Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory coronavirus 2 (SARS-CoV-2), has become a global health threat [Reference Guan1–Reference Nozaki and Miyano3]. Since the first case of COVID-19 was identified in Wuhan, China [Reference Huang4], there have been various changes in the approaches to clinical management of COVID-19. The risk factors for severe disease now seem to have been identified [Reference Terada5, Reference Asai6] and the pharmaceutical treatments for COVID-19 cases are at least somewhat established [7–9]. However, the SARS-CoV-2 B.1.1.529 variant of concern (VOC) that causes COVID-19 has somewhat different clinical characteristics from the pre-existing variants. This variant, which was first identified in South Africa on 24 November 2021 [10] and was subsequently named Omicron, has now spread worldwide. Fewer patients develop serious illness with this variant and vaccines are less effective [Reference Modes11–Reference Lauring13].
In Japan, the Omicron VOC has spread rapidly, as in many countries, although there have been fewer severe cases than when the Delta variant was dominant [14]. Nevertheless, the higher infectivity of the Omicron VOC compared with previous variants led to a huge number of new confirmed cases and a corresponding increase in the number of severe cases. This has burdened our health systems and society further [15].
Although previous studies have identified the risk factors associated with severe illness caused by other variants [Reference Terada5, Reference Chen16–18], the same factors may not be applicable to the disease caused by the Omicron VOC. Examining the risk factors associated with severe COVID-19 caused by the Omicron VOC is therefore desirable especially since, throughout the entire pandemic period, older Japanese adults have accounted for a large proportion of the severe COVID-19 cases that required hospitalisation [Reference Matsunaga19, 20]. Because Japan is fast becoming an extremely aged society, an evaluation of the risk factors specific to the elderly population would be extremely valuable. For instance, the US Centers for Disease Control and Prevention (CDC) reported that living in a long-term care facility was an independent risk factor for mortality [Reference François Watkins21]. Moreover, dementia or pre-existing Alzheimer's disease was reported to be associated with late mortality due to COVID-19 [Reference Vlachogiannis22, Reference Kim23]. According to Steenkamp et al., a moderate or high level of physical activity had a preventive effect on severe COVID-19 [Reference Steenkamp24]. However, the influence of these factors on the severity of COVID-19 caused by the Omicron VOC is not clear.
The main objectives of this study were to identify the risk factors for severe COVID-19 caused by the Omicron VOC and to assess the relevance of three factors specific to the elderly population – dementia, living in a long-term care facility, and physical activity status – for the severity of the disease.
Methods
Study population and data
Healthcare facilities that voluntarily participated in the COVID-19 Registry Japan (COVIREGI-JP) [Reference Matsunaga19, Reference Matsunaga25], which is managed by the REBIND (Repository of Data and Biospecimen of Infectious Disease) project, enroled the patients. Research collaborators in each facility manually entered the data into the registry. The inclusion criteria for enrolment were (i) a positive SARS-CoV-2 test result and (ii) admittance to a healthcare facility between 1 January 2022 and 16 May 2022. The exclusion criteria were positive test results for any of the N501Y, E484K, E484Q and L452R mutants in SARS-CoV-2 genome tests. Also, although not all the patients had the results of genome tests in our registry, most cases in January 2022 in Japan were caused by the Omicron VOC and some other studies regarded all the patients from the beginning of 2022 as having a disease caused by the Omicron VOC [Reference Shoji26, Reference Shoji27]. Therefore, we also excluded patients who showed any evidence suggesting a disease caused by strains other than the Omicron VOC (i.e. N501Y, E484K, E484Q and L452R during the study period).
Statistical analysis
As a descriptive analysis of the whole data, which included patients younger than 65 years old, continuous variables are presented as median and interquartile range (IQR) and categorical variables as number of cases and percentages. We then performed an exploratory analysis using logistic regression to identify risk factors for severe illness for elderly patients (age 65 or older). In this study, we defined severe illness as a need for supplementary oxygen during admission. The following variables were included in the regression model: age, sex, vaccinated at least twice, current smoking habit, cardiovascular disease, cerebrovascular disease, chronic lung disease, asthma, liver disease, renal failure and/or dialysis, diabetes mellitus, solid tumour, blood cancer, collagen disease, physician-diagnosed obesity, dementia, admission from a long-term care facility and physical activity. In this study, we use the term ‘long-term care facility’ to refer to not only healthcare facilities but also nursing homes and other facilities that provide accommodation for elderly people, including those who are healthy, because there is no equivalent English term to cover this category of patients used in Japan. We defined physical activity as a dichotomous variable and patients were considered to have good physical activity status if they could (i) eat a normal diet, (ii) walk independently and (iii) take care of themselves; otherwise, they were considered to have poor physical activity status. Each of the three criteria was assessed by a treating physician at each facility, and then we judged the physical activity of each patient based on these three factors.
