Introduction
Bacterial meningitis (BM) is a severe infectious disease of the central nervous system occurring mainly in young children. It makes a bad effect on children's hearing and learning abilities [Reference Somand and Meurer1, Reference de Jonge2]. Although tremendous advance in antimicrobial therapy has been emerging, BM is still responsible for considerable mortality worldwide. However, the exact aetiology of BM remains unknown.
Studies have demonstrated that genetic variations in microbial recognition genes may be relevant with altered host responses to infection [Reference Brouwer, Read and van de Beek3]. The pathogen recognizing receptors (PRRs) can generate increased or decreased inflammatory response to infection in epithelial and macrophages cells [Reference Becker and O'Neill4]. Toll-like receptors (TLRs) is an important family member of PRRs. They are composed of transmembrane proteins and expressed mainly on human immune cells including macrophages and dendritic, B, T and some other nonimmune cells [Reference Jimenez-Dalmaroni, Gerswhin and Adamopoulos5, Reference Underhill6]. The above studies have confirmed that TLRs could adjust inflammatory reactions and activate immune response to eliminate infectious pathogens [Reference Jo7]. As a key member of TLRs, TLR2 plays an important role in recognising a variety of bacterial lipoproteins. A single nucleotide polymorphism of TLR2 + 2477G/A has been most widely studied and considered related to reduced cellular activation in the presence of the TLR2 ligand lipopeptide. And it is well-known that host genetic factors are closely related to the susceptibility to infectious disease caused by infectious pathogens.
In recent years, several literatures focused on the association between TLR2 + 2477G/A polymorphism and BM susceptibility. However, the results of previous studies were controversial. Hence, in the present study, we perform this meta-analysis on all published case-control studies to derive a more precise estimation of TLR2 + 2477G/A polymorphism with BM risk.
Materials and methods
Search strategy
PubMed, Embase and CNKI (China National Knowledge Infrastructure) databases were searched using the terms as follows: (‘TLR2’ or ‘Toll-like receptor 2’) in combination with (‘polymorphism’ or ‘variant’ or ‘mutation’) and in combination with ‘Bacterial meningitis’ updated on December 2017 for all publications on the association between TLR2 + 2477G/A polymorphism and BM risk. To identify other relevant studies, additional literatures were identified through scanning the references of original literatures which were included in the present Meta-analysis. Review articles were also examined and inspected to find other eligible literatures.
Inclusion and exclusion criteria
The issues below were inclusion criteria for the present literature selection: (a) a case-control study; (b) assessment of the relationship between TLR2 + 2477G/A polymorphism and BM risk; (c) offering distribution of genotypes or other data to compute odds ratios (ORs) and 95% CIs. Accordingly, studies were excluded if one of the following exclusion criteria existed: (a) studies that contained overlapping data; (b) not offering necessary data such as the distribution of alleles or genotypes; (c) studies in which family members had been investigated because of linkage disequilibrium.
Data extraction
All the data were independently reviewed and extracted by two investigators (Xu Chen and Xiaochun Jin). And the result was reviewed by a third investigator (Youtao Zhang). From each study, the following information was recorded: first author, publication year, country, ethnicity; the number of cases and controls, allele frequency and genotype distribution in cases and controls, genotyping methods and evidence of Hardy–Weinberg equilibrium (HWE) in control subjects. Different ethnic descents were categorised as Caucasians, Asian, African or Mixed population.
Statistical analysis
The OR and corresponding 95% confidence interval (95% CI) were calculated to assess the association strength between TLR2 + 2477G/A polymorphism and BM risk. As the genotype AA was rare in the Caucasian population, we evaluated the BM risk with TLR2 + 2477G/A polymorphism by two genetic models including allele contrast (A vs. G) and recessive genetic model (AA vs.AG/GG).
The χ 2-test based Q-statistic and I2 statistics were applied to assess heterogeneity among eligible studies [Reference Higgins8, Reference Higgins and Thompson9]. If there was no obvious heterogeneity, the fixed-effects model would be applied to calculate summary OR [Reference Mantel and Haenszel10]. If not, the random-effects model would be used [Reference DerSimonian and Laird11]. To detect the possible source of heterogeneity, we examined the sources of heterogeneity such as publication year, the source of control, genotyping method, type of BM and sample size.
