INTRODUCTION
Acute gastroenteritis is the second most common disease worldwide [Reference Butterton, Calderwood and Kasper1]. The prevalence of diarrhoea, a major symptom of gastroenteritis, in selected communities of Canada and the United States, was found to be the highest in several developed countries (Australia, Canada, Ireland, United States) [Reference Scallan2]. A study in Hamilton (Ontario, Canada) estimated the mean annual cost per case of gastroenteritis at Can$1089. Although it might not be representative of Canada, those numbers highlight the importance of this illness and the need for prevention efforts [Reference Majowicz3].
Manure is known for its benefit as a fertilizer. However, in livestock farming areas, it has also been considered an important environmental issue. Manure may carry a variety of bacterial and protozoan pathogens which can cause acute gastroenteritis in humans (also known as zoonoses) such as Cryptosporidium parvum, Giardia spp., Campylobacter spp., Escherichia coli O157:H7, and Salmonella spp. [Reference Hooda4, Reference Pell5]. Hutchison et al. [Reference Hutchison6] studied the levels of zoonotic pathogens in British livestock manure and found that over 30% of livestock manure examined contained at least one pathogen. When contaminated run-off from manure-applied fields reaches the drinking-water supply, it could be a threat to public health especially in the case of water-treatment deficiency or inadequate monitoring. In fact, a study in Ontario (Canada) identified that the application of manure to land was associated with endemic human Shiga toxin-producing E. coli infection [Reference Valcour7].
A surplus occurs when manure produced on intensive livestock farms far exceeds agronomic requirements. A significant association between manure surplus and acute gastroenteritis hospitalization (AGH)-zoonotic has been shown in our previous study [standardized rate ratio 1·4, P⩽0·01] [Reference Lebel8]. However, the relation of the disease with a specific type of animal production has not yet been investigated because the only indicator used was the phosphorus index, which is not an animal-specific indicator. Different animals might carry different pathogens. As the number of animals on a farm increase, the probability of pathogens being present in the manure will increase as well [Reference Hooda4, Reference Pell5, Reference Cliver, Fayer and Cotruvo9].
The 2001 Canadian Census of Agriculture has shown an increasing number of livestock in Canada compared with the previous census in 1996: cattle increased by 4·4%, pigs by 26·4% and sheep by 46%. There was also an increase of 5·5% on the total manure spread in Canada [10]. Therefore, it is important to improve our earlier study with more recent data by adding a more specific indicator, livestock density.
The main objective of the present study is to evaluate the association between manure surplus or livestock density indicators and human AGH. Furthermore, because the risk of gastroenteritis may vary according to water source [Reference Birkhead and Vogt11] and subjects' age [Reference Majowicz, Horrocks and Bocking12], we aimed to assess the modifying effects of water source and subjects' age.
METHODS
Study design
An ecological study design was used in Quebec agricultural municipalities. In this study, the exposure data was measured at municipal level but the analysis was carried out at an individual level by inferencing individual exposure from the municipal data. An agricultural municipality was defined as a municipality with at least 25% of its surface area used for agricultural purposes. The agricultural municipalities with intensive farming activities – manure surplus and high animal density – were selected and compared with those without intensive farming activities.
Study population
The study population consisted of subjects living in Quebec (Canada) agricultural municipalities which were classified into two groups based on the manure surplus status. The first group consisted of subjects living in municipalities in seven watersheds considered by the Ministère du Développement durable, de l'Environnement et des Parcs du Québec (MDDEP) to be in ‘manure surplus’ (the exposed group). These watersheds involve the following rivers: Chaudière, Etchemin, Boyer, L'Assomption, Bayonne, Yamaska and Nicolet. The second group consisted of subjects living in Quebec municipalities located outside the watersheds (the unexposed group). All Quebec agricultural municipalities (n=306) out of 1476 Quebec municipalities were included in the study. Among them, 139 municipalities were considered to be exposed to manure, while 167 were unexposed.
Data sources
Cases
Cases of AGH were obtained from the Med-Echo database for the period January 2000–December 2004. Med-Echo is the only hospital database available in Quebec in which all hospitalizations are registered. The following variables were obtained from the database for each case: age, sex, municipality code, admission date, date of discharge, main diagnosis [according to Classification of Diseases, 9th revision (ICD-9) codes], 15 secondary diagnoses (ICD-9 codes) and encrypted health insurance number.
We used a modified list of ICD-9 codes for acute gastroenteritis previously used in a study of the association between water quality and gastrointestinal illness in Vancouver (Canada) [Reference Aramini13] to select gastroenteritis cases from the Med-Echo database (see Appendix). A hospital admission was counted as an acute gastroenteritis case if it fitted the appropriate ICD-9 code. We classified the codes into four categories:
(1) acute potentially zoonotic gastroenteritis,
(2) acute non-zoonotic gastroenteritis,
(3) acute gastroenteritis with unknown aetiology,
(4) various symptoms related to gastroenteritis.
