INTRODUCTION
Leptospirosis is a vastly underreported bacterial disease that occurs worldwide [1]. Many different animal species are chronic carriers of the bacteria and contaminate the environment by shedding the organism in their urine [Reference Faine2]. Infection with Leptospira in humans can lead to severe disease, causing multi-organ failure and death [Reference Bharti3]. The disease has a wide spectrum of symptoms resembling many other infectious diseases; therefore it is often misdiagnosed as another febrile illness. Techniques of detection and clinical diagnosis of leptospirosis are inadequate for prompt and specific treatment. Leptospirosis is rare, but when outbreaks do occur, they can be large and debilitating. Ninety per cent of patients who acquire leptospirosis develop only mild illness that is often self-limiting. However, 5–10% of patients develop severe leptospirosis with multi-organ failure, resulting in high costs of hospitalization and supportive treatments. Many patients who do seek treatment for the infection are hospitalized [Reference Levett4]. Because high-risk environments may lead to the exposure of hundreds or thousands of individuals simultaneously, strategies for avoiding the severe complications that may occur in a few individuals are of great interest.
Published literature is inconclusive regarding effective treatment and prophylaxis of leptospirosis, especially since late leptospirosis is largely immune-mediated [Reference Abdulkader5]. Only three randomized clinical trials met the inclusion criteria established by the Cochrane Review database that evaluated antibiotic treatment for leptospirosis [Reference Weir6, Reference Guidugli, Castro and Atallah7]. One study of penicillin initiated after 4 days of illness found no benefit to antibiotic treatment [Reference Costa8]. Similarly, Edwards et al. [Reference Edwards9] found no significant differences in patients with icteric leptospirosis who were treated with penicillin compared to those who were not given penicillin. However, Watt et al. [Reference Watt10] found that penicillin reduced the duration of fever and length of hospital stay in severe and late leptospirosis. Currently, penicillin is recommended only in diagnosed leptospirosis cases and it is the drug of choice. Azithromycin has recently been mentioned as an alternative to penicillin or doxycycline for the treatment of leptospirosis and may be as effective as penicillin with fewer drug reactions and population limitations [Reference Phimda11–Reference Moon14].
In a Cochrane Review of prophylaxis for leptospirosis, only two randomized clinical trials were found; both compared doxycycline to placebo [Reference Guidugli, Castro and Atallah15]. Based on this limited information and because the two clinical trials only looked at the effectiveness of doxycycline for antibiotic prophylaxis, the reviewers concluded that prophylaxis of leptospirosis may be achieved by administration of doxycycline to soldiers training in endemic areas [Reference Guidugli, Castro and Atallah15]. Prophylactic studies have found that doxycycline prophylaxis does not prevent people from acquiring leptospirosis, but shortens the duration of disease and lessens the severity of symptoms [Reference Sehgal16, Reference Gonsalez17]. The question has also been raised by Gilks et al. of whether or not prophylaxis with penicillin delays the onset of disease rather than prevents or shortens the duration of illness in the case of a laboratory exposure to leptospirosis [Reference Gilks18]. Azithromycin may be an alternative to doxycycline for prophylaxis since it has fewer population restrictions [Reference Phimda11, Reference Moon14].
Optimal treatment for leptospirosis remains undefined [Reference Griffith, Hospenthal and Murray19] and guidelines for the practice of antibiotic treatment and prevention of leptospirosis are unclear [Reference Guidugli, Castro and Atallah20]. Therefore, the question of the cost effectiveness of treating this rare but serious illness must be raised, along with the question of whether or not early treatment will avert serious illness. There are a number of researchers who highlight the challenges of appropriate and cost-effective treatment [Reference Griffith, Hospenthal and Murray19, Reference Vinetz21–Reference John23]. Randomized controlled trials aimed at addressing these questions for a rare infection such as leptospirosis may take years to complete at significant cost. This study applies a decision modelling approach to evaluate the effectiveness and the cost effectiveness of early antibiotic therapy in patients with leptospirosis infections.
