INTRODUCTION
Hepatitis C virus (HCV) infection is a considerable public-health problem and an important cause of liver disease. The global prevalence of HCV is 3% with 200 million people infected worldwide [Reference Soriano1]; an estimated 80% of individuals infected by HCV become chronic carriers, a significant proportion of which develop cirrhosis and even hepatocellular carcinoma during their lifetime [Reference Chuang2, Reference Rehermann and Nascimbeni3]. Current standard treatment for chronic HCV involves pegylated interferon (Peg-IFN) in combination with ribavirin. However, this treatment produces sustained virological response (SVR) rates in only about 40–50% of patients with HCV genotype 1 and ~60% in those infected with genotype 4, whereas 70–80% of patients with genotypes 2 or 3 achieve SVR [Reference Hadigan and Kottilil4]. In India HCV genotype 3 is the most prevalent genotype, and a significant number of patients fail to respond to treatment and experience significant side-effects to Peg-IFN/ribavirin, highlighting the need for accurate prediction of an individual's response to treatment before initiating therapy.
The pathogenesis of HCV infection remains unclear but it is possible that not only the virus but also the interaction between the virus and the host's immune system is important in determining the course of the infection and the response to Peg-IFN-based therapy [Reference Rehermann5]. Many studies show that the immunity level of the host correlates with the relevant gene polymorphisms in the promoter region that regulates gene expression. Recently, functional gene polymorphisms have been identified in inflammatory cytokines such as IFN-λ, which indicates possible relationships between these genotypes and the clinical outcome of HCV-related liver disease [Reference McCaughan and George6–Reference Zenewicz10].
Interleukin-10 (IL-10) is a multifunctional cytokine and a potent regulator of immune function through its anti-inflammatory and anti-fibrotic action [Reference Moore11, Reference Tsukamoto12]. It has been suggested that IL-10 can down-regulate the expression of major histocompatibility complex (MHC) class I and II molecules and the production of type-1 helper T cells (Th1) which are important in the host's defence against infection by viruses [Reference Moore11, Reference Del Prete, Maggi and Romagnani13]. An imbalance in Th1 and Th2 cytokine also plays and important role in the pathogenesis of chronic HCV infection [Reference Prezzi14]. Hence, IL-10 is believed to be a principal mediator cytokine in the host's immune response to HCV infection. Several studies in Caucasian and Oriental populations have shown that functional polymorphisms in the promoter region of the IL-10 gene (–592C/A, –819C/T, –1082G/A) determine the susceptibility of an individual to inflammatory disease [Reference Bidwell15]. The role of these IL-10 polymorphisms in the clearance of genotypes 1 and 4 HCV infection has been investigated in Caucasian, Oriental and Egyptian populations but inconsistent results were obtained [Reference Constantini16–Reference Shaker and Sadik19].
In contrast to the association of functionally important polymorphisms in the IL-10 gene with chronic HCV genotypes 1 and 4, infection data is scarce regardimg HCV genotype 3, which is more prevalent in the Indian population; the present case-control study was therefore initiated to investigate the association of functionally important polymorphisms in the IL-10 gene with chronic HCV genotype 3 infection in a North Indian population. Attempts were also made to investigate the association of these polymorphic variants with combined Peg-IFN/ribavirin therapy response.
METHODS
A case-control study was initiated to investigate the association of functionally important polymorphisms in the IL-10 gene (–592A/C, –819C/T, –1082G/A) with HCV infection. The study groups consisted of 150 patients suffering from genotype 3 HCV infection visiting the OPD facility of the Gastroenterology Department of Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGIMS), Lucknow, India, during May 2011 to April 2013. All patients were positive for HCV antibodies and HCV RNA in the serum for more than 6 months and displayed elevated serum alanine aminotransferase (ALT) levels. Patients were excluded if they were known to have injected drugs or abused alcohol within the last 6 months, had poorly controlled psychiatric illness, were cirrhotic, or were suspected of having hepatitis B surface antigen (HBsAg) or human immunodeficiency virus (HIV) antibodies or renal insufficiency infections and autoimmune liver disease. They were also excluded if they suffered from other significant concurrent medical conditions including chronic liver diseases of aetiologies other than HCV infection. Patients were included in the current analysis if they were of North Indian origin, infected with HCV genotype 3, and had been treated according to protocol (>12 weeks of treatment with >80% of the prescribed dose of combined Peg-IFN/ribavirin therapy and had a follow-up sample available to assess SVR).
