a prevalence of 2.4% and annual 28-day incidence of 0.64 cases per person-year (Table 2). The prevalence for AGI was 1.6% and the annual incidence was 0.43 cases per person-year, while the prevalence and incidence for the more severe form of AGI (sAGI) were 0.25% and 0.065 cases per person-year. For the milder form of GII (mGII), the reported prevalence was 0.81% and incidence was 0.21 cases per person-year. When the incidence was estimated by using the recall of the last 14 days, the annual incidence was 16–21% higher for sdGII, AGI and mGII, and 12% higher for sAGI (Table 2).
Table 2. Two-week prevalence (14 daysa) and yearly incidence rate (cases per person-year, last 28 days and last 14 days) of gastrointestinal illness cases reported by monthly SMS (2348 participants, 23 961 SMS responses) presented by symptom definition, year, season, age and gender
sdGII: all symptoms, clinical profile of last reported episode; AGI: vomiting and/or three loose stool sample, clinical profile of all reported episode; sAGI: vomiting and five loose stool samples, clinical profile of all reported episode; mGII: sdGII, minus AGI, clinical profile of all reported episode.
a The 14-day recall estimate arrives from the 14- and 28-day recall estimates in the SMS questionnaire, thus estimating 0–14 and 15–28 days from each SMS send-out.
We observed seasonal differences in the prevalence, with the highest prevalence during winter and the lowest during summer (Table 2). The exception was for mGII, for which the prevalence was similar independent of season, although there were indications of a slightly higher prevalence during summer. We also observed differences in the prevalence between gender and age. The prevalence, for all case definitions, was generally highest among the age group 18–39 years and lowest among the age group 56–80 years. While the prevalence of most GII case definitions was similar between genders, there were some differences in the prevalence of mGII (higher prevalence among men).
The duration of illness among sdGII was on average 2.3 days (Table 3). The duration of illness for AGI and sAGI was slightly higher, 2.6 and 2.8 days, respectively, while the duration of mGII was 1.9 days. Fifty-seven per cent of the sdGII episodes lasted at least 1 day, while only 9% lasted more than 3 days. Similar durations were seen for AGI and mGII. For sAGI, most episodes lasted at least 2 days (64%), especially during summer (84%). GII episodes lasting >6 days were few, for all case definitions.
Table 3. Duration of gastrointestinal illness by symptom definitions, expressed as average (days and standard error) or proportion (%) of self-defined gastrointestinal illness (sdGII) cases by their reported days of duration
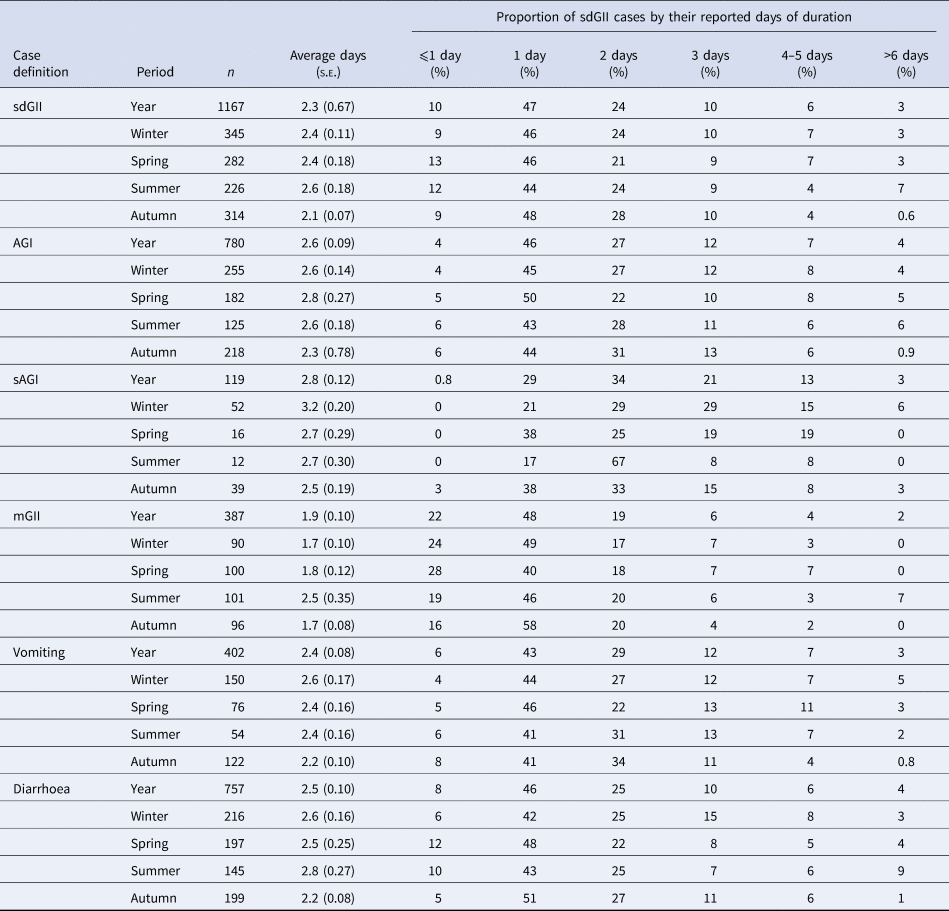
s.e., Standard error; sdGII: all symptoms, clinical profile of last reported episode; AGI: vomiting and/or three loose stool sample, clinical profile of all reported episode; sAGI: vomiting and five loose stool samples, clinical profile of all reported episode; mGII: sdGII, excluding AGI, clinical profile of all reported episode.