Sensitivity analysis was conducted to appraise the stability of the results and identify potentially influential studies. Any single study from the meta-analysis was deleted each time to calculate the influence of the individual dataset to the pooled OR. Funnel plots and Egger's linear regression test were applied to detect the potential publication bias [Reference Begg and Mazumdar12]. An asymmetric plot infers a possible publication bias. The significance of the intercept was determined by the Student t test suggested by Egger (P < 0.05 was considered representative of statistically significant publication bias). All analyses were conducted using Stata software (version 12.0; StataCorp LP, College Station, TX, USA).
Results
Eligible studies
A flow diagram of the search process is shown in Figure 1. We searched all the eligible studies using three databases including PubMed, Embase and Chinese National Knowledge Infrastructure (CNKI). Based on the predefined search strategy and inclusion criteria, a total of six studies were finally included in our meta-analysis [Reference Gowin13–Reference van Well18]. And all of the six studies came from PubMed and Embase. None of them came from CNKI. All of the six studies investigated the Caucasian population. Several genotyping methods were used in the eligible studies such as TaqMan probe, PCR-RFLP and direct sequencing. The genotype frequency in controls of all studies was consistent with HWE (P > 0.05). The main characteristics of all the case-control studies included in our meta-analysis were listed in Table 1.

Fig. 1. Flow diagram for identification of eligible studies for this meta-analysis.
Table 1. Main characteristics of all case-control studies included in meta-analysis

PB. population-based; HWE, Hardy-Weinberg equilibrium; RFLP, Restricted Fragment Length Polymorphism.
Quantitative synthesis of data
The summary results for the association of TLR2 + 2477G/A polymorphism with BM risk are shown in Table 2. Overall, no significant association between TLR2 + 2477G/A polymorphism and BM risk were found under allele contrast (A vs. G: OR = 1.15, 95% CI = 0.93–1.43, P = 0.202) (Fig. 2), recessive genetic model (AA vs. AG/GG: OR = 1.12, 95% CI = 0.90–1.41, P = 0.313) (Fig. 3). The significant association was found between TLR2 + 2477G/A polymorphism and pneumococcal meningitis (PM) risk under allele contrast (A vs. G: OR = 1.54, 95% CI = 1.01–2.36, P = 0.046) (Fig. 2), recessive genetic model (AA vs. AG/GG: OR = 1.63, 95%CI = 1.03–2.57, P = 0.035) (Fig. 3).
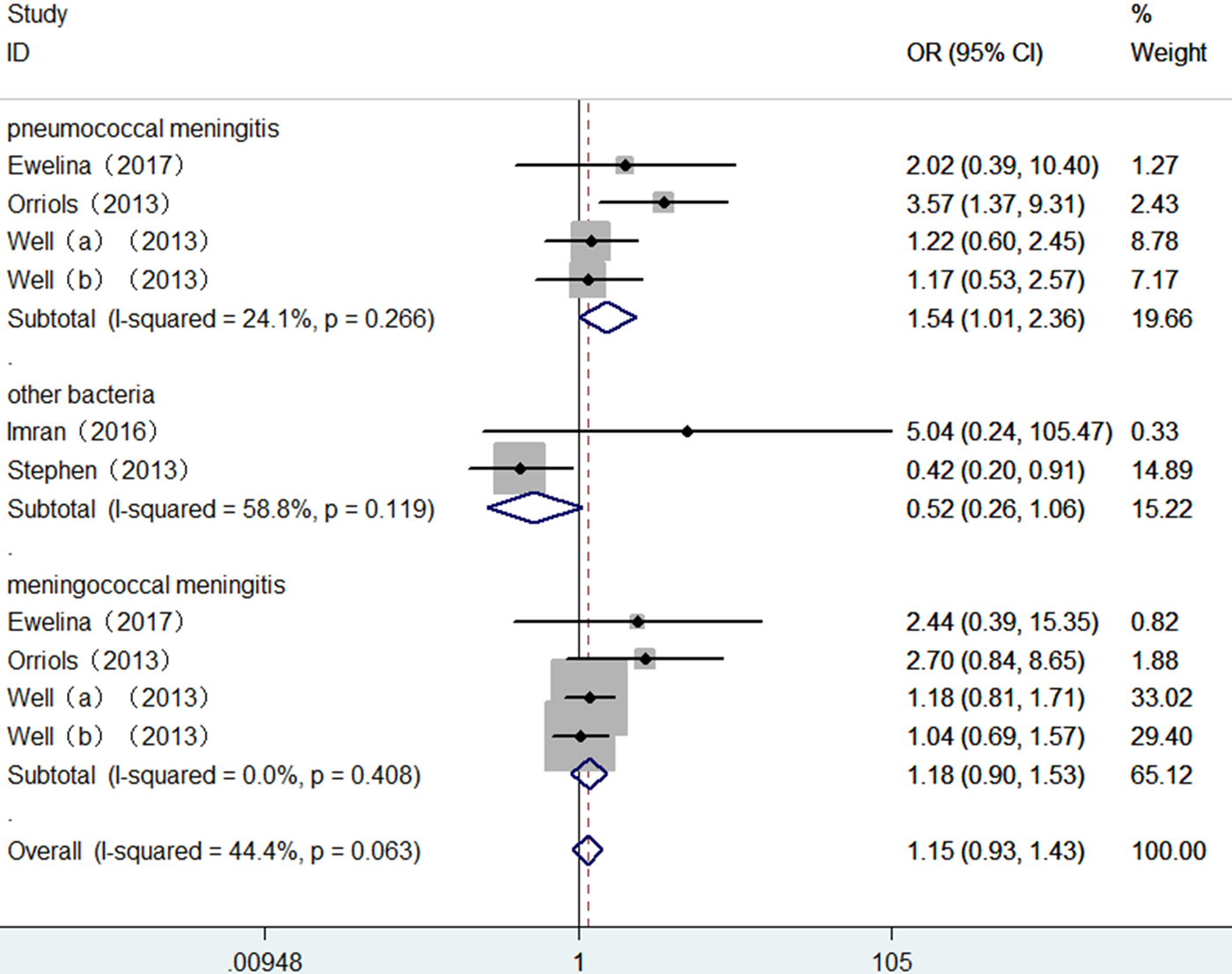
Fig. 2. Forest plot of TLR2 + 2477G/A polymorphism on BM risk (allele contrast A vs. G).
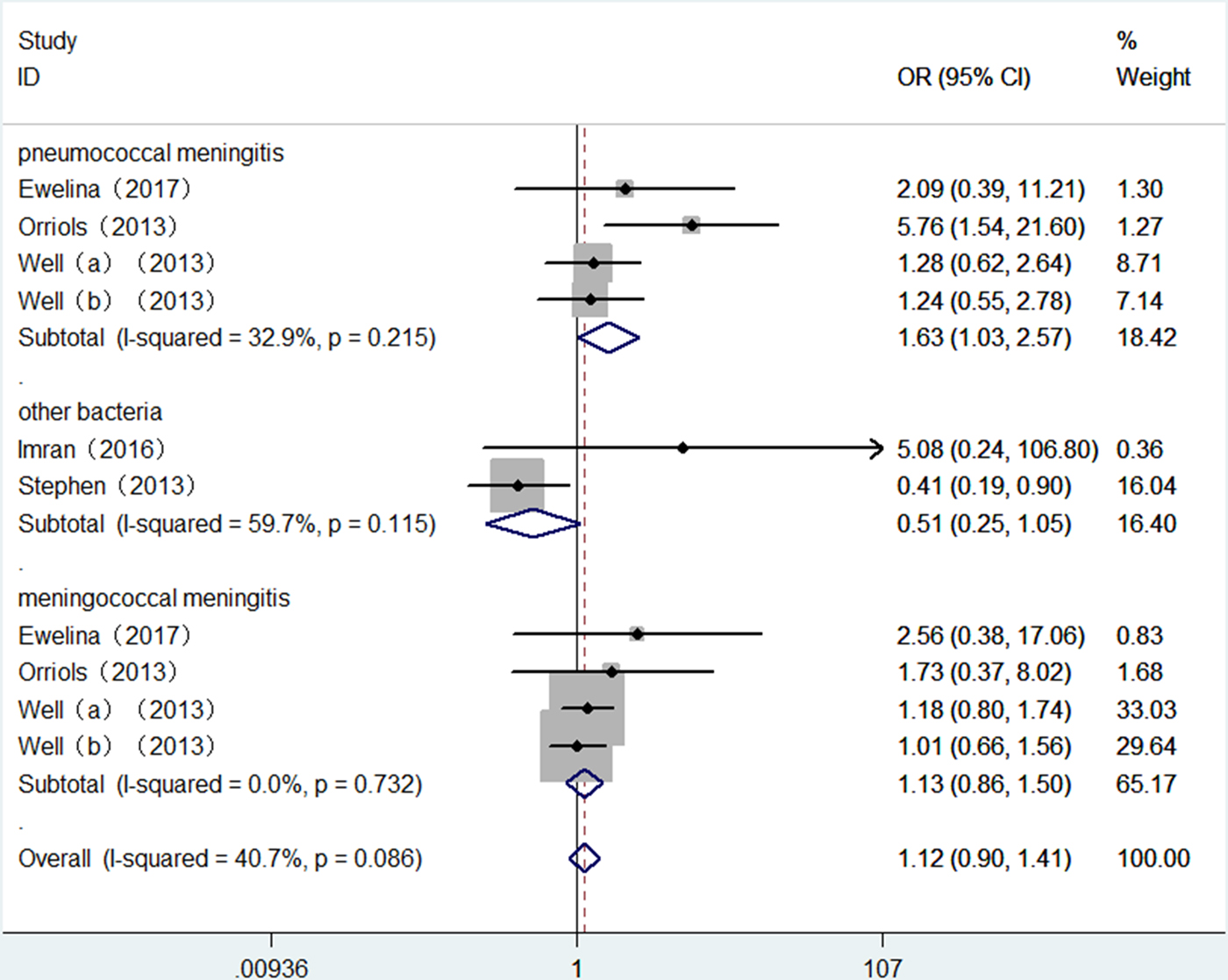
Fig. 3. Forest plot of TLR2 + 2477G/A polymorphism on BM risk (recessive genetic model: AA vs. AG/GG).