Next, we conducted one-to-one propensity score (PS) matching [Reference Rosenbaum and RUBIN28] using three categorisations: presence/absence of dementia, admission from a long-term care facility/home or an acute healthcare facility; and good/poor physical activity status. The PS was calculated by logistic regression analysis with the same variables included in the exploratory analysis. All matching processes were based on nearest neighbour matching with a caliper width of 0.05 and no replacement was allowed. The standardised difference was used to measure the covariate balance, and an absolute standardised difference above 0.1 was interpreted as a meaningful imbalance. Fisher's exact test was used to compare the proportion of severe and fatal COVID-19 cases in matched data. In addition, we estimated the average treatment effect on treated (ATT) in each PS matching analysis.
Two-sided P values of < 0.05 were considered to show statistical significance. All analyses were conducted with R version 4.1.3 [29].
Results
The characteristics of participants are shown in Table 1. We included 4,868 patients in the analysis, 1,380 of whom had severe COVID-19. The age distribution of the severe group was about 20 years older than that of the non-severe group (median 79.0 and 57.0 years old, mean 74.3 and 53.9 years old, respectively). Both the severe and non-severe groups showed comparatively high vaccination coverage, with 61.1% and 65.0% vaccinated at least twice, respectively. The severe group showed a higher proportion of past medical history and comorbidity; for instance, 14.3% of the severe group had cardiovascular disease, compared with only 5.8% of the non-severe group. Poorer physical activity and higher case fatality rate were other characteristics of the severe group.
Table 1. Demographic characteristics of hospitalised patients for COVID-19 caused by the Omicron VOC

IQR, interquartile range; LTCF, long-term care facility; NA, not applicable.
Table 2 and Figure 1 show the results of the logistic regression analysis. Age, male sex, cardiovascular disease, cerebrovascular disease, chronic lung disease, physician-diagnosed obesity, admission from a long-term care facility and poor physical activity status were identified as risk factors for severe illness (i.e. need for supplementary oxygen during admission). Vaccination was identified as the only factor associated with non-severe illness.

Fig. 1. Results of multivariable logistic regression analysis. Black circles indicate median. Whiskers indicate 95% confidence intervals. LTCF, long-term care facility.
Table 2. Results of logistic regression analysis

LTCF, long-term care facility.
Figures 2a–2c show the standardised mean difference before and after the matching procedure for dementia, admission from a long-term care facility and physical activity status, respectively. For all categorisations, neither group showed significant differences in each item included in the model. The details of the three datasets after matching are available in Tables S1–S3 in the supplementary file.
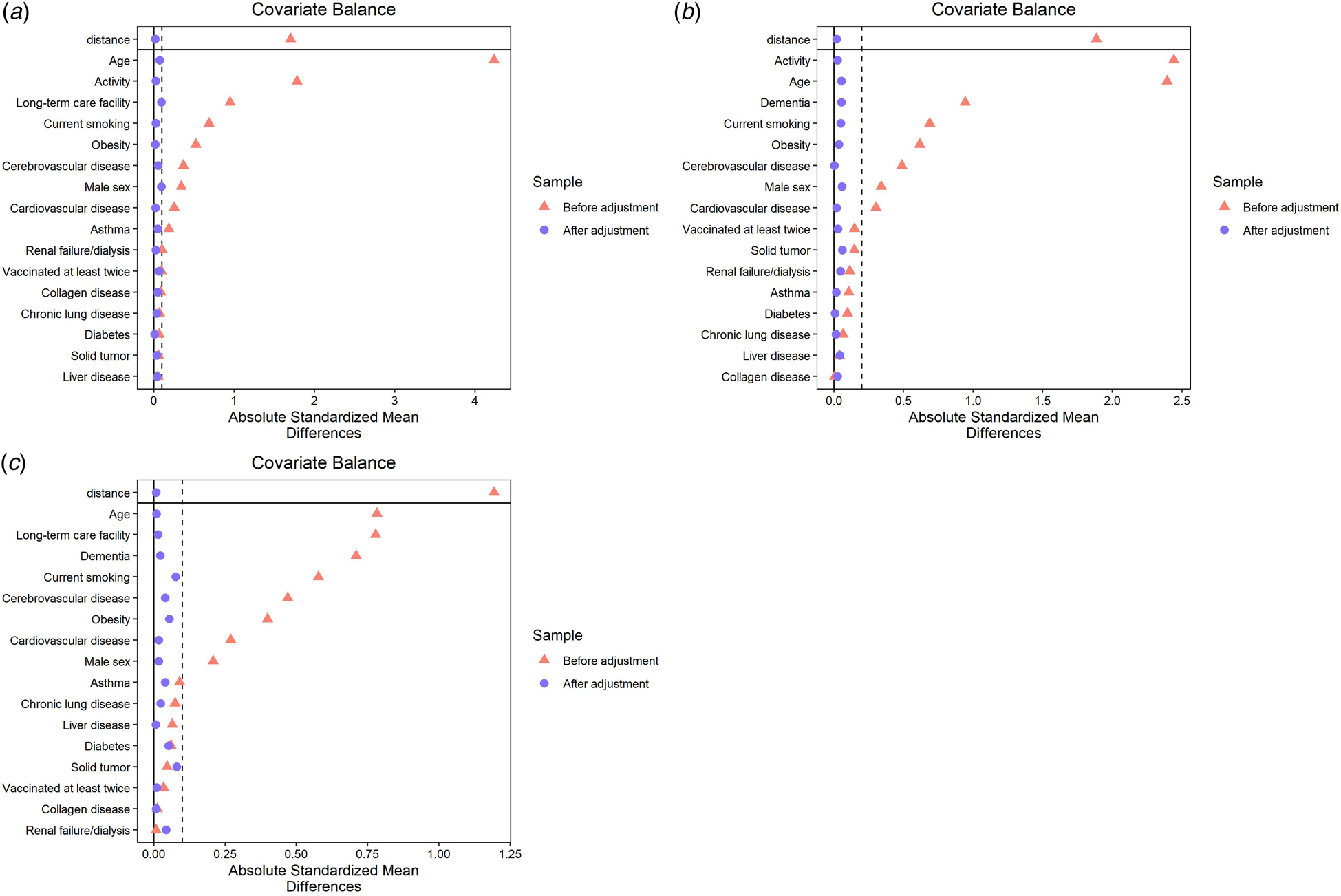