Validation of SMS
When estimating the intra-individual correlation for SMS responses and responses from the validation telephone study for the corresponding periods, we observed that responses for 28-day recall were highly correlating for most symptoms and case definitions, especially the case definitions on severe illness like AGI and sAGI (Table 4). In addition, the prevalence for different GII case definitions reported by SMS (prospectively) (Table 2) was only slightly higher compared to the prevalence reported from the retrospective telephone interviews in Ale and nationwide (Table 4), confirming the nationwide representability of the SMS cohort.
Table 4. Prevalence of gastrointestinal illness by data collection method, recall time and symptom definition
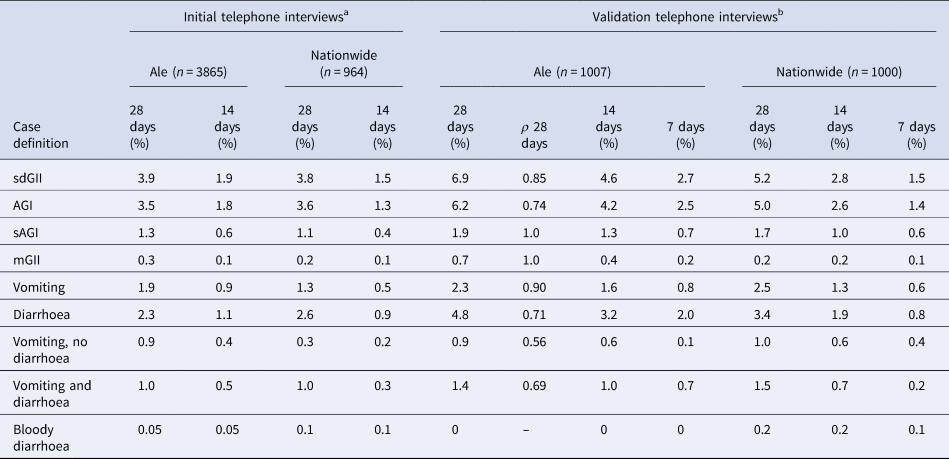
The correlation coefficient (ρ) between the validation telephone interviews in Ale and the corresponding SMS questionnaires for the same persons in the SMS cohort.
sdGII: all symptoms; AGI: vomiting and/or three loose stool sample; sAGI: vomiting and five loose stool samples; mGII: sdGII, excluding AGI.
a Clinical profile of last reported episodes.
b Clinical profile of all reported episodes.
Discussion
In this 1-year prospective SMS cohort, we estimated an annual incidence of sdGII and AGI among adults of 0.64 and 0.43 cases per person-year, respectively. With a 14-day recall, instead of 28 days, the incidence was about 20% higher. We also found that responses collected by SMS highly correlated with responses collected by telephone.