The control group consisted of 150 healthy individuals, recruited during same period with no evidence of any liver disease as judged by physical examination and normal liver function test. HCV patients and controls were age-matched and the same numbers of male and females were recruited for both controls and cases. All the patients and controls included in the study were from the same geographical location (Northern India) and were of the same ethnicity. The clinical data of the patients and the controls are given in Table 1.
Table 1. Distribution of demographic and biochemical variables of healthy controls and chronic HCV patients
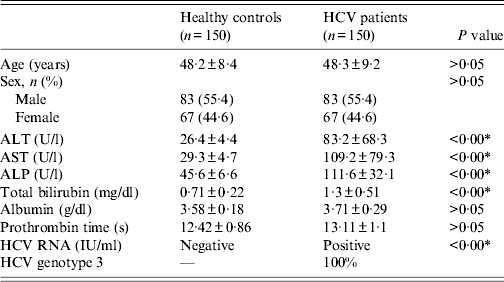
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase.
Data are represented as means ± standard deviation (s.d.).
* P < 0·05 is considered statistically significant.
The protocol for the research work was approved by the Institutional Bioethics Committee (IBC) of SGPGIMS, Lucknow and conforms to the provisions of the Declaration of Helsinki (1995). Informed consent was obtained from the study subjects prior to inclusion in the study; before collection of blood samples it was ensured that subject anonymity was preserved. Information concerning dietary habits, family history of disease, smoking, tobacco chewing, and alcohol consumption was obtained from the questionnaire completed by the cases and controls.
Genomic DNA isolation
One millilitre of blood was drawn into citrate-containing tubes from all patients and controls. DNA was isolated from whole blood with the Flexi Gene DNA kit (Qiagen, USA) following the manufacturer's protocol. Isolated DNA was subsequently used for genotyping studies.
IL-10 genotyping
The three biallelic IL-10 single nucleotide polymorphisms (SNPs) at promoter sites –592, –819, and –1082 were detected by PCR using primers that amplified a short fragment of DNA containing the polymorphism. The primers for the –592 SNP were: FP5′-CCTAGGTCACAGTGACGTGG-3′, RP5′-GGTGAGCACTACCTGACTAGC-3′; for the –819 SNP: FP5′-TCATTCTATGTGCTGGAGATGG-3′, RP5′-TGGGGGAAGTGGGTAAGAGT-3′; and for the –1082 SNP: FP5′-CTCGTCGCAACCCAACTGG-3′, RP5′-TCTTACCTATCCCTACTTCC-3′. The PCR mixture contained 500 ng genomic DNA, 0·8 mm dNTP, 0·5 μ m of each primer, and 0·6 U Taq polymerase in a 25 μl final volume. Amplification of the specific DNA fragments was performed using the Gene Amp PCR system 9700 of Applied Biosystems (USA) according to the following thermocycler conditions: denaturation at 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 64°C for 45 s, and extension at 72°C for 1 min. This was followed by a final extension at 72°C for 10 min. For amplification of the –1082 SNP, an initial activation step of 94°C for 10 min preceded the cycling programme.
IL-10 –592A/C, –819C/T and 1082G/A SNPs were genotyped by restriction fragment-length polymorphism (RFLP). RFLP assays were performed in a 20 μl reaction volume containing PCR products and specific restriction enzymes RsaI, MaeIII or MnII. For the presence of the IL-10 –592A allele, RsaI restriction enzyme was used to cut the 412 bp PCR product into two bands of 236 bp and 176 bp. For the presence of the IL-10 –819C allele, MaeIII was used to cut the 209 bp PCR product into two bands of 125 bp and 84 bp. For the presence of the IL-10 –1082 G allele, MnII was used to cut the 139 bp PCR product into two bands of 106 bp and 33 bp. The digestion products were stained with ethidium bromide and visualized on a 3% agarose gel.