Fig. 2. Balance of demographic characteristics of older COVID-19 inpatients before and after PS matching in relation to (a) with dementia or without dementia, (b) admission from a long-term care facility or from elsewhere and (c) poor or good physical activity status. LTCF, long-term care facility.
Table 3 shows the estimated ATT of each cohort for the risk of having severe disease. All three factors were significantly associated with disease severity. Dementia showed a negative relevance to the severity. Both admission from a long-term care facility and poor physical activity status were associated with severe illness, with the latter showing a larger ATT. Fisher's exact test determined similar results for admission from a long-term care facility and poor physical activity status (P = 0.014 and < 0.001, respectively), whereas dementia showed no significant difference (P = 0.435).
Table 3. Average treatment effect on the treated for each matched cohort on supplementary oxygen requirement

ATT, average treatment effect on the treated; LTCF, long-term care facility.
Table 4 shows the estimated ATT of each cohort for the risk of death. Similar to the estimated ATT for having severe disease, poor physical activity was positively associated with mortality, and dementia was negatively associated with it (P < 0.001 and = 0.004, respectively). Living in a long-term care facility showed no significant association with death. As for the results of Fisher's exact test, poor physical activity status showed significant difference in mortality (P < 0.001), whereas dementia and admission from a long-term care facility did not (P = 0.236 and 0.582, respectively).
Table 4. Average treatment effect on the treated for each matched cohort on death
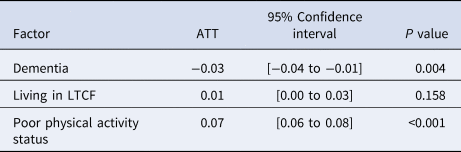
ATT, average treatment effect on the treated; LTCF, long-term care facility.
Discussion
This study identified the risk factors for severe illness due to COVID-19 caused by the Omicron VOC in older adults. There has been little evidence relating to these factors in Japan until now, and our results show that the risk factors for severe illnesses due to the Omicron VOC are very similar to those of other variants (e.g. Alpha and Delta variants). As suggested previously, older age, male sex, cardiovascular disease, chronic lung disease and obesity are among the factors associated with severe COVID-19 [Reference Terada5, 9, Reference Bouzid12, Reference Booth17, 18]. In addition, these risk factors are similar to those specific to the elderly population [Reference Asai6]. These facts may suggest that the original pathology of SARS-CoV-2 infection is inflammation of the respiratory tract, regardless of the strain or variant of the causative organism.
At least two vaccine doses is strongly associated with non-severe illness for the Omicron VOC, as well as for other strains [Reference Lauring13, Reference Dagan30–Reference Polack32]. This result supports the need to promote vaccination at the population level, even though there has been concern about the efficacy of the currently available COVID-19 vaccines against the Omicron VOC [Reference Lauring13].