The ICD-9 codes classified as acute potentially zoonotic gastroenteritis were the codes for infections which were considered by the World Health Organization experts as potentially zoonotic and possibly water-related [Reference Cliver, Fayer and Cotruvo9].
The case definition in this study was also adapted from the Vancouver study [Reference Aramini13]. A hospital admission for a case of gastroenteritis was defined as any individual from the studied municipality admitted to hospital with a main diagnosis of any of the ICD-9 codes from categories 1, 2 or 3 or with the main diagnosis of any of the ICD-9 codes from category 4 with at least one secondary diagnosis of any of the ICD-9 codes from category 1. A main diagnosis is a diagnosis determined by a physician based on clinical and/or laboratory testing, and considered as the leading cause of a hospitalization. A secondary diagnosis is another diagnosis which is not the leading cause of hospitalization, and for which a patient could have been diagnosed or received treatment during hospitalization [14].
Individuals who were admitted to and discharged from a hospital on the same day were excluded. In order to ensure that we included only incident cases, if the same individual was hospitalized more than once for the same diagnosis within 30 days from the date of discharge of the last hospitalization, only the first hospitalization was retained.
Risk factor variables
Two exposures as the indicators of livestock farming intensity were assessed: manure surplus and livestock density. Both indicators were measured at the municipal level.
The definition of ‘manure surplus’ was based on the phosphorus index used to assess the overproduction of manure in a municipality by the MDDEP. It is the soil-surface phosphorous balance resulting from the application of animal manure to farmland and its absorption by cultivated plants in a given municipality. The phosphorous index (kg/ha per year) was calculated as the quantity of phosphorus produced by farm animals in a municipality (kg/year), after subtracting the estimated quantity of phosphorous consumed by cultivated plants (kg/year) in the municipality, divided by the total cultivated area of the municipality (ha). If the phosphorous index was >0 kg/ha per year then it was considered a positive index, otherwise as a negative index [Reference Lebel8]. The phosphorous index data for each selected municipality were obtained from the MDDEP.
Livestock density data of each municipality were obtained from the Ministère de l'Agriculture des Pêcheries et de l'Alimentation du Québec for the period 2000–2003. Livestock density was defined as the number of animal units relative to total cultivated area (a.u./ha) within a municipality. One animal unit is equivalent to ~500 kg of animal body weight [15]. Five variables of livestock density were developed: (1) total animal density, (2) dairy cattle density, (3) beef cattle density, (4) swine density, and (5) poultry density.
Municipality water-source data were obtained from the Drinking Water Database of MDDEP updated on October 2002. We used three categories of water source in analysis: (1) surface water, (2) ground water, and (3) private well. The term ‘private well’ was applied to the municipalities where there were no community waterworks or where community waterworks was supplied to ⩽49% of the population.
The deprivation index, developed by Pampalon & Raymond [Reference Pampalon and Raymond16], was used as a socioeconomic level indicator. The index is based on the smallest geographic unit for which census data are available in Canada, namely the enumeration area (EA). One EA has ~750 persons. It is considered to be socioeconomically homogeneous. In the selected municipalities, the average number of EAs in each municipality was 3·02 (range 1–22). To estimate the deprivation level of AGH cases, EAs were linked to the postal code of each case, as recorded in the Med-Echo database. The index combines six indicators which are related to a large number of health and welfare determinants: the proportion of persons without a high-school diploma; the ratio of employment to population; average income and the proportion of persons who are separated, divorced, or widowed; the proportion of single-parent families; and the proportion of people living alone. The indicators represent two forms of deprivation represented by two variables: material and social deprivation variables. Each variable was classified according to quintiles, ranging from the least deprived (quintile 1) to the most deprived (quintile 5) segments of the Quebec population [Reference Pampalon and Raymond16].
In addition, the changes of municipality codes during the study period were taken into account while gathering AGH cases. We used the 2001 Canadian census of population to estimate the size of population of each municipality by age and sex and to obtain the socioeconomic data of the population.
Statistical analysis
We compared the baseline characteristics between the exposed and unexposed study population groups. Binomial regression was used to compare the proportion of each baseline characteristic except for average income, to which a Student's t test was applied.
The AGH rate as outcome was defined as the number of AGH cases that occurred during the study period divided by the person-years over the study period [Reference Rothman, Greenland, Rothman, Greenland and Lash17]. The AGH rates were calculated for the 2000–2004 period: (1) by 5-year age groups, (2) by sex group, (3) for total AGH cases and by categories of acute gastroenteritis. All those rates were calculated separately using exposure groups except for the first one, where the rate was calculated for the whole population. Univariate Poisson regression was used to compare the hospitalization rate according to sex and age.
AGH events were discrete counts and rare events, which is a characteristic of a Poisson distribution [Reference Ott and Longnecker18]. The group-level data, either municipal or EA level, were assigned to each subject within the same group. Therefore, a Poisson regression model with the individual as the smallest unit of analysis was chosen to evaluate the association between AGH and farming indicators. Since each indicator of livestock farming intensity was a single variable measured on ‘cluster’ of subjects (municipality), we incorporated the generalized estimating equation approach into the regression model and we entered municipality as a repeated effect [Reference Hanley19]. Exponentiation of the main effect coefficient in the Poisson models estimates the adjusted hospitalization rate ratio (aHRR) for the effect.