The incidence of AGI among the total population in Sweden has previously been estimated in a retrospective study, using a 1-year recall [Reference Hansdotter10] and a prospective cohort, using a 1-week recall [Reference Edelstein11]. The studies reported an incidence rate of 0.31 and 0.36 episodes of AGI per person-year, with the highest incidence rate among the youngest age groups. Among the population over 15 years, the incidence ranged from 0.09 to 0.33 episodes of AGI per person-year depending on the age group. Although the incidence reported previously is lower compared to the present study, the estimates are still in line with previous estimates, especially when comparing with the prospective cohort with the 7-day recall time [Reference Edelstein11].
European studies have reported incidence ranging from 0.27 to 1.4 AGI episodes per person-year [Reference Hansdotter10–Reference Van Cauteren18]. Most of the previous estimates are for the total population, including children, thus partly explaining the low AGI reported in our study. Other differences may be linked to factors affected by geographical location. Studies from Norway, Denmark and Canada have reported an incidence among adults of 0.2–2.3 episodes of AGI per person-year, depending on gender and age [Reference Muller, Korsgaard and Ethelberg12, Reference Kuusi13, Reference Majowicz, Horrocks and Bocking19]. In comparison, the highest reported AGI incidence in the present study was 0.62 cases per person-year (females <40 years). Also the study design in the present study, especially the inclusion criteria, may have affected the GII incidence; however, few were excluded due to not mastering Swedish, not residing in Ale more than 5 days a week or having chronic/recurring GII. Excluding travel-related GII might however have affected the GII incidence, thus the GII incidence reported in the present study should be seen as domestic.
As reported in previous studies [Reference Muller, Korsgaard and Ethelberg12, Reference Viviani14], the GII incidence in the present study was affected by recall time. This may be linked to a recall bias commonly known as telescoping, where participants remember disease episodes as occurring more recently than they actually were. We also believe that the difference may additionally be linked to the duration of GII episodes. Although we clearly defined how episodes should be reported, it is likely that GII episodes with a longer duration will be reported during multiple recall periods. However, in the present study, the difference in annual incidence between 14- and 28-day recall for the SMS responses was 12–21%, depending on the case definition, while retrospective studies have reported differences of 42%, comparing 7- and 14-day recall [Reference Muller, Korsgaard and Ethelberg12] and 65%, comparing 7- and 28-day recall [Reference Viviani14]. In the estimates collected by telephone interviews in the present study, the difference between the prevalence for 14- and 28-day recall was low, while the 7-day recall yielded a 10–15% higher estimate compared to 14-day recall. Therefore, using the 14-day recall in the SMS study may potentially underestimate the incidence to some extent.
As reported in previous studies, we observed gender- and age-related differences in the GII prevalence. The highest prevalence of most GII case definitions was among the age group of 18–40 years old and especially women. The high prevalence in this age group may be due to life style and especially due to having small children. Children are typically more prone to contracting GII and may transmit the disease to their parents. In Sweden, there is a national, publicly funded parental leave insurance. In 2013, the majority of the parental leave days were taken out by women [20], potentially resulting in a higher risk of exposure to child-related GII.
In high-income countries, the GII prevalence often follows a bimodal distribution [Reference Majowicz, Horrocks and Bocking19, Reference Thomas21]; however, in several studies, especially from Nordic countries such as Denmark and Sweden, a unimodal distribution of GII is observed with the peak occurring during the cold season [Reference Edelstein11, Reference Muller, Korsgaard and Ethelberg12, Reference Sargeant, Majowicz and Snelgrove22]. This is also the case in the present study. The bimodal distribution is often explained by a high prevalence of viral infections during winter and bacterial infections during summer. The lack of a GII peak during summer may therefore be a result of high hygiene standards, reducing bacterial infections, but to smaller extent viral infections. The unimodal distribution of GII is also reflected in the Swedish statistics of reported foodborne outbreaks of GII [Reference Lindblad23], strengthening this assumption. It should also be mentioned that the present study encouraged non-response from participants in case of travel, thus intentionally reducing foreign and domestic travel-related GII.
Although this was a 1-year cohort, there may be inter-annual differences in the GII incidence. Although the statistical signal for winter vomiting disease started at week 43 in 2012, compared to weeks 46–48 in 2013–2015 [24], the annual peak during 2012 showed a similar pattern to following years, indicating that 2012 was representative in terms of endemic viral infections.