Table 2. Meta-analysis of the TLR2 + 2477G/A polymorphism with BM risk
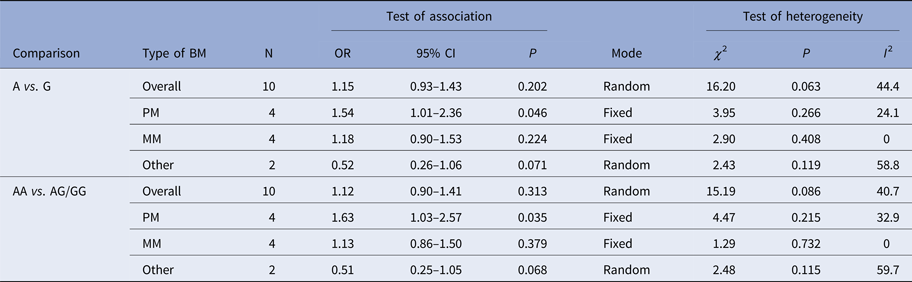
PM, pneumococcal meningitis; MM, meningococcal meningitis.
Test of heterogeneity
There was no significant heterogeneity in TLR2 + 2477G/A polymorphism with BM risk under allele contrast (P heterogeneity = 0.063, I 2 = 44.4%) and recessive genetic model (P heterogeneity = 0.086, I 2 = 40.7%). To explore the sources of tiny heterogeneity, we evaluated allele contrast by considering possible sources including publication year, genotyping methods, type of BM, the source of control and sample size. We found that type of BM (χ 2 = 4.39; df = 1; P = 0.036) could substantially influence the initial tiny heterogeneity.
Sensitivity analysis
Sensitivity analysis was conducted by sequential removal of individual eligible studies which were included in the present meta-analysis. In the present meta-analysis, any single study could not influence the overall results qualitatively, indicating robustness and reliability of our results.
Publication bias
Begg funnel plot was created to assess the publication bias of selected literatures. Although slightly asymmetrical funnel plots were detected in our results (P = 0.107) (Figs 4 and 5), Egger's test did not exhibit obvious publication bias in the allele comparison model (P = 0.194) and the recessive model quantitatively (P = 0.167).