Treatment and treatment response
The study group consisted of 150 patients suffering from genotype 3 HCV infection visiting the OPD facility of the Gastroenterology Department of SGPGIMS, Lucknow, India. All patients were positive for HCV antibodies and HCV RNA in the serum for more than 6 months and displayed elevated serum ALT levels. HCV patients were treated with combination therapy, which was composed of Peg-IFN [either 180 μg Peg-IFN α2a (Pegasys®, Roche Co., USA) or 100 μg Peg-IFN α2b (Peg-Intron®, Schering-Plough Co., USA)] administered subcutaneously once per week (dosage reduced to 80% in case of cytopenia or symptomatic adverse events during treatment) and 600–1200 mg of oral ribavirin daily (dosage adjustment according to body weight or anaemia during treatment), for 24–48 weeks. After discontinuation of treatment, the patients were followed for another 24 weeks. To determine the response to combination therapy, serum was tested for HCV RNA using a qualitative test at the end of treatment and 6 months off therapy. Response-relapse (RR) was defined as clearance of HCV RNA from serum at the end of treatment, but HCV returning during the follow-up period. SVR was defined as clearance of HCV RNA from serum at the end of treatment and for 24 weeks after the end of treatment. Patients who achieved neither RR nor SVR were defined as non-responders (NRs). NRs were patients with persistent HCV infection treated with Peg-INF who never lost HCV RNA during treatment. The total number of RR and NR patients are defined as non-SVR patients. Baseline characteristics of SVR, RR and NR chronic HCV patients are given in Table 2.
Table 2. Baseline characteristics of sustained virological response (SVR), response-relapse (RR) and non-responder (NR) chronic HCV patients
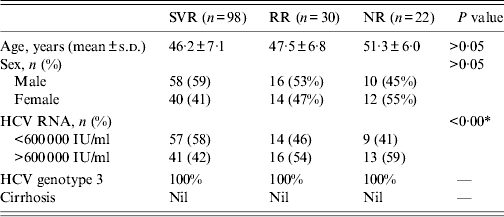
P value represent the comparison of data between SVR and RR patients.
* P < 0·05 is considered statistically significant.
Statistical analysis
The statistical data are reported as the mean ± s.d. of the original values. A comparison of numerical variables between the study groups was performed using Student's t test to compare independent samples from the two groups when the samples were normally distributed, and the Mann–Whitney U test to compare independent samples when the samples were not normally distributed. The Pearson χ 2 goodness-of-fit test was used to test the distribution of genotypes and allele frequencies for deviations from Hardy–Weinberg equilibrium between the patient and control groups. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to measure the risk associated with variant genotypes by the unconditional logistic regression method. Multiple logistic regression analysis was performed to assess the impact that genotypes and other clinical variables had on treatment response. The differences were considered significant at P < 0·05. All statistical analysis was performed using SPSS software version 13.0 for Windows (SPSS Inc., USA). The haplotype analyses (haplotype frequency estimation and pairwise linkage disequilibrium between SNPs) were performed using Haploview software (www.broad.mit.edu/mpg/haploview/). The power of the study was found to be >80% as analysed by power genetic association analysis software (http://dceg.cancer.gov/bb/tools/pga) at a level of significance α = 0·05 with a sample size of 150 for each of controls and HCV patients.
RESULTS
The distribution of demographic and biochemical variables of healthy controls and chronic HCV genotype 3 patients is described in Table 1. The biochemical profiles including serum ALT, aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, and HCV RNA in patients with chronic hepatitis C were significantly higher than those in the healthy controls (Table 1).
The distribution of genotype and allele frequencies of the IL-10 gene is summarized in Table 3. Both the genotype and allele frequencies of the IL-10 gene in controls were in Hardy–Weinberg equilibrium. The frequency of GG genotypes of IL-10 −1082A/G (MnII) was higher in HCV patients (12·5%) compared to controls (7·5%). The OR for GG genotypes was found to be significantly increased (OR 2·5, 95% CI 1·09–5·79, P = 0·025) when comparing the frequency of the GG genotype in HCV patients to controls (Table 3). The frequency of AG genotypes (heterozygous) of IL-10 –1082A/G (MnII) was also higher (45·5%) in HCV patients compared to controls (36·5%). When comparing the frequency of the AG genotype of IL-10 –1082A/G (MnII) in HCV patients vs. controls an increased risk was observed in HCV patients (OR 1·6, 95% CI 0·99–2·62, P = 0·05) which was statistically significant (Table 3).
