INTRODUCTION
Campylobacteriosis is the leading cause of bacterial gastroenteritis in the developed world [Reference Allos1]. In the United States it causes an estimated 2 million infections every year, but is highly underreported with only 43 000 cases confirmed annually [Reference Samuel2]. This is probably due to the generally mild clinical symptoms that are frequently seen and the sporadic nature of transmission [Reference Friedman3–Reference Gould5]. Data from FoodNet, the Center for Disease Control and Prevention's enhanced surveillance system, estimated the US rate to be 12·63 cases/100 000 people in 2008 [6]. In Arizona, which is not part of FoodNet, rates have historically been higher and in 2008 the reported rate was 15·4 cases/100 000 [7]. Racial disparities are consistent across the United States and Arizona, with Hispanics having higher rates of disease than non-Hispanic whites (United States, 10·73 vs. 8·07; Arizona, 14·3 vs. 7·5) [6, 8]. Younger ages are also at increased risk with children aged <4 years having very high rates of disease [Reference Lampel, Al-Khaldi and Cahil9]. In Arizona, children aged <4 years experience rates over 2.5 times the overall incidence (39·3 cases/100 000) [10].
Identification of risk factors for Campylobacter has largely been conducted using case-control studies of sporadic cases as outbreaks are rare. Common risk factors identified from these studies are foreign travel [Reference Friedman3, Reference Eberhart-Phillips4, Reference Neal and Slack11–Reference Domingues13], consumption of poultry, both cooked and undercooked [Reference Domingues13, Reference Stafford14], as well as raw dairy products [Reference Langer15], barbeque meat [Reference Kapperud16], contact with puppies [Reference Eberhart-Phillips4, Reference Saeed, Harris and DiGiacomo17, Reference Tenkate and Stafford18] or animals with diarrhoea [Reference Saeed, Harris and DiGiacomo17, Reference Adak19], and contact or consumption of untreated water [Reference Kapperud20]. The majority of these studies have been conducted in Northern European countries and across FoodNet sites which are not generally representative of a more diverse ethnic profile.
The Arizona Department of Health Services conducted a collaborative statewide case-control study to determine what common or unique risk factors might be associated with reported Campylobacter infections in Arizona and whether the risk factors varied by ethnicity.
METHODS
Overview
The study was designed as a matched case-control study where cases were matched to controls (1:2) on age group, gender, and residential location. All cases were identified from the state's routine passive surveillance system.
Study population
Cases
Individuals with a positive culture for Campylobacter jejuni reported though the state's routine laboratory surveillance system were included in the study if they were (a) not part of a recognized outbreak, (b) reported to the health department as a laboratory-confirmed case from 1 April 2010 to 31 December 2010 and (c) able to be interviewed over the telephone within 3 weeks of the report date. Five attempts on various days and times were made for each case before being considered as a non-contact. A representative sample of 20% of cases from Maricopa County was randomly selected for recruitment due to the county's large population size and number of cases (selection took place each week of the study period). In all other 14 counties, recruitment was attempted for all reported cases [7, 21].
Controls
The goal was to match cases to controls (1:2) on age group (0–11 months, 1–9, 10–19, 20–29, 30–59, ⩾60 years), gender and residential location. Reverse lookup was used to identify controls closest to the case residence. Households with home phone numbers on the same street within two blocks either direction were first called and, if no one was available, the households on neighbouring streets of the case street were then called. Controls were excluded if they (a) had a Campylobacter infection in the last 30 days, (b) experienced diarrhoea or abdominal pain with a fever in the past 30 days, or (c) were not able to be interviewed within 2 weeks of the case interview. Students at the University of Arizona who were part of the SAFER (Student Aid for Field Epidemiology Response) team conducted interviews with those cases identified from Maricopa County and their associated controls. All other interviews were conducted by county and state health department epidemiologists. Subjects were excluded if they did not speak either English or Spanish.