We assessed the association between the two farming indicators and AGH with cases retained from all four categories of AGH. Furthermore, associations between the farming indicators and AGH-zoonotic, with cases retained from the AGH potentially zoonotic category, were also assessed separately. The following variables were considered as potential confounders: age, sex, deprivation index, and water source. Age and water source were also considered as modifiers prior to evaluating their confounding effect.
To evaluate the association between AGH (or AGH-zoonotic) and manure surplus as well as the modifying effects of age and water source on the association, we created three models: (1) one model included manure surplus, age, sex and deprivation index variables, (2) one model included manure surplus, age, sex, deprivation index and an interaction term between manure surplus and age variables, (3) a third model included manure surplus, age, sex, deprivation index, water source and an interaction term between manure surplus and water-source variables. The modifying effects were tested using the Wald test for the interaction term included in the models. In the absence of the modifying effect, we assessed the possible confounding effects.
In order to evaluate the association between AGH and livestock density, the two groups of municipalities previously defined as ‘exposed’ and ‘unexposed’ to manure surplus were pooled. They were then classified according to quartile breaks for each livestock-density variable based on the number of municipalities under study. The lowest quartile (Q1) was chosen as the reference group. Variance inflation factors were calculated to assess the possibility of multicollinearity between livestock-density variables. Since we did not observe any multicollinearity, we placed all livestock-density variables into one model, except for the total animal-density variable which we ran in a separate model because, by nature, it is correlated with the other livestock-density variables. The trends of risk ratio across the quartiles of livestock-density variables were tested. We used the Wald statistic to test the trend by using the median of density in each quartile as a continuous variable in the model. Furthermore, effects of specific zoonotic pathogens were also evaluated for exploratory purposes. However, due to an insufficient number of cases, we were only able to assess the association between livestock density and Salmonella, Campylobacter and E. coli infections.
The variables entered in the models are shown in Table 1. Only the variables with significant and/or confounding effects were kept in the final model. A P value of ⩽0·05 was considered statistically significant. A confounding effect was assessed by calculating the difference between the HRR before and after a selected variable was entered. If the difference was ⩾10%, the variable was considered as a confounder. SAS statistical software version 9.1 was used for analysis [20].
Table 1. Distribution of the value of each variable included in the Poisson regression models used to evaluate the association between human acute gastroenteritis and both manure surplusFootnote * and livestock density in Quebec (Canada) agricultural municipalitiesFootnote †, 2000–2004
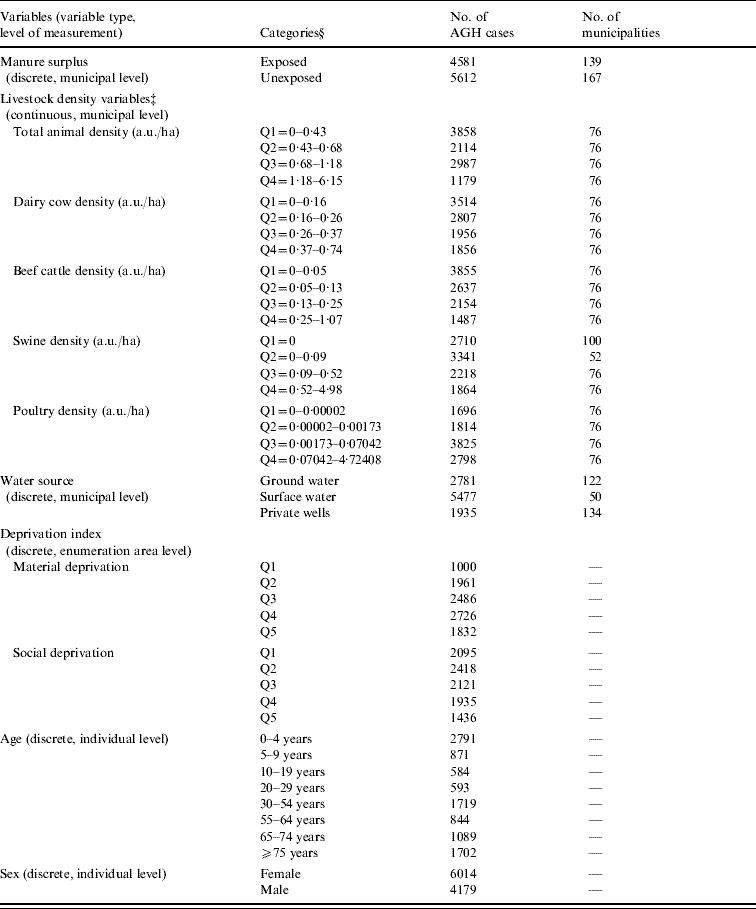
AGH, Acute gastroenteritis hospitalization; a.u./ha, animal unit/hectare.
* Manure surplus was indicated by a positive phosphorous index.