Table 3. Distribution of genotype and allele frequencies of IL-10 in controls (n = 150) and HCV patients (n = 150)
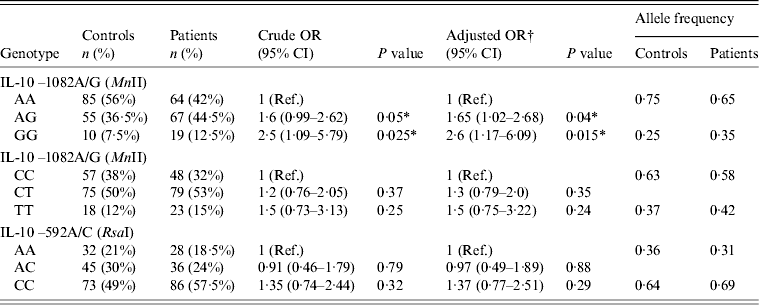
Ref., Reference category; OR, Odds ratio; CI, confidence interval.
† OR adjusted with multivariate logistic regression models.
* P < 0·05 is considered statistically significant.
As evident from Table 3, the frequency of both the TT and CT genotype of IL-10 –819C/T (MaeIII) was observed to be slightly higher in HCV patients compared to controls, although the risk associated was not statistically significant (Table 3). Similarly to the IL-10 –819C/T polymorphism, the frequency of the CC genotype of IL-10 –592A/C (RsaI) was higher in HCV patients (57·5%) than in controls (49%); however, no significant risk was detected in HCV patients compared to controls (OR 1·35, 95% CI 0·74–2·44, P = 0·32) (Table 3). Similarly, when comparing the frequency of the AC genotype of IL-10 –592A/C (RsaI) in HCV patients and controls no risk was found (OR 0·91, 95% CI 0·46–1·79, P = 0·79) (Table 3).
The haplotype approach was also followed to study the association of the combined effect of three SNPs (–1082A/G, –819C/T, –592A/C) of the IL-10 gene with HCV infection. The haplotype A-C-A was considered to be the reference carrying allele of all three SNPs. The frequency of haplotype G-T-C, carrying the G allele of –1082A/G, the T allele of –819C/T and the C allele of –592A/C, was higher in HCV patients (15%) compared to controls (6·5%). The higher frequency of the G-T-C haplotype in HCV patients resulted in a significant increase in risk (OR 3·3, 95% CI 1·66–6·61, P = 0·00) compared to controls (Table 4). Although the frequency of other haplotypes (A-C-C, A-T-C, A-T-A, G-C-C, G-C-A, G-T-A) were also higher in HCV patients than in controls, the increase in OR associated with these haplotypes was not statistically significant (Table 4).
Table 4. Distribution of IL-10 haplotypes in controls and HCV patients
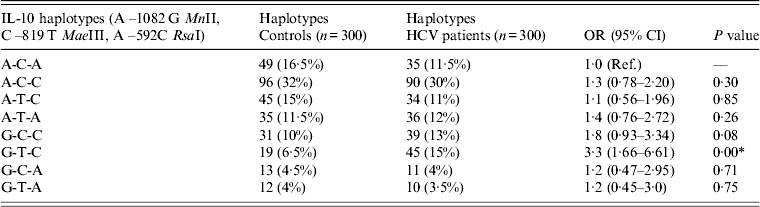
Ref., Reference category; OR, Odds ratio; CI, confidence interval.
* P < 0·05 is considered statistically significant.
Table 5 summarizes the genotype comparisons between SVR and RR in HCV patients with IL-10 genotypes. As shown in Table 5, the frequency of RR patients for the GG genotype (23%) of IL-10 –1082A/G (MnII) was higher than for SVR patients (5%). The increase in frequency of GG genotypes of IL-10 –1082A/G (MnII) resulted in a significant increase in OR (OR 6·6, 95% CI 1·73–25·01, P = 0·00) in RR patients compared to SVR patients (Table 5). The frequency of the CC genotype of the IL-10 –592A/C (RsaI) polymorphism was also found to be higher (67%) in RR patients compared to SVR patients (49%), this increase in frequency of CC genotype was associated with a significant increase in OR (OR 5·0, 95% CI 1·07–23·18, P = 0·02) in RR patients compared to SVR patients (Table 5).
Table 5. Comparison between sustained virological response (SVR) and response-relapse (RR) in HCV patients with IL-10 genotypes
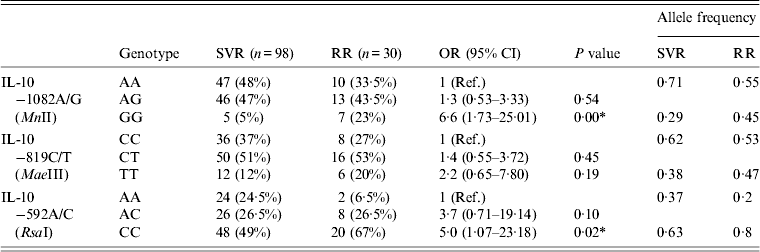
Ref., reference category; OR, odds ratio; CI, confidence interval.