Questionnaire design
Individuals with a laboratory-confirmed case of Campylobacter are reportable to county and state health departments under Arizona law within 5 days of confirmation (A.A.C. R9-6-202). Cases reported through this system are contacted and interviewed by the county health department in which the case resides. The standard questionnaire covers some of the commonly associated risk factors associated with Campylobacter infection reported in the literature. A more extensive questionnaire was designed for this study using a Campylobacteriosis questionnaire from the World Health Organization's ‘Control and Prevention of Campylobacter Infections’ [Reference Kapperud22] as a guide. The questionnaires collected demographic information, 2-week travel history (both domestic and international), 2-week food history asking about specific foods based on previous studies of both sporadic and outbreak-related infections, kitchen and food-handling practices, as well as animal and water exposure information. For cases, additional information was collected on symptoms and any medical care received. This document was also the basis for the age groups used in control recruitment.
Data management and analyses
A standardized database and data entry protocol were created and disseminated to all participating sites conducting interviews. Data from all sites were merged and binary variables were generated for analysis. All analyses were conducted at The University of Arizona using Stata v. 11.0 (Stata Corporation, USA).
The distribution of demographic factors for all cases and controls was calculated. Differences in factors between unmatched cases (cases without at least one matched control) and matched cases (cases with at least one matched control) were determined using Fisher's exact test or Student's t tests.
For all foods and many environmental exposures, the time period participants were asked to recall was 2 weeks prior to illness onset for cases and 2 weeks prior to the interview for controls. Participants were asked if they could definitively recall (yes/no response), or were not sure (do not remember), or if they frequently ate that item or had a particular exposure but could not say for certain. Ultimately, only ‘yes’ responses were used in the regression models to ensure a higher degree of confidence in the exposures of interest.
As only 34% of cases were successfully matched to at least one control, random-effects logistic regression modelling was used. This method was chosen because it allows for the inclusion of unmatched cases, in addition to the matched case/control pairs [Reference Ten Have23]. When generating these models, a random-effect variable was first created that identified each matched set [a case and its matched control(s) received the same number] and unmatched cases received a separate unique identifier. This variable was then included in each model to adjust for the variability in correlation introduced if both unmatched and matched sets were included. Univariate random-effects logistic regression was utilized to estimate magnitude of effect for each risk factor. Age and gender were included in all models to adjust for the original matching factors. Odds ratios (OR) and associated 95% confidence intervals (95% CI) for each risk factor were calculated. Both conditional and random-effects logistic regressions were performed. The magnitudes of effect were the same although the sample size was much smaller for the conditional analyses, resulting in wider confidence intervals. Results from the random-effects models are presented.
A goal of this analysis was to build a final model that included all statistically significant risk factors that were associated with campylobacteriosis in Arizona. The strategy to select risk factors for potential inclusion included variables previously identified in the literature and variables identified in the Arizona database that were associated with disease at P ⩽ 0·10. Backward, stepwise regression was then used to develop the final predictive model; the variable with the highest P value was dropped from the model and the fit of each model was compared (P < 0·05) using the likelihood ratio test. As variables were removed from the models their impact on variables remaining in the model were assessed for confounding. If the parameter estimates changed by >10%, the variable was kept in the model. Concordance among variables in the final model was tested to ensure there were no high levels of collinearity between risk factors. Specific interaction terms were evaluated between key behavioural risk factors and were included if statistically significant. Age and gender were forced into the final model to adjust for the matching in the study design. Age, rather than age group, was included as a continuous variable to account for any residual confounding that may have been introduced due to the broad age groups.
Initial analyses found increased crude ORs for both Hispanic ethnicity and travel. To further explore whether Hispanic (an uncommonly reported risk factor) was simply a surrogate marker for travel or an independent risk factor, a joint variable was created and included in the models: Hispanic/travellers, Hispanic/non-travellers, non-Hispanic/travellers and non-Hispanic/non-travellers (reference group).
Finally, as a validation step and to evaluate the potential effects from these response and control matching problems, a subsample of the data collected at The University of Arizona was analysed (23 controls/23 cases, 14 matched, 9 unmatched, all cases and controls from Maricopa County). This subsample represented the ‘best’ matching site in the study, meaning a higher percentage of controls were recruited to cases. Both random-effects and exact logistic regression models were run on this smaller dataset.