METHODS
Literature review
The PubMed database was searched using the search terms ‘leptospirosis’ and ‘treatment’. The same terms were used for a Medline search of EBM reviews and the Cochrane Library Database, from 1966 to 2007. The search was limited to articles published in the English language and studies using human subjects. The search included clinical trials, but was not limited only to clinical trials due to the small number of clinical trial studies conducted evaluating early treatment. Articles and/or information contained in them were excluded from the study based on lack of treatment data or lack of data needed to determine early vs. conventional antibiotic treatment. Single case-reports and articles that focused on alternative, non-antibiotic treatment strategies (dopamine, plasma exchange, methyl prednisolone, etc.) were excluded. The definition of early treatment, or empirical treatment, in this study was used as defined by the authors of each selected study (between 4 and 7 days after onset of symptoms). When early treatment was not specifically defined by the author, antibiotic treatment administered within 4–7 symptomatic days was considered early treatment for that study. Conventional treatment consisted of supportive therapy but no antibiotic treatment or late (after 7 symptomatic days) antibiotic treatment. Results of the literature review were used to define the probabilities utilized in the model. A list of articles included in the cost-effectiveness studies are found in Table 1.
Table 1. List of included studies

Decision model
TreeAge Pro software (Williamstown, MA, USA) was used to create decision trees comparing the strategy of early empirical treatment (within 4–7 symptomatic days) or prophylaxis to conventional antibiotic treatment (Fig. 1). The first branch of the tree was divided into those who acquired leptospirosis and those who did not become ill following exposure. Subsequent branches included recovery, hospitalization due to fever of unknown origin (FUO), respiratory failure, renal failure, or multi-organ failure (MOF). Terminal nodes were survival or death. Effectiveness of treatment was measured using probabilities of survival for each terminal node.
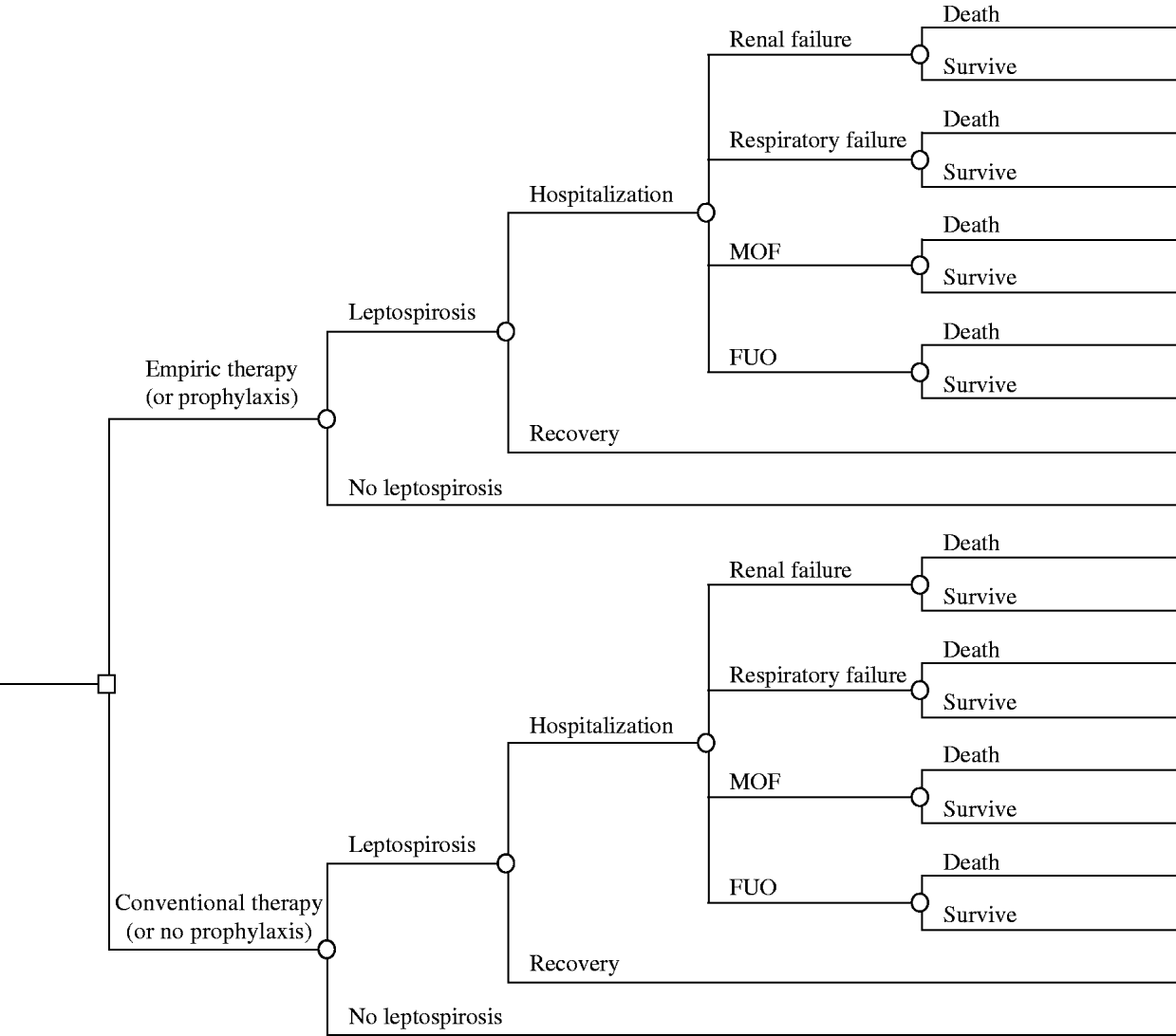
Fig. 1. Decision tree for treatment of leptospirosis, comparing empirical therapy to conventional treatment or prophylaxis to placebo. MOF, Multi-organ failure; FUO, fever of unknown origin.