† An agricultural municipality was a municipality with at least 25% of its surface area used for agricultural purposes.
‡ Data for two municipalities were missing.
§ Q, Quartile or quintile.
RESULTS
Baseline characteristics of the study population
The 306 municipalities under study had a population of 1 135 790 in 2001 and represent about 16% of the Quebec population (7 237 480 people). A total population of 521 630 (46%) was considered to be exposed to manure while a population of 614 160 (54%) was unexposed.
The baseline characteristics of the study population are shown in Table 2. Most variables were statistically different between the exposed and unexposed groups (P<0·01), except for the proportion of the population aged ⩾15 years (P=0·31), the employment rate (P=0·53) and the proportion of persons aged 15–24 years without a school degree (P=0·36). All variables except for the proportion of the population aged ⩾15 years, participation rate, and unemployment rate are incorporated in the deprivation index.
Table 2. Comparison of baseline characteristics of the exposed and the unexposed groups based on 2001 Canadian Census of Population (Statistics Canada)
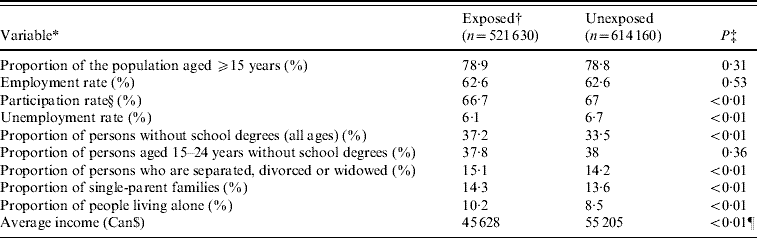
* Terms rate and proportions were used instead of real numbers to ensure comparability between municipalities.
† Exposed group refers to subjects living in municipalities with a positive phosphorous index and at least 25% of their surface area used for agricultural purposes.
‡ Obtained from binomial regression model or Student's t test.
§ ‘Participation rate in labour market activities refers to the labour force in the week (Sunday to Saturday) prior to Census Day (15 May 2001), expressed as a percentage of the population 15 years of age and over’ [21].
¶ Student's t test.
Number of cases and AGH rate
A total of 10 457 cases of AGH for the study population was recorded in the Quebec hospital database for the period 2000–2004. Despite universal health insurance in Quebec, 264 cases did not have a health insurance number and were then excluded. Consequently, 10 193 cases were included in the study. The majority of the AGH cases (88%) were gastroenteritis of unknown aetiology. Of all cases, 526 were categorized as AGH-zoonotic. This included: 186 cases of Salmonella infections (other than typhoid and paratyphoid fever), 23 cases of Giardia infections, two cases of other specified protozoal intestinal diseases, 90 cases of E. coli infections (all strains), 220 cases of Campylobacter infections, and five cases of Yersinia enterocolitica infections.
AGH rate varies by age (P<0·01) with the highest rates occurring in the youngest (0–4 years) and the oldest (⩾85 years). In the older population, rates tended to increase by age group (Fig. 1). We also found that females had higher rates than males in both the exposed and unexposed groups (P<0·01) (Table 3). Of the four acute gastroenteritis categories, acute gastroenteritis with unknown aetiology had the highest rate, regardless of its exposure status (155·2/100 000 person-years for the exposed group and 161·7/100 000 person-years for the unexposed group) (Table 3).
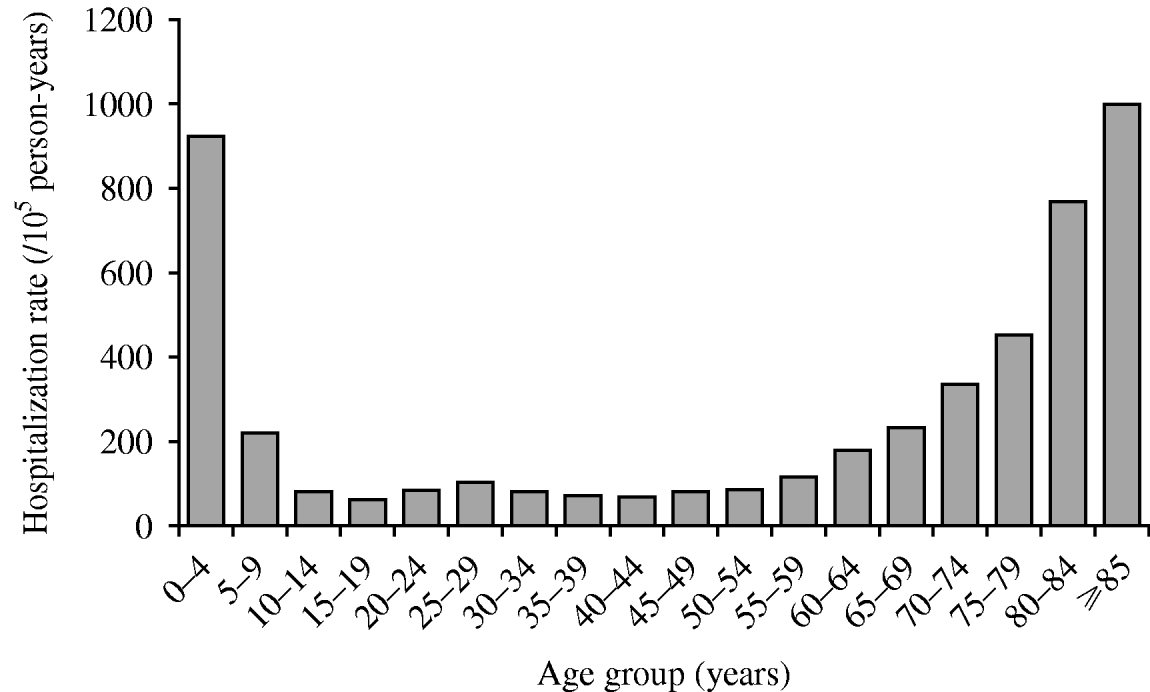
Fig. 1. Age-specific acute gastroenteritis hospitalization rates in Quebec (Canada) agricultural municipalities (municipalities with at least 25% of their surface areas used for agricultural purposes), 2000–2004.