RESULTS
Demographics and symptoms
During the study period, 781 laboratory culture-confirmed cases of C. jejuni were reported to the state health department [24]; 424 cases were selected as eligible for the study [20% of Maricopa cases selected at random (N = 90), all cases from other counties (N = 334)]. Of these 110 (26%) cases participated. Of the non-Maricopa County cases, 20·4% were interviewed and included in the study (N = 68). Of the Maricopa County cases, 42 (46·7%) were interviewed.
A total of 61 control interviews were completed, resulting in 37 matched sets (a set consisted of a case and either one or two matched controls). Demographics for all controls and cases are shown in Table 1 as well as for matched and unmatched cases; 40% of the cases were male with an overall mean age of 32·4 years (median 39·5 years); 57% of all cases resided in either an urban or suburban area.
Table 1. Demographic characteristics for Campylobacter cases and controls
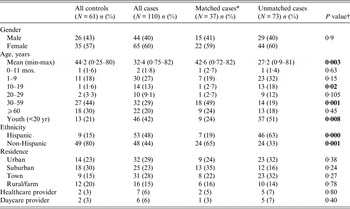
* Matched cases only included if at least one respective matched control interviewed.
† t test or Fisher's exact test of the difference between matched and unmatched cases.
Symptoms for all cases and stratification by matched status are given in Table 2. All cases experienced diarrhoea and many reported abdominal pain (75%), fever (68%), nausea (55%) and headache (46%). At least 27% of cases reported bloody diarrhoea and 35% were hospitalized, indicating that these reported cases were more severe than average compared to the national estimated rate of hospitalization for Campylobacter of 17% [Reference Scallan25]. The average duration of illness was 13·9 days (mode 7 days), with longer term effects (symptoms lasting >2 weeks) reported by 40% of cases. Medical risk factors, including chronic conditions and medications taken prior to the illness, were also enquired of, with 43% of cases reporting a pre-existing chronic condition. Only 10% and 13% reported taking antibiotics and antacids prior to illness, respectively, both reported risk factors for Campylobacter infection [Reference Neal and Slack11, Reference Gallay26, Reference Koningstein27]. The only two conditions that differed between matched cases and non-matched cases were vomiting (P = 0·002) and pre-existing chronic conditions (P < 0·001).
Table 2. Characteristics of illness in Campylobacter cases
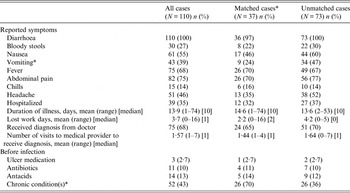
* Statistically different difference between matched and unmatched cases, P < 0·05.
Risk factors for Campylobacter in Arizona
Table 3 reports results for various risk factors related to ethnicity and probable environmental and behavioural exposures. The largest OR was for Hispanic ethnicity (OR 6·01, 95% CI 2·7–13·5). The only behavioural risk factor found to be strongly associated with the odds of developing disease was travel history in the last 2 weeks (OR 3·9, 95% CI 1·3–11·8) and more specifically, travel to Mexico (OR 4·2, 95% CI 1·2–14·8). Contact with untreated water, by ‘swimming in a river, lake or pond’, was elevated but not statistically significant. A history of handling raw chicken was associated with infection (OR 2·5, 95% CI 1·2–5·3) (attributable risk 71·8%). While a history of washing cutting boards (OR 0·4, 95% CI 0·2–0·8) and counter tops with soap and water following raw meat preparation (OR 0·5 95%, CI 0·3–1·08) were both found to be protective against disease.
Table 3. Association between selected univariate behavioural exposures for Campylobacter in Arizona and case-control status
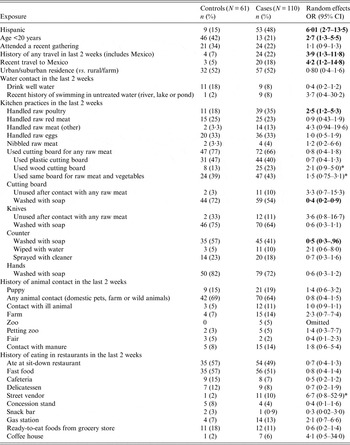
OR, Odds ratio; CI, confidence interval.