Primary assumptions of the model were: patients misdiagnosed with a non-bacterial illness (e.g. viral) were not treated with antibiotics, patients with both respiratory and renal failure experienced MOF, recovery was full and complete without complications, studies that did not specify renal failure or respiratory failure in study patients were assigned a diagnostic code of FUO, and the regimen of antibiotic treatment was standard.
Probabilities of chance outcomes (probabilities of acquiring leptospirosis, hospitalization due to FUO, renal failure, respiratory failure, or MOF) were compiled and pooled from published literature. Table 2 lists the calculated probabilities and their ranges (based on their 95% confidence intervals) for both the empirical treatment analysis and the prophylaxis studies. Utility scores, or pay-offs, were assigned to terminal outcomes based on death (score=0) or full recovery (score=1) [Reference Torrance51], since patients who survive leptospirosis typically recover with no chronic complications [Reference Daher Ede, Zanetta and Abdulkader52].
Table 2. Probabilities used in the models for empirical and prophylactic treatment obtained from published literature
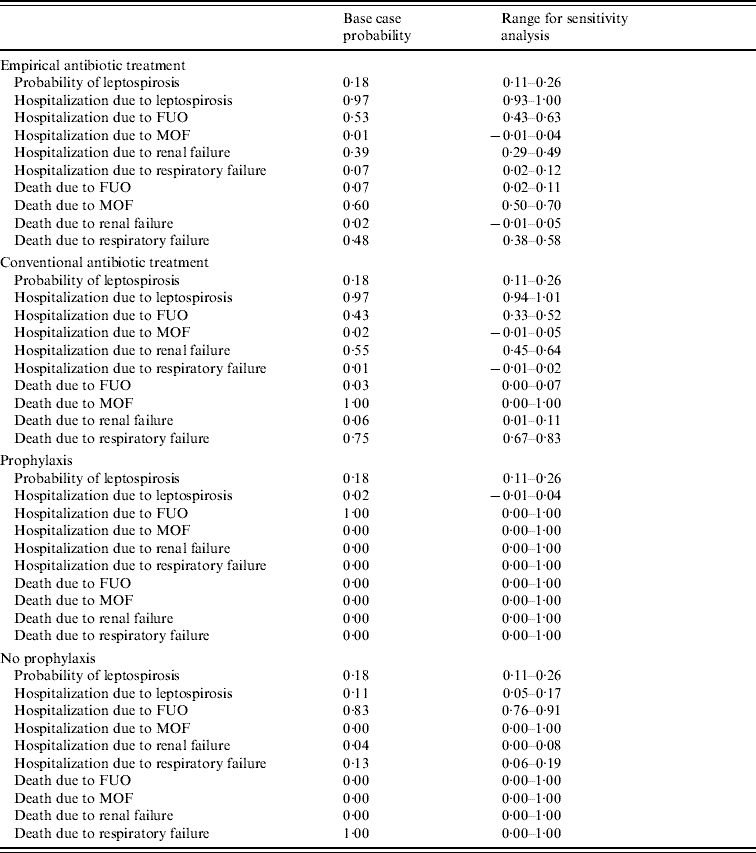
FUO, Fever of unknown origin; MOF, multi-organ failure.