* P < 0·05 is considered statistically significant.
The distribution of genotype frequencies of the IL-10 gene in SVR and NR HCV patients is summarized in Table 6. The frequency of GG genotypes of IL-10 –1082A/G (MnII) was found to be higher (32%) in NR patients than in SVR patients (5%). The OR for GG genotypes was found to be higher (OR 9·4, 95% CI 2·32–37·95, P = 0·00) when the frequency of the GG genotype in NR patients was compared to SVR patients (Table 6). The frequency of the CC genotype of IL-10 –592A/C (RsaI) was higher in NR patients (82%) compared to SVR patients (49%), this increase in frequency resulted in a significant increase in OR (OR 4·5, 95% CI 0·96–21·0, P = 0·04) when comparing the frequency of the CC genotype of IL-10 –592A/C (RsaI) of NR patients to SVR patients (Table 6).
Table 6. Comparison between sustained virological response (SVR) and non-responder (NR) HCV patients with IL-10 genotypes
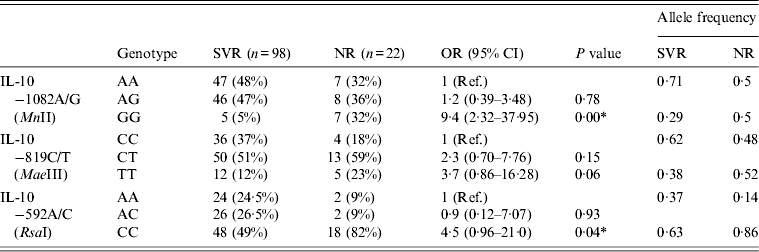
Ref., reference category; OR, odds ratio; CI, confidence interval.
* P < 0·05 is considered statistically significant.
Table 7 summarizes the genotype comparisons between SVR and non-SVR (RR + NR) in HCV patients with IL-10 genotypes. Almost similar results were detected when comparing the frequency of variant genotypes of IL-10 –1082A/G, IL-10 –819C/T and IL-10 –592A/C in non-SVR patients with SVR patients (Table 7), as was observed when comparing the frequency of the variant genotypes of IL-10 –1082A/G, IL-10 –819C/T and IL-10 –592A/C in RR or NR patients with SVR patients (Tables 5 and 6).
Table 7. Comparison between sustained virological response (SVR) and non-SVR in HCV patients with IL-10 genotypes
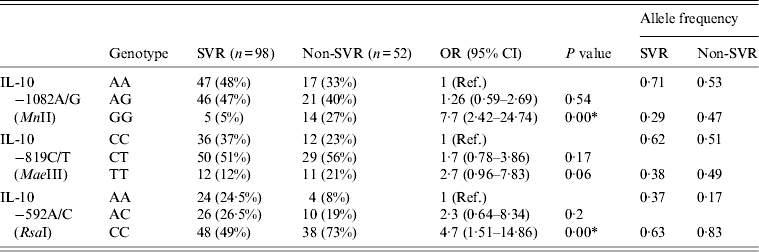
Ref., reference category; OR, odds ratio; CI, confidence interval.
* P < 0·05 is considered statistically significant.
No significant change was observed in OR when comparing the frequency of variant genotypes of IL-10 –1082A/G, IL-10 –819C/T and IL-10 –592A/C in NR and RR patients (data not shown).
DISCUSSION
Consistent with earlier studies [Reference Chuang2, Reference Knapp17, Reference Shaker and Sadik19 ], our data have shown that polymorphisms exist in the IL-10 gene in the North Indian population. The frequency of the G allele (25%) of IL-10 –1082A/G (MnII) was found to be slightly lower than that reported in the Caucasian population (37–42%) [Reference Knapp17, Reference Lech-Maranda20, Reference Poli21], while the Oriental population carry a much lower frequency (3·5%) of the G allele [Reference Chuang2, Reference Wang22]. Similarly, the T allele frequency (37%) of IL-10 –819C/T (MaeIII) was similar to that reported previously in a Caucasian population (29–36%) [Reference Lech-Maranda20, Reference Poli21, Reference Visentainer23], while this frequency was shown to be relatively higher (70–81%) in an Oriental population [Reference Chuang2, Reference Wang22]. Our data further reveal that the frequency of the A allele (36%) of IL-10 –592A/C (RsaI) is similar (36%) to the Caucasian population (31–33·5%) [Reference Knapp17, Reference Lech-Maranda20, Reference Corchado24], while this frequency is relatively higher (52–77%) in the Oriental population [Reference Chuang2, Reference Wang22].