Odds ratios in bold are significant at the 0·05 alpha level; those with an * indicate a value 0·05 < P value ⩽0·1.
The odds of disease associated with specific food items are given in Table 4. During the study period, only consumption of cantaloupe (OR 2·8, 95% CI 1·2–5·7) (AR 69·4%) and queso fresco, a traditional, often unpasteurized, white cheese (OR 4·4, 95% CI 1·4–13·3) (AR 84%) were statistically significant at the alpha = 0·05 level. Consumption of some foods were found to be ‘protective’ against the disease including delicatessen chicken, roast beef, raw peas, tomatoes, blueberries and pasteurized dairy.
Table 4. Food specific risk factors for Campylobacter in Arizona
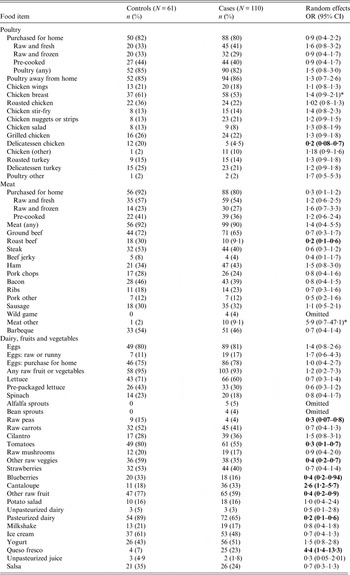
OR, Odds ratio; CI, confidence interval.
Odds ratios in bold are significant at the 0·05 alpha level, those with an * indicate a value 0·05 < P value ⩽0·1.
The final multivariate model is shown in Table 5. The largest risk factors were a history of eating cantaloupe (OR 7·64, 95% CI 2·0–28·6), handling raw poultry (OR 4·88, 95% CI 1·50–15·9) and eating queso fresco (OR 7·11 95% CI 0·9–55·2). Protective risk factors included a history of washing the cutting board following use for raw meat (OR 0·14, 95% CI 0·04–0·45), and a history of consuming blueberries (OR 0·15, 95% CI 0·04–0·60) or roast beef (OR 0·10, 95% CI 0·02–0·49). This model also included the joint relationship between Hispanic and travel history. Compared to non-Hispanic/non-travellers, the highest risk group were Hispanic/non-travellers (OR 7·27, 95% CI 1·7–31·5), and Hispanic/travellers (OR 5·87, 95% CI 0·8–43·5). Non-Hispanic/travellers had decreased odds (OR 0·59, 95% CI 0·3–11·7) although the sample size for this group was very small (n = 4).
Table 5. Final multivariate model* for risk factors associated with Campylobacter infections in Arizona
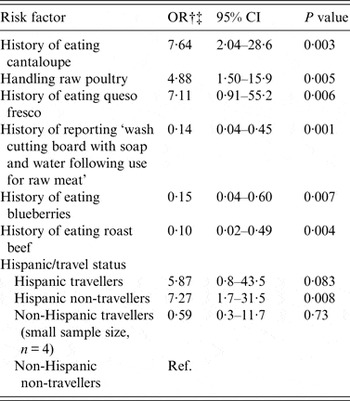
OR, Odds ratio; CI, confidence interval.
* Variables included in the initial model, but not found to be statistically significant include: History of eating raw peas, tomatoes, other raw vegetables, other raw fruit, pasteurized milk, unpasteurized milk, chicken breast, other meat, washing kitchen counters after preparing raw meat, using the same board for raw meat and vegetables, using a wood cutting board and eating from a street vendor.
† Random effects logistic regression of all cases and all controls, backwards stepwise regression.
‡ Adjusted for age and gender.
Not shown in the table, Hispanic and travel history were analysed individually in separate models (not using dummy variables) along with inclusion and exclusion of interaction terms. Being Hispanic was an elevated risk factor, but travel and the interaction between travel and Hispanic were not. No other interaction terms between ethnicity or travel with other risk factors were significant.