Table 3. Hospitalization rate for acute gastroenteritis [per 100 000 person-years (p-yr)] in the agricultural municipalities* exposed and unexposed to manure surplus†, Quebec, Canada, 2000–2004

* An agricultural municipality was a municipality with at least 25% of its surface area used for agricultural purposes.
† Manure surplus was indicated by a positive soil-surface phosphorous index.
‡ The population exposed to manure surplus.
§ Wald confidence interval.
¶ P value obtained from univariate analysis of sex variable by Poisson regression.
Association between AGH and surplus of manure
For analysis with cases retained from all four categories of AGH, the aHRR of AGH for the manure surplus indicator was 1·03 (95% CI 0·76–1·40). Furthermore, we observed that age modified the association between AGH and manure surplus (P for the interaction term <0·01). Risk ratio was higher for young children with a significant HRR for the 5–9 years age group (aHRR 1·45, 95% CI 1·02–2·05). An apparent protective effect was observed in adults aged ⩾20 years. However, the effect was only significant in age groups 55–64 and 65–74 years. Moreover, we observed a modifying effect of water source (P for the interaction term=0·03). A significant risk ratio of manure surplus was observed in municipalities supplied by private wells and ground water (Table 4). Finally, since we found the modifying effect of subjects' age, we verified the age-specific effect for each water-source type. The significant risk ratio of manure surplus in municipalities supplied by private wells and ground water was especially observed in children aged 0–4 years (private wells: aHRR 1·81, 95% CI 1·30–2·54; ground water: aHRR 1·73, 95% CI 1·25–2·38).
Table 4. Evaluation of modifying effect of subjects' age and water source on the association between hospitalization rate for acute gastroenteritis and manure surplusFootnote * in the Quebec (Canada) agricultural municipalitiesFootnote †, 2000–2004
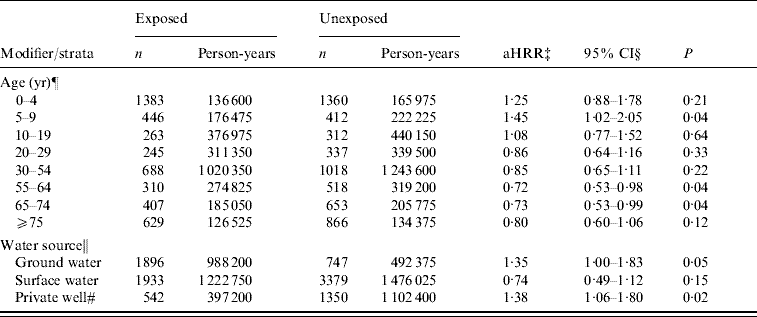
* Manure surplus was indicated by a positive soil-surface phosphorous index.
† An agricultural municipality was a municipality with at least 25% of its surface area used for agricultural purposes.
‡ aHRR, Adjusted hospitalization rate ratio of manure surplus for acute gastroenteritis. The modifying effect of age and water source was assessed in two separate models.
§ Confidence intervals were based on empirical standard error estimates.
¶ The model was adjusted for sex and deprivation index. There was a modifying effect of age on the association between AGH and manure surplus (P value of the interaction term <0·01).
‖ The model was adjusted for age, sex, and deprivation index. There was a modifying effect of water source on the association (P value of the interaction term=0·03).
# The term ‘private wells’ was applied to the municipalities where there were no community waterworks or where community waterworks was supplied to ⩽49% of the population.
For analysis with cases retained from the category of AGH potentially zoonotic, the aHRR of AGH-zoonotic for the manure surplus indicator was 1·06 (95% CI 0·86–1·31). However, we found a borderline significant effect modification of age (P for the interaction term=0·06) with a significant risk ratio in children aged 0–4 years (HRR 1·93, 95% CI 1·21–3·09) (Table 5). Finally, we did not find any modifying or confounding effect of water source on the association between the manure surplus and AGH-zoonotic. Sex and deprivation index were finally excluded from this analysis because they did not have any significant or confounding effect on the association (data not shown).