The significant increase in frequency of the GG genotype of IL-10 –1082A/G (MnII) in chronic HCV patients compared to controls suggests that the polymorphism in IL-10 –1082A/G (MnII) may modify the susceptibility of an individual to chronic HCV infection. Explanation for the association of the GG genotype of IL-10 –1082A/G (MnII) with chronic HCV patients is derived from the experiment showing that the G allele of IL-10 –1082A/G (MnII) produces higher levels of cytokine which may compromise the cellular immune response to HCV [Reference Eskdale25, Reference Reuss26]. Further, a small study conducted in a Sicilian population showed the IL-10 –1082A/G GG genotype to be highest in a very small number of patients with self-limiting infection compared to those with persistent infection, and the frequency of the GG genotype in those with persistent infection and self-limiting infection was found to be higher compared to healthy controls [Reference Lio18]. A significant association of the GG genotype of IL-10 –1082A/G (MnII) with chronic HCV infection has been shown in the Caucasian population [Reference Knapp17, Reference Vidigal, Germer and Zein27]. However, this association was not found to be present in the Oriental population [Reference Chuang2, Reference Chen28] and has been partly attributed to the smaller sample size, lower frequency of GG genotype, and ethnic variations among these groups [Reference Sun29]. Interestingly, Gao et al. showed that the IL-10 –1082AA genotype and –1082A allele, which produce low IL-10, were associated with an increased risk of HCV RNA replication in a Chinese population [Reference Gao30]. By contrast, a recent study showed that significantly higher IL-10 production was observed in HCV patients with the IL-10 –1082GG genotype in a Chinese population [Reference Li31].
A meta-analysis conducted on eight studies that contained 992 cases and 1123 controls found that the IL-10 –1082GG genotype was associated with an increased risk of susceptibility to chronic HCV infection [Reference Zhang32]. A recent meta-analysis conducted on 26 studies that contained 4039 cases and 2902 controls further demonstrated that the presence of the IL-10 –1082GG genotype significantly increased the risk of chronic HCV infection in the general population [Reference Sun29]. A meta-analysis conducted on a subgroup further showed that the IL-10 –1082GG genotype significantly increased the risk of chronic HCV infection in a Caucasian population but not in African or Oriental populations [Reference Sun29].
Similarly to that observed in our study, IL-10-819C/T (MaeIII) and IL-10 –592A/C (RsaI) polymorphisms were not found to be associated with chronic HCV infection compared to healthy controls in Caucasian and Oriental populations [Reference Chuang2, Reference Knapp17]. Recent meta-analysis further showed no significant association between IL-10-819C/T (MaeIII) and IL-10 –592A/C (RsaI) polymorphisms with chronic HCV infection compared to healthy controls in Caucasian and Oriental populations [Reference Sun29]. By contrast, Afzal et al. reported suggestive evidence of an association with hepatitis C for the IL-10 –819C/T (–592C/A) promoter polymorphism at the allele level but not in genotype distribution in a Pakistani population [Reference Afzal33].
Haplotype analysis has revealed that IL-10 –1082A/G (MnII), IL-10 –819C/T (MaeIII) and IL-10 –592A/C (RsaI) polymorphisms of the IL-10 gene do not exhibit linkage disequilibrium in either controls or patients in a North Indian population. Haplotype data also demonstrated that the haplotype G-T-C, which contains G allele IL-10 –1082A/G (MnII), was associated with a several-fold increased risk (OR 3·3, 95% CI 1·66-6·61, P = 0·00) for chronic HCV infection compared to healthy controls. A significant association of haplotype-containing G allele of IL-10 –1082A/G (MnII) with chronic HCV infection has also been reported in Caucasian and Pakistani populations [Reference Knapp17, Reference Afzal33].