For the subsample (Maricopa data interviewed at The University of Arizona), few results were different from the full dataset (data not shown). Consumption of cantaloupe remained statistically significant and had an OR of 7·5 in the reduced set compared to an OR of 3·6 in the full dataset for the entire state.
DISCUSSION
Overall, the results of this study are similar to previous case-control studies of Campylobacter with two notable exceptions. First, the role of ethnicity has not been examined in other published studies. Most Campylobacter case-control studies have been performed in Northern European countries [Reference Schorr12, Reference Kapperud16, Reference Adak19], Australia [Reference Stafford14] and New Zealand [Reference Eberhart-Phillips4]. In the one large case-control study in the United States only 8% [Reference Friedman3] of the cases were Hispanic compared to 48% in this Arizona study.
While initially hypothesized that Hispanics were at greater risk due to more frequent travel to Mexico, it was found that while travel was a general risk factor (crude OR 3·9, 95% CI 1·3–11·8), compared to non-Hispanic/non-travellers, Hispanics who did not travel actually had the highest odds of disease (OR 7·27) followed by Hispanic/travellers (OR 5·87). This differential among Hispanics could possibly be due to increased exposure and higher levels of immunity in this Arizona Hispanic population that travel to Mexico frequently; the possible development of immunity to Campylobacter has been reported in other highly exposed populations [Reference Havelaar28]. Questions about the frequency of travel and length of time residing in the United States would be important in future studies to determine what role, if any, this factor plays for populations in this region. However, these analyses indicate that Hispanics in Arizona have a higher risk of disease that is not related to travel history
Differences in ethnicity due to dietary practices such as the consumption of queso fresco and cantaloupe, the younger age distribution of the population and kitchen maintenance practices might explain some of these differences, although none of these variables were statistically significant when stratified analyses were conducted (data not shown). Future studies should examine additional factors such as food preparation techniques and the role of socioeconomic status as possible explanations for these differences by ethnicity.
The second result that has not been reported in other studies was the increased risk associated with cantaloupe consumption. Most studies reported consumption of fresh fruits and vegetables to be protective [Reference Domingues13], although it was not reported if any of these studies asked specifically about cantaloupe. Potentially, the source of cantaloupe in Arizona might differ from other regions of the country and be more vulnerable to contamination. Only one reported outbreak in 1985 of C. jejuni had been linked to cantaloupes [Reference Bowen29], although more recent outbreaks of Listeria [30] and Salmonella [31] were both linked to the fruit. History of consumption was found to have statistically significant elevated ORs in the univariate and multivariate models.
This current study identified many risk factors that were similar to other studies. Those statistically significant risk factors included youth (aged <20 years) [Reference Stafford14, Reference Tenkate and Stafford18], travel history [Reference Friedman3, Reference Eberhart-Phillips4, Reference Neal and Slack11–Reference Domingues13], handling raw poultry [Reference Eberhart-Phillips4, Reference Neimann32], and consumption of queso fresco [Reference Eberhart-Phillips4, Reference Domingues13, Reference Langer15, Reference Neimann32, Reference Headrick33].
For the observed ‘protective’ exposures, washing a cutting board following meat preparation follows other findings that washing cutting boards can decrease cross-contamination and risk of disease [Reference Christensen34]. Consuming roast beef may simply be an effect of choosing beef over chicken, which is known to be a riskier food exposure [Reference Kapperud20, Reference Harris35, Reference Wingstrand36]. For blueberries, this may also be an indicator of general dietary practices. Another possibility is that antibacterial properties against other enteric pathogens such as Salmonella have been identified for blueberries [Reference Park37]. The three strongest risk factors in this study, handling raw poultry, consumption of cantaloupe and consumption of queso fresco all had high attributable risk percentages (72%, 69%, 84%, respectively). This indicates that focusing on behaviour modification surrounding these few risk factors could have a large impact on reducing the burden of disease overall in this population.