Table 5. Evaluation of modifying effect of age on the association between hospitalization rate for acute potentially zoonotic gastroenteritis and manure surplusFootnote * in the Quebec (Canada) agricultural municipalitiesFootnote †, 2000–2004
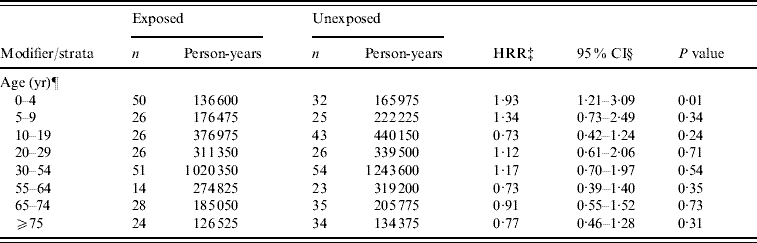
* Manure surplus was indicated by a positive soil-surface phosphorous index.
† An agricultural municipality was a municipality with at least 25% of its surface area used for agricultural purposes.
‡ HRR, Hospitalization rate ratio of manure surplus for acute potentially zoonotic gastroenteritis.
§ Confidence intervals were based on empirical standard error estimates.
¶ The modifying effect of age on the association between AGH-zoonotic and manure surplus was borderline significant (P value of the interaction term=0·06). Sex and deprivation index variables were excluded because they did not have significant or confounding effect.
Association between AGH and livestock density
Because the association between AGH and manure surplus was especially observed in children aged 0–9 years, we decided to evaluate the association between livestock density and AGH/AGH-zoonotic in this age group. Furthermore, pathogen-specific effects were assessed for the same age group.
In this age group, we observed a small increasing trend of HRR across the quartiles of all types of livestock density, except for beef cattle. However, in the AGH model, the trend was only significant for poultry density (P=0·04). For the AGH-zoonotic model, the trend was significant for total animal density (P=0·04) and swine density (P=0·04) (Table 6). Further analysis was conducted for AGH-zoonotic in children aged 0–4 years and similar trends were observed in this age group without significant effect for total animal and swine density. However, we observed a significant increasing trend with an apparent dose–response effect for poultry density (aHRR from Q1 to Q4=1, 2·33, 2·53, 2·98, respectively; P=0·03).
Table 6. Association between acute gastroenteritis hospitalization and livestock density [animal unit/hectare (a.u./ha)] [hospitalization rate ratio (HRR) and 95% confidence intervals (CI) obtained from multivariate Poisson regression model] in children aged 0–9 years, Quebec, Canada, 2000–2004
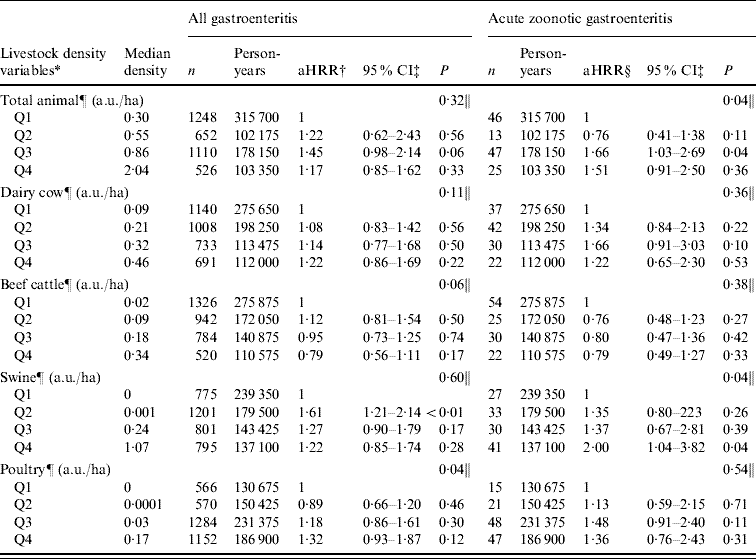
* All livestock-density variables, except total animal density, were assessed in the same model.
† aHRR, Adjusted hospitalization rate ratio adjusted to the other livestock-density variables (except the total animal density), age, sex, and deprivation index.
‡ Confidence intervals were based on empirical standard error estimates.
§ aHRR, Adjusted hospitalization rate ratio adjusted to the other livestock-density variables (except the total animal density), age, and deprivation index.
¶ Categorized variable.
‖ P trend obtained by Wald statistics.
In children aged 0–9 years, increasing trends of HRR were observed between Campylobacter infections and dairy-cow density (aHRR from Q1 to Q4=1, 1·08, 2·90, 1·72, respectively; P=0·05), E. coli infections and swine density (aHRR from Q1 to Q4=1, 1·15, 4·32, 6·06, respectively; P=0·03). In children aged 0–4 years, a significant increasing trend of HRR of Salmonella infections was only observed in poultry density (aHRR from Q1 to Q4=1, 3·95, 5·20, 7·37, respectively; P=0·05).