Our study further shows that the GG genotype of IL-10 –1082A/G (MnII) may influence the outcome of combined Peg-IFN/ribavirin therapy. The GG genotype of IL-10 –1082A/G (MnII) was found to present in much higher frequency in RR patients (23%) compared to SVR patients (5%) which results in a significant increase in OR (OR 6·6, 95% CI 1·73–25·01, P = 0·00) in RR patients compared to SVR patients. This increase in GG genotype frequency in RR patients indicates that the GG genotype may be responsible for the failure of combined Peg-IFN/ribavirin therapy to maintain the SVR in chronic HCV patients. The GG genotype of IL-10 –1082A/G (MnII) is associated with a higher level of IL-10 production which compromises the cellular immune response to HCV which leads to the development of chronic HCV infection at the time of initial infection, as demonstrated by our data, and may lead to the suppression of combined Peg-IFN/ribavirin therapy which favours RR when patients are treated with combined therapy [Reference Reuss26, Reference Turner34, Reference Tso35]. That the GG genotype of IL-10 –1082A/G (MnII) may be associated with the suppression of combined Peg-IFN/ribavirin therapy is further shown by a statistically significant increase in risk (OR 9·4, 95% CI 2·32–37·95, P = 0·00) in NR patients compared to SVR patients carrying the same GG genotype.
A previous study in a Caucasian population also showed that patients with IL-10 –1082GG and transforming growth factor (TGF)-β1 + 29CC genotypes may be associated with susceptibility to chronic HCV infection and failure to combined Peg-IFN/ribavirin therapy [Reference Vidigal, Germer and Zein27]. However, a conflicting result was reported in another study which identified a significantly higher frequency of the GG genotype in SVR patients compared to NR patients, which influences the outcome of IFN therapy [Reference Knapp17]. This may be due to IFN monotherapy which was used in the study by Knapp and colleagues [Reference Knapp17].
As observed with IL-10 –1082A/G (MnII), there was a statistically significant risk for the CC genotype of IL-10-592A/C (RsaI) in RR patients compared to SVR patients. It is likely that the CC genotype of IL-10 –592A/C (RsaI) is associated with a higher level of IL-10 production which leads to the suppression of combined Peg-IFN/ribavirin therapy which favours RR when patients are treated with combined therapy. A significant association of the CC genotype of IL-10 –592A/C (RsaI) with treatment response has also been shown in other studies [Reference Edwards-Smith36, Reference Yee37]. Edwards-Smith et al. showed an association of the IL-10 –592 CC genotype with NR patients compared to initial responders and an association of the corresponding G-C-C haplotype with NR patients [Reference Edwards-Smith36]. Yee et al. reported an association of the IL-10 –592 AA genotype with SVR and NR patients who had been treated with IFN/ribavirin combination therapy [Reference Yee37]. However, Vidigal et al. did not observe any associations with the IL-10 promoter polymorphisms and outcomes of combination therapy in a study of SVR and NR patients [Reference Vidigal, Germer and Zein27]. Our study further shows that the AA genotype frequency of IL-10 –592 is much higher in SVR patients (24·5%) compared to RR (6·5%) or NR (9%) patients which indicates that patients carrying the AA genotype of IL-10 –592 are more likely to clear HCV when treated with combined Peg-IFN/ribavirin therapy. Decreased IL-10 production has been observed in individuals with the –592A allele [Reference Clerici38]. Therefore, patients carrying the IL-10 –592A allele are more likely to remove the virus when treated with combined Peg-IFN/ribavirin therapy. A recent meta-analysis conducted on 26 studies containing 4039 cases and 2902 controls also demonstrated the role of the AA genotype of IL-10 –592 in HCV clearance [Reference Sun29].
In conclusion, our data suggest that the GG genotype of IL-10 –1082A/G (MnII) is associated with an increased susceptibility to chronic HCV genotype 3 infection. Haplotype analysis revealed that haplotype G-T-C, carrying the GG allele of IL-10 –1082A/G, was associated with increased risk for chronic HCV genotype 3 infection. Results of treatment response further indicate that the GG genotype of IL-10 –1082A/G (MnII) and CC genotype of IL-10 –592A/C (RsaI) may influence the outcome of combined Peg-IFN/ribavirin therapy. Further studies with larger sample sizes are needed to clarify these conclusions.
ACKNOWLEDGEMENTS
The authors are grateful to the Director, Sanjay Gandhi, Post Graduate Institute of Medical Science (SGPGIMS), Lucknow for his keen interest and support in carrying out the study. Dr A. J. Khan is grateful to Indian Council of Medical Research (ICMR), New Delhi for providing a Research Associate Fellowship.
DECLARATION OF INTEREST
None.