In the present study, the GII episodes reported were generally short in duration and there was no clear difference in the duration of illness between seasons. Based on present knowledge on the clinical profiles of different pathogens from mainly primary care visits primarily in the Nordic countries [Reference Bylund, Toljander and Simonsson25], the short duration of GII is likely due to a high proportion of viral infection, like norovirus or adenovirus. While some of the cases may be due to bacterial infections, it is unlikely that a large proportion is due to, for example, salmonellosis, which generally has a longer duration of illness. Bloody diarrhoea was included in the telephone surveys, but not in the SMS study, thus there is limited information to draw any conclusion about the proportion of infections associated with pathogens giving rise to bloody diarrhoea, e.g. STEC (Shiga toxin-producing Escherichia coli). While the duration indicates that a majority of the infections have a viral aetiology, the proportion of cases including vomiting as a symptom in the present study was lower than previously reported for viral infections [Reference Kaplan26]. In a previous study of the clinical profiles of common GII pathogens, 76% of norovirus cases reported vomiting [Reference Bylund, Toljander and Simonsson25]; however, a large part of these cases were health care visits. Endemic viral GII infections may therefore be milder compared to cases resulting in visiting primary care, emphasising the need to use community-based studies when assessing the societal burden of infections.
Based on the results of our study, SMS seemed to be a promising tool for the collection of repeated estimates of GII, although the method has both strengths and limitations. First, as mobile phones are owned by 98% of all adult Swedes [27] and 92% of the cohort participants reported to use SMS at least on a monthly basis, we believe that any bias linked to ownership of mobiles and use of SMS is likely to be low. Second, based on the results from the validation study, the prevalence of GII was similar for SMS and the telephone interviews, with a high intra-individual correlation between the data collection tools. Third, we experienced a high response rate in the SMS cohort. Considering that the participants were instructed not to answer the SMS questionnaire in case of travel, the true response rate is likely even higher. For future studies using SMS as data collection, we therefore recommend to include a possibility for the participants to indicate a, for the study, legitimate reason for not answering the questionnaires, like using keywords. Some of the non-responses were also due to technical difficulties (about 1% of the participants in the validation telephone interview reported that they had experienced this). With regard to the response rate, it should be noted that Sweden had experienced several large-scale drinking-water-related outbreaks [Reference Widerstrom28, Reference Bjelkmar29], which may have increased the interest in participating. However, the low response rate in the telephone interviews, having similar questionnaires as the SMS cohort, indicated that the data collection method is affecting response rate more than the research subject. Despite differences in the response rate, it should be mentioned that non-response generally introduces limited bias [Reference Shahar, Folsom and Jackson30, Reference Kreiger and Nishri31]. Fourth, the participants replied to the SMS questionnaires instantly or very shortly after they received the questionnaires. This gave fast indications of any technical problems and gave the opportunity to send reminders exclusively to those that had forgotten to answer. The fast response also assured that the estimates from the sub-panels were corresponding in time, thus none of the SMS send-outs hade more than 48 h between the first and the last response. Fifth, although a shorter recall may have been preferable, monthly questionnaires on the 14- and 28-day GII prevalence were sent out, to reduce the risk of dropout among the study participants. Using a 14- and 28-day recall gave a good estimate of the prevalence and yearly incidence, which to a small extent differed from estimates based on a shorter recall time. The present study design also resulted in a high response rate with few dropouts, thus increasing the validity of the study. In case of more frequent SMS questionnaires being sent out in other studies, we therefore recommend to assess how this would affect the response rate. In case of GII during the last 28 days, the participants received additional 3–4 SMS questionnaires, to estimate the GII for 14 days and to estimate the symptom, duration and number of loose stools during the last episode. This may have introduced a bias, as participants who frequently experience GII episodes may choose not to report GII episodes. However, as the GII prevalence in the SMS cohort was higher compared to the prevalence in the telephone interviews, we believe the aforementioned bias is limited.
Conclusions
In this 1-year prospective SMS cohort, we estimated an annual incidence of sdGII and AGI among adults of 0.64 and 0.43 cases per person-year, respectively. With a 14-day recall, instead of 28 days, the incidence was about 20% higher. We also found that responses collected by SMS highly correlated with responses collected by telephone.
Financial support
The study was financed by the Swedish Civil Contingencies Agency (a Swedish non-profitable governmental agency, grant number 2012-172) and the EU programme Interreg IV A Öresund-Kattegatt-Skagerak (VISK – Reduced vulnerability to waterborne viral infection).
Conflict of interest
None.