DISCUSSION
Several studies have shown associations between livestock farming intensity and acute gastroenteritis incidence [Reference Valcour7, Reference Potter, Kaneene and Gardiner22, Reference Haus-Cheymol23]. We found that the association between livestock farming intensity and AGH as well as AGH-zoonotic was modified by subjects' age and water source.
Morbidity from gastroenteritis mostly involves young children [Reference Butterton, Calderwood and Kasper1] and has been observed in several studies [Reference Majowicz, Horrocks and Bocking12, Reference Potter, Kaneene and Gardiner22, Reference Kuusi24]. This may be due to lack of immunity because the immune system in children may not be well developed yet. Protective immunity resulting from repeated low-level exposure from infected persons or other sources including drinking water has recently been suggested as an important consideration for the lower risk in adults [Reference Kuusi24–Reference Frost26]. Persons exposed to contaminated water in their normal drinking water may also develop some protective immunity [Reference McAnulty27, Reference Licence28].
Variation of risk of gastroenteritis according to water-source type has been shown in some studies. Birkhead & Vogt [Reference Birkhead and Vogt11] observed higher risks of Giardia infections in municipalities supplied by ground water [relative risk (RR) 1·8, 95% CI 0·9–3·4], non-filtered surface water (RR 1·9, 95% CI 1·1–3·3), and private water supply (RR 2·2, 95% CI 1·3–3·6) compared to filtered surface water. Drinking water from a private supply has also been identified as a risk factor for gastroenteritis in children [Reference Kuusi24]. The higher probability of water contamination in municipalities receiving ground water but not surface water was also observed in a local study conducted on the seven watersheds exposed to manure surplus. In addition, all surface water in the exposed territories received complete treatment (filtration and disinfection) before distribution [Reference Tremblay29]. Therefore, lack of association between manure surplus and AGH in municipalities receiving surface water supply may be due to the high quality of drinking water provided by surface water systems. In contrast, not all waterworks distributing ground water used the treatment [Reference Tremblay29].
In our study, the association observed between livestock density and AGH-zoonotic in children should be interpreted with caution since only a small number of cases were available for analysis and some analyses were exploratory. Associations between livestock density, especially cattle, and acute gastroenteritis have been observed in previous studies in Canada and other countries [Reference Valcour7, Reference Potter, Kaneene and Gardiner22, Reference Haus-Cheymol23, Reference Nygard30]. Valcour et al. [Reference Valcour7] found a strong association between ratio of beef cattle number to human population and human Shiga toxin-producing Escherichia coli (STEC) infection in Ontario. Haus-Cheymol et al. [Reference Haus-Cheymol23] found that the paediatric incidence of haemolytic–uraemic syndrome (HUS) was associated with dairy-cattle density and the ratio of calves to children aged <15 years in France (P<0·01). Nygard et al. [Reference Nygard30] observed a significant association between ruminant density and Campylobacter incidence in Sweden [incidence rate ratio (IRR) 1·08, 95% CI 1·05–1·11]. Surprisingly, in the present study, the associations observed were mostly with swine and poultry density. Association with poultry density has been investigated by Potter et al. [Reference Potter, Kaneene and Gardiner22]. A higher incidence of C. jejuni enteritis in the counties with high poultry density than those with low poultry density was observed (IRR 1·31, 95% CI 1·21–1·42). In our study, the association observed between poultry density and AGH-zoonotic in children was mostly due to Salmonella infections. It is well known that poultry is a major source of Salmonella infections in humans, although cattle can also be an important reservoir [Reference Cliver, Fayer and Cotruvo9]. The association with swine density may be due to the high production of pigs in Quebec as this province is the main producer of pigs in the country [10]. It is widely known that ruminants are considered to be the main reservoir for E. coli especially E. coli O157 [Reference Cliver, Fayer and Cotruvo9]. However, our results may indicate that swine could be another reservoir, as observed in other countries and recently in the United States [Reference Feder31]. Above all, it is also possible that the observed associations were due to the multiple comparisons issue in which the null hypothesis is incorrectly rejected [Reference Savitz and Olshan32].
As already known, acute gastroenteric-causing pathogens have several routes of transmission. They may spread from person to person or may be transmitted by food or environmental vehicles or they may also result from exposure to animals [Reference Musher and Musher33]. In the present study, direct and indirect contact with farm animals or animal excreta may have been important routes of transmission. Contact with animal faeces: living on, working on, or visiting a farm has been found to be associated with gastroenteritis [Reference Voetsch34, Reference Locking35].
Our study has several strengths. The AGH cases obtained from Med-Echo were clinically and/or laboratory confirmed and the database covers all hospitals in the province of Quebec that offers universal health insurance. Furthermore, we evaluated the modifying effect of age on the association between manure surplus and AGH and controlled for the confounding effect of sex. Both of these are important demographic determinants of acute gastroenteritis [Reference Majowicz, Horrocks and Bocking12]. We also introduced a deprivation index because socioeconomic characteristics may influence host susceptibility. The importance of this index in health research has recently been demonstrated [Reference Martinez, Pampalon and Hamel36].