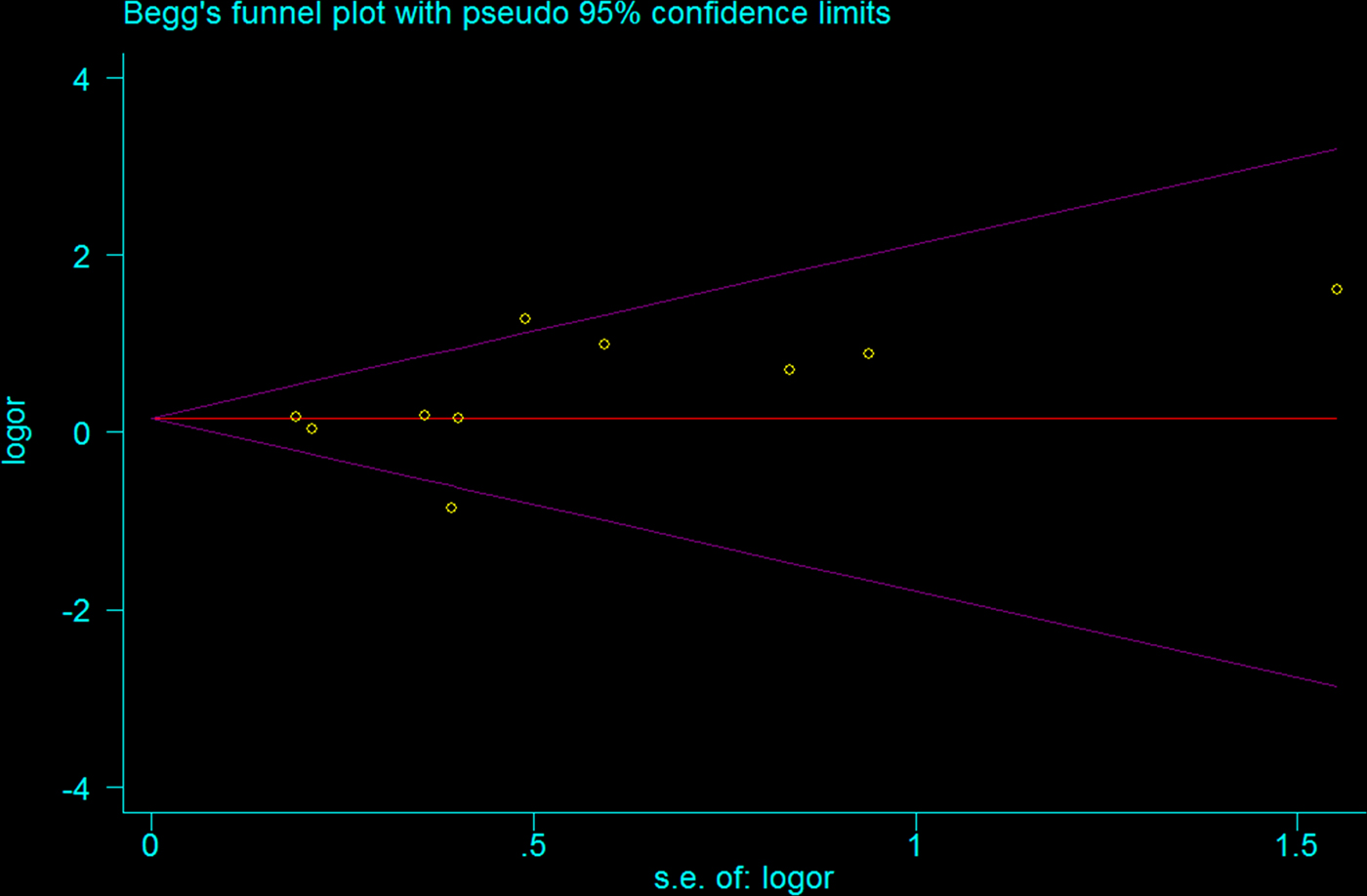
Fig. 4. Funnel plot of TLR2 + 2477G/A polymorphism on BM risk (allele contrast A vs. G).