Cost data
Cost data related to treatment and hospitalization were determined using available information contained in the selected studies. We utilized diagnosis-related group (DRG) codes from the Medicare Fee Schedule 2008 to assess hospital costs [53], and current procedural terminology (CPT) codes from the data file Ingenix ICD-9-CM to assess physician fees, hospital services, and clinical services [54] (Table 3). Drug therapy costs for empirical and prophylactic doxycycline treatment were obtained through the AHFS Drug Information 2008 comparative pricing list maintained by the American Society of Health System Pharmacists, Inc. [55]. Standard treatment with doxycycline is US$4.66 for a 1-week supply of 100-mg generic capsules, two capsules per day. Azithromycin is US$23.54 for a 3-day supply of one dose of 1000 mg followed by two doses of 500 mg each. In order to apply the model to other countries with publicly funded health-care systems, we obtained cost data from Bayview Hospital, a private hospital in Barbados, a country that experiences a higher incidence of leptospirosis. All hospital codes and costs are listed in Table 3. For each comparison, the incremental cost effectiveness ratio (ICER) was calculated, which represents how efficiently early treatment or prophylaxis can produce an additional increase in percent survival. All costs are expressed in US dollars (US$).
Table 3. Hospital and physician codes and cost ranges
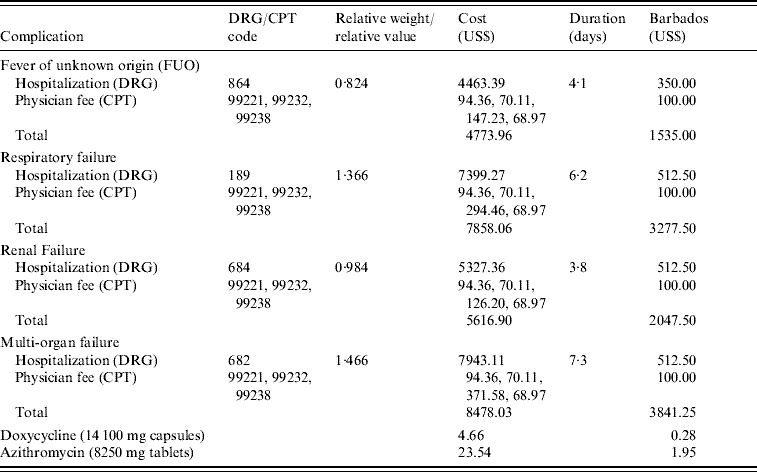
DRG, Diagnosis related groups; CPT, current procedural terminology.
Sensitivity analysis
Univariate sensitivity analyses were performed using a range of values calculated from the base value [Reference Hanley and Lippman-Hand56, Reference Doubilet57] to determine the extent to which variation in the model influenced outcomes. This sensitivity analysis generated threshold values, at which the model recommends a change in management strategy.
RESULTS
Literature review
A total of 34 studies contained information relevant to this analysis (Table 1). These 34 studies analysed antibiotic treatment for 907 patients diagnosed with leptospirosis and 335 individuals who contracted leptospirosis in the prophylaxis studies. Publication dates ranged from 1978 to 2007. Thirty studies were used for empirical antibiotic treatment information, and four studies were used for prophylactic analysis. Eleven studies contained environmental exposure information, from which attack rates were determined. The attack rate was 18·3% (252/1374) among those individuals who were exposed to an environmental source of leptospirosis contamination from the list of studies consulted (Table 1). Among individuals who received placebo in prophylaxis studies conducted in highly endemic areas, the attack rate was 19·2% (214/1116) from the pooled literature. Combining environmentally exposed persons and those in endemic areas receiving placebo, the attack rate was 18·7% (466/2490). Persons in endemic areas taking doxycycline prophylactically had an attack rate of 13·7% (125/915) from the pooled literature.
Probabilities obtained from the literature review were utilized in the model. The probabilities showed similar hospitalization rates and recovery rates from leptospirosis, regardless of antibiotic treatment (Table 2). In empirically treated patients, most of the hospitalized patients were admitted due to FUO (52·8%) or renal failure (39·1%). However, among the hospitalized patients, the highest proportion of deaths occurred in those with MOF (60·0%), which only represented 0·8% of hospitalized patients who received early treatment. The majority of patients in the empirical group survived renal failure (97·9%), while 93·4% survived FUO.