As an ecological study, our analysis was based on data at group level rather than an individual level. This has some limitations, particularly for the indicators of livestock farming intensity data which were measured at the municipal level. Individuals living in a municipality with manure surplus or with high animal density are not always exposed to the same level of manure pathogens. For example, individuals living where the manure is only spread 100 m from their residence may be more exposed than those where the manure is spread 1 km from their house. The water-source variable was also measured at the municipal level which may not reflect the water source consumed by individuals. The possibility of misclassification of exposure due to the use of these data measurements is not negligible. Moreover, we were unable to measure several potential confounders which were available only at the individual level, such as contact with farm animals, farm (where the manure was spread) to house distance, consumption of possibly contaminated food, water consumption, water treatment applied by individuals, travel history, etc. Despite limitations, ecological studies remain useful especially when the influence of environmental variables is difficult to measure at an individual basis. Additionally, they are inexpensive, take little time, and can cover a wide study area.
CONCLUSIONS
We have evaluated the association between livestock farming intensity and hospitalization for acute gastroenteritis. We noted a significant association between manure surplus and acute gastroenteritis as well as acute potentially zoonotic gastroenteritis in young children. Moreover, we found an increased trend of risk of hospitalization for acute potentially zoonotic gastroenteritis in children, especially Salmonella infections, with increasing levels of poultry density. Further study with more individual data on exposure and risk factors is needed to better explain the observed associations.
ACKNOWLEDGEMENTS
We thank Robert Pampalon for providing deprivation index data and Elhadji Anassour Laouan Sidi for statistical advice. Thanks are also due to the Ministère de l'Agriculture des Pêcheries et de l'Alimentation du Québec and the Ministère du Développement durable, de l'Environnement et des Parcs du Québec. We also thank the anonymous reviewers of the journal of Epidemiology and Infection for their important comments on previous versions of this paper. This project was supported by Le Fonds québéçois de la recherche sur la nature et les technologies (FQRNT).
APPENDIX
List of ICD-9 codes for acute gastroenteritis (modified from Aramini et al. [Reference Aramini13])
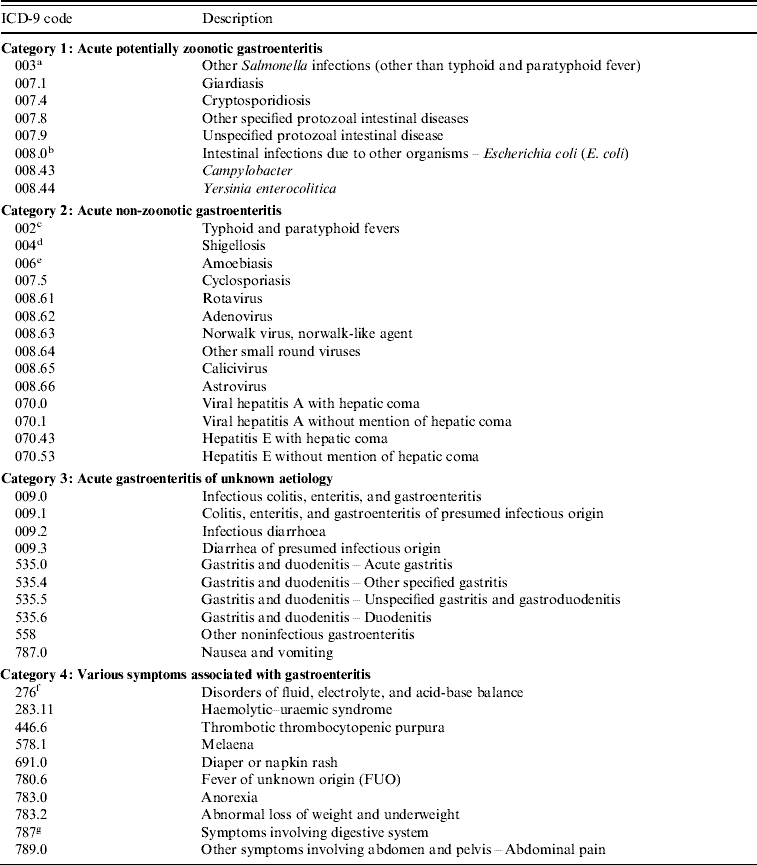
a Including subcategories: 003·.0, 003·.1, 003·.2, 003·.8, and 003·.9.
b Including subcategories: 008·.00, 008·.01, 008·.02, 008·.03, 008·.04, 008·.09.
c Including subcategories: 002·.0, 002·.1, 002·.2, 002·.3, and 002·.9.
d Including subcategories: 004·.0, 004·.1, 004·.2, 004·.3, 004·.8, and 004·.9.
e Including subcategories: 006·.0, 006·.2, 006·.3, 006·.8, and 006·.9.
f Including all subcategories: from 276·.0 to 276·.9.
g Including all subcategories: from 787·.1 to 787·.9
DECLARATION OF INTEREST
None.