The results pertaining to dementia should be carefully interpreted. Although Fisher's exact test did not show significant differences in the risk of severe disease in the data after PS matching, logistic regression analysis did not identify dementia as one of the risk factors for severe illness. In addition, the ATT for dementia showed a negative relevance to its severity. These results are not in agreement with those of previous studies [Reference Vlachogiannis22, Reference Kim23, Reference July and Pranata33, Reference Wang34], and dementia itself would not intuitively seem to have a positive effect on the severity of COVID-19. However, two reports from Japan stated that dementia was not a significant risk factor for severe illness due to COVID-19 [Reference Asai6, Reference Miyashita35]. One possible reason is that dementia is easily confounded by other factors specific to the elderly population, such as poor physical activity, and so the true effect of dementia is difficult to determine by simple logistic regression analysis. Good physical activity is likely an important factor in preventing severe illnesses, and dementia itself does not impair physical activity directly. Identifying the reason for this discrepancy is a future challenge.
According to our results, both living in a long-term care facility and poor physical activity status were associated with severe COVID-19, but the ATT for poor physical activity status was the larger of the two. This may suggest that poor physical activity status has a greater relevance to the severity of COVID-19 than living in a long-term care facility; in other words, older adults who live in such facilities may also have different risk. Those who have a good physical activity status may have a lower risk of severe illness even if they live in a long-term care facility. This finding could be valuable when we consider how long-term care facilities manage COVID-19, particularly in super-aged societies like Japan. For instance, there is likely to be substantial diversity in activities of daily living among the residents of long-term care facilities, and physical activity status could be a potential determinant for the triage of hospitalisation requests from such facilities when healthcare resources are limited.
The similar results of ATT for mortality should also be noted. The negative association seen between dementia and mortality, and the positive association seen between poor physical activity and mortality, suggest that physical activity is important to prevent poor clinical outcomes. Furthermore, living in a long-term care facility did not show any significant difference in mortality. Given these results, the place of residence itself might be an indirect factor for prognosis, whereas physical activity may be more directly associated with it.
Several limitations of this study should be noted. First, this was a retrospective cohort study and not a randomised controlled trial. Although we adjusted for various factors using PS matching, not all factors were included in the model. Next, we excluded a large number of patients from the final analyses because if any two groups (i.e. presence/absence of dementia, living in a long-term care facility/elsewhere, good/poor physical activity status) had substantially different demographic characteristics, then numerous patients had to be removed to adjust the background of both groups. Although this would strengthen the internal validity of our results, the external validity and generalisability would be sacrificed to some extent. Furthermore, because our registry data did not include information directly assessing the activities of daily living of each patient (e.g. Barthel Index), we had to adopt a new indicator comprising several factors associated with physical activity. These factors – normal diet, independent walking, and ability to take care of oneself – were assessed by each physician in each facility participating in our registry, and subjectivity may have affected the result. Last, we defined severe disease as having required any supplementary oxygen therapy. Given that physicians sometimes use supplementary oxygen for mild cases, we cannot rule out that some mild cases were included in the severe group in this study, leading to potential bias of the results. However, our analyses of mortality showed similar results, so we believe it is reasonable to conclude that poor physical activity would be associated with a poor clinical outcome and dementia might be associated with a good clinical outcome.
Conclusions
Our results suggest that physical activity and living in a long-term care facility have a substantial association with severe illness caused by COVID-19 owing to the Omicron-19 variant, whereas dementia may be associated with non-severe illness. We should take these factors into consideration in the management of older adults with COVID-19.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268822001686
Acknowledgements
The authors thank all of the participating facilities for their care of patients with COVID-19 and their cooperation with data entry into the registry. The data used for this research were provided by the REBIND (Repository of Data and Biospecimen of Infectious Disease) project, which was commissioned for the National Center for Global Health and Medicine by the Ministry of Health, Labour and Welfare of Japan.
Author contributions
ST and NO conceived the study. TA curated the data. ST and TA analysed and interpreted the data. ST wrote the first draft of the manuscript. All authors critically reviewed the manuscript and approved the final version.
Financial support
This research was supported by the Health and Labour Sciences Research Grant, ‘Research on Emerging and Re-emerging Infectious Diseases and Immunisation’ (grant number 20HA2003) and JSPS KAKENHI (grant number 20K10546).
Conflict of interest
All authors have no conflicts of interest to be disclosed.
Ethical standards
Our study data were provided by Research Electronic Data Capture, a secure, Web-based data capture application hosted at the JCRAC Data Center of the National Center for Global Health and Medicine. The opt-out recruitment method was applied, and informed consents for individuals were waived, as approved by the National Center for Global Health and Medicine Ethics Review Board. Information about the entire research is available through the COVID-19 Registry Japan website (https://covid-registry.ncgm.go.jp/). This study was approved by the National Center for Global Health and Medicine Ethics Review Board (approval number: NCGM-G-003494-0).
Availability of data
The data supporting the findings of this study are not publicly available due to the privacy of research participants and sites but are available upon reasonable request. Data on an individual level are shared with limitations to participating healthcare facilities through application to the REBIND project.