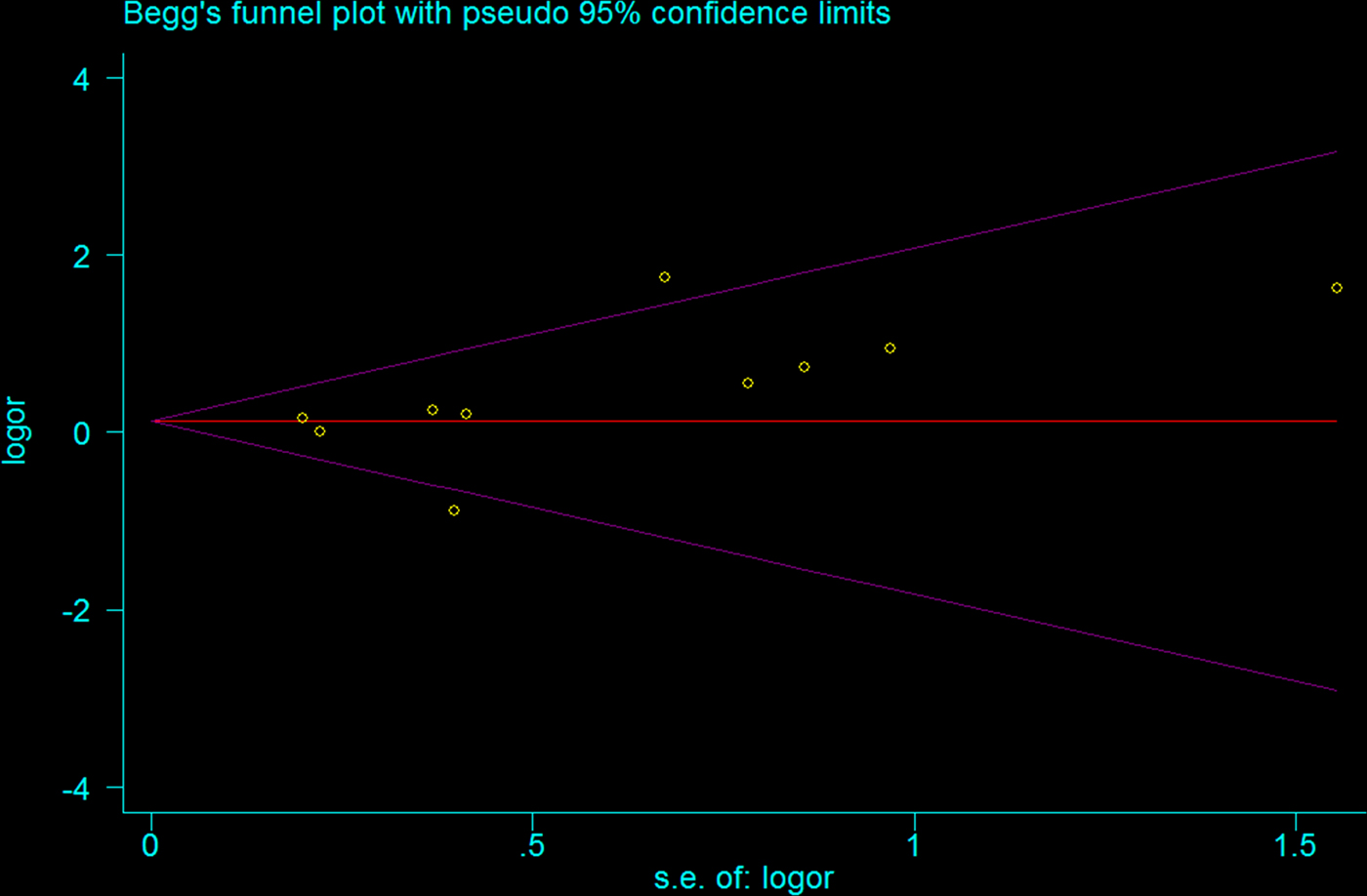
Fig. 5. Funnel plot of TLR2 + 2477G/A polymorphism on BM risk (recessive genetic model: AA vs. AG/GG).
Discussion
In the present study, we critically performed a meta-analysis of TLR2 + 2477G/A with BM risk. To the best of our knowledge, the present meta-analysis was the first to explore the association between TLR2 + 2477G/A polymorphism and BM risk. The results of our meta-analysis showed TLR2 + 2477G/A polymorphism was not associated with meningococcal meningitis risk but contributed an increased risk of pneumococcal meningitis in the Caucasian population.