Limitations
Logistical barriers impeded the ability of the various recruitment sites to acquire matches for all cases. Lack of dedicated resources at several interview sites led to differential matching success. Recruitment of Hispanic controls could have been improved by using only bilingual interviewers but that option was not possible given the unfunded nature of the study. While differences in recruiting Hispanic cases (48%) and controls (15%) may appear marked at first, by matching on the other three variables, the controls were still well matched to cases.
The next two limitations were related to gender and age. Only 40% of those interviewed were male compared to 52% of reported cases during the study period statewide being male [38], although cases and controls recruited into the study were matched well on gender. Matching on gender may have affected control recruitment; however, this step likely ensured that we had a higher number of men then we would have had without this as part of the protocol. Moreover, there was a substantial difference between cases and controls by age group. For cases aged >30 years, the matching to controls was successful; however, recruitment in the younger groups was problematic. For younger children, parents were more often home and able to answer questions. For school-age children, parents were less often available during the day, and for children aged >14 years it was difficult to convince both the child and the parent to answer the 30-min questionnaire. For the control group aged 20–29 years, the greatest obstacle was the protocol-prescribed use of only landline numbers. National survey estimates during this time period reported that 23% of adults did not have a landline and these rates were much higher for younger adults [Reference Blumberg39]. Given that low level of landline use, it was not surprising that few controls in the 20–29 years age group were recruited [Reference Blumberg39]. Future studies would need to review the benefits of matching by neighbourhood to the limitations of using only listed landlines numbers, or determine how to get access to cell phone numbers by address [Reference Blumberg39].
A final limitation is due to the number of potential risk factors (over 100) assessed and the possibility that some of our results are due to chance. However, given the large ORs and highly significant results of the more novel risk factors identified in the full model, namely being Hispanic (P = 0·018) and consumption of cantaloupe (P = 0·003), we are confident these results would hold in a larger study or under more conservative multiple comparison methods.
Strengths
This study is one of a few statewide population-based case-control studies of enteric disease in the United States. All steps in the process from design, implementation and data analysis and interpretation were completed by a collaborative partnership of local, county, state, and university public health professionals and numerous departments and jurisdictions. This study utilized a more extensive questionnaire than has typically been utilized for Campylobacter providing enhanced information for broader characterization of risk factors within this region. In addition, despite the length of the questionnaire, overall the study had very complete data, with <20% missing data from any one variable, most around ⩽10%. Finally, Arizona represents a unique and highly diverse population that has experienced higher rates of infection, making it important to determine the contributing factors.
The results of the smaller subset analyses also helped to validate the findings of the overall study. In addition, it was unlikely that differences in the cases had any effect on the ability to recruit a matched control. There were very few differences in symptomology by matched and unmatched cases and the differences by chronic condition were not due to age group and gender.
Many public health case-control studies suffer the consequences from low response rates due to lack of incentives for participation, particularly in community-wide studies. For studies that choose to match as part of their study design, low response rates yield very low numbers of paired matches, thus decreasing study power and the likelihood of observing statistically significant results. In these circumstances, using a random-effects model allows all of the cases, both matched and unmatched, to be included in the analyses which increases the precision of the estimated fixed effects and also may strengthen the generalizability of the results to a broader community setting.
CONCLUSION
In this statewide study of C. jejuni, results suggest there are some unique risk factors that may be contributing to the higher rates of disease seen in Arizona. It is clear that the high rate of reported infections in Hispanics should be investigated further. Future studies should focus on understanding the role ethnicity might have on either exposure frequency, symptoms or possible differential reporting of disease. The result from this study will help to inform public health officials of the varying risk factors for Campylobacter in this region and beyond and can be used to modify the routine questionnaires to address these risks.
ACKNOWLEDGEMENTS
The authors would like to acknowledge all state and county health department epidemiologists and communicable disease investigators who helped to conduct the enhanced questionnaire required for this study. We also thank members of the Student Aid for Field Epidemiology Response (SAFER) team who also conducted many of the interviews. Finally, we thank Dr Denise Roe for her statistical advice and contributions. No financial support was provided for this study.
DECLARATION OF INTEREST
None.