How might the TLR2 + 2477G/A polymorphism affect susceptibility to BM? A gene function study demonstrated that TLR2 + 2477G/A polymorphism could weaken tyrosine phosphorylation, dimerization with TLR6, MyD88 recruitment and nuclear factor κB activation [Reference Xiong19, Reference Lorenz20]. All of the above actions bring about abnormal intracellular signaling and impaired cytokine secretion in response to lipopeptides, peptidoglycan and other ligands, which may result in the development of BM. Additionally, animals models have indicated that defective TLR2 signaling should be responsible for the increased risk to BM. All of these findings accord to the TLR2 + 2477G/A polymorphism with PM. Nevertheless, how could one explain the negative association between TLR2 + 2477G/A polymorphism and MM? The streptococcus pneumoniae belongs to the gram-positive bacterium, whose cell wall contains peptidoglycan and teichoic acid. And the Neisseria meningitides is a part of gram-negative bacterium, whose cell wall consists of lipoprotein, lipopolysaccharide and peptidoglycan. The different structure of cell wall may explain the difference between PM and MM. But no matter what happens, we should be cautious about the present results as the sample size may be insufficient.
The heterogeneity is an important factor when performing meta-analysis. Finding the sources of heterogeneity and solving the heterogeneity are of great significance to final results. Overall, the heterogeneity is not significant. The publication year, genotyping methods, type of BM, the source of control and sample size were considered as the possible source of heterogeneity. We found the type of BM contributed substantial heterogeneity to the final results. Then, we carefully evaluated literature quality of every included study and found that the heterogeneity may come from Stephen et al. who focus on population including Black non-Hispanic, White non-Hispanic and Hispanic [Reference Spector15]. It is well-known that ethnicity exerts a tremendous influence on allele frequency and genotype distribution. Nevertheless, the sample size of Stephen et al. is small. Future large, well designed epidemiological studies are required to investigate the polymorphism in other ethnicities such as Asian, African populations. Sensitive analysis demonstrated that any single study could not affect the final result of the present meta-analysis, indicating that our results were stable and reliable. The publication bias is another important factor that may influence the reliability of meta-analysis. We failed to find any evidence to demonstrate the existence of publication bias, suggesting that publication bias have little effect on the results of our study and the results of our meta-analysis are relatively stable.
Studies demonstrated that inevitable selection bias is existent in hospital-based controls, which were just represent a sample of ill-defined reference population and may not be representative of the study population or general population. Furthermore, it was easier to be influenced when the genotypes under investigation were associated with a disease condition. Hence, it is crucial and necessary to select population-based controls, which contributes to eliminating selection biases in such genetic association studies. In the present meta-analysis, the source of control brings little heterogeneity to the final results.
As we all know, HWE plays an important role in genetic association studies and gene polymorphism investigations. The genetic association studies which disobey the law of HWE may derive from genetic reasons such as nonrandom mating and the alleles reflect recent mutations. All of the above reasons may lead to the unreliable results of genetic association studies. In the present meta-analysis, all eligible studies conformed to the law of HWE, which contributes to the reliable results of the present meta-analysis.
Although comprehensive meta-analysis was conducted to demonstrate the association between TLR2 + 2477G/A polymorphism with BM risk, there are still some limitations that should be pointed out. Firstly, the primary studies included in our meta-analysis mainly investigated Caucasian population. Since TLR2 + 2477G/A polymorphism substantially varies across different ethnicities, more primary studies which focused on other ethnicities such as Asian population, African population, the mixed population should be carried out. Secondly, we should be cautious to the results because only six eligible studies were included in our meta-analysis. The sample size was relatively small and the only Caucasian population was investigated. Thirdly, because of lack of sufficient primary data, hence, subgroup analysis according to age, gender, radiation exposure and other confounding factors could not be performed in the present meta-analysis.
In spite of the limitations above, our meta-analysis had also several advantages. Firstly, a meta-analysis of the association of TLR2 + 2477G/A polymorphism on BM risk is statistically more powerful than any other single study. Secondly, the quality of our eligible studies was relatively high and the sensitivity analysis and publication bias analysis suggested the stability and credibility of the meta-analysis, which leads to a more convincing result. More important, the process of literature selection, data extraction and data analysis in the meta-analysis was well designed and conducted.
In conclusion, this is the first meta-analysis which investigates the association between TLR2 + 2477G/A polymorphism and BM risk. We conclude that TLR2 + 2477G/A polymorphism is not associated with meningococcal meningitis risk but contributes an increased risk of pneumococcal meningitis.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818000298
Declaration of Interest
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
Author contributions
XC, XJ conceived and designed the experiments; XC, XJ, SY, YZ performed the experiments; SY, YZ analysed the data; XC, XJ, SY, YZ contributed reagents/materials/analysis tools; and XC, XJ, SY, YZ wrote the paper.