In the model, conventionally treated patients were most often hospitalized for renal failure (54·7%) and FUO (42·7%). Survival rates for each were high, 93·9% and 96·7%, respectively. All patients who were diagnosed with MOF (representing 1·7% of hospitalized patients in the conventional group) died. A high proportion of deaths (75·0%) was also seen in those patients who had respiratory failure. However, these patients represent only 0·6% of hospitalized patients who were conventionally treated.
Analysis of prophylactic studies showed a benefit with doxycycline prophylaxis compared to placebo. A smaller proportion of patients receiving prophylaxis acquired leptospirosis (13·7% vs. 19·2%). Of those patients who did acquire the infection, only a very small proportion of patients on prophylaxis were hospitalized (1·6%) compared to the placebo group (11·2%). Only one hospitalized patient who received prophylaxis developed complications and all patients survived. There were also very few complications in the placebo group compared to the prophylaxis group – only 11·2% were hospitalized due to leptospirosis. Hospitalization rates for the treatment studies were in the 90th percentile for both treated and untreated groups. Most patients in the placebo group were hospitalized with FUO (83·3%), and the only deaths (n=3, representing a proportion of 14·3% among hospitalized patients taking placebo) resulted from patients with respiratory failure.
Costs, effectiveness, and cost effectiveness
The model showed that empirical antibiotic treatment was slightly less effective than conventional treatment (Table 4). Early doxycycline treatment was actually less costly than conventional treatment, although azithromycin was more costly. Cost-effectiveness analysis for doxycycline and azithromycin showed that if empirical treatment with an antibiotic was administered, costs per patient would be $948.56 and $951.89, respectively, with an effectiveness of 98·5% survival. Conventional treatment resulted in a cost of $950.32 per patient at a slightly higher effectiveness (98·7% survival).
Table 4. Effectiveness and costs of the treatment strategies
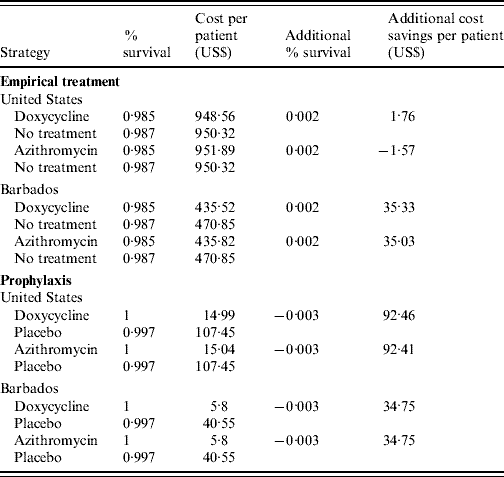
The models comparing prophylaxis with placebo favoured prophylaxis as the dominant strategy. Cost-effectiveness analysis showed that if prophylaxis was administered to populations in areas endemic to leptospirosis, US costs would be $14.99 per patient for doxycycline and $15.04 per patient for azithromycin with 100% effectiveness. No prophylaxis resulted in a much higher US cost for both doxycycline and azithromycin ($107.45 per patient) at a slightly lower effectiveness (99·7%). The cost per patient in Barbados for prophylaxis was the same for both doxycycline and azithromycin ($5.80 per patient at 100% effectiveness); whereas placebo cost $40.55 per patient at 99·7% effectiveness.
The ICER for early doxycycline treatment in the US and Barbados is a cost savings of $880.00 and $17 665.00, respectively, per percent increase in survival. The ICER for early azithromycin treatment in the United States is a loss of $785.00 per percent increase in survival. However, in Barbados there is a cost savings of $17 515.00 with azithromycin. In the prophylaxis models, the ICER resulted in a cost savings for both antibiotics in each area: $30 820.00 and $11 583.33 for doxycycline in the United States and Barbados, respectively, per percent increase in survival; and $30 803.33 and $11 583.33 for azithromycin in the United States and Barbados.
Univariate sensitivity analysis revealed no variables that had an effect on the model when each value was varied.
DISCUSSION
Although this study is the first of its kind for infections due to leptospirosis, it is not without a number of limitations. Many of the published studies used in this analysis lacked complete information, or may have contained limited information with regard to symptom history, treatment history, treatment regimen, hospital stays, complications, or chronic complications. A small portion of the data was extracted from survey data and therefore may be incomplete or biased. The data gathered were not limited to a specific geographical area, although most leptospirosis cases occur in tropical regions where the illness is endemic. Given the prevalence of different serovars in different areas, potential differences in virulence in different areas, and the variable incidence of leptospirosis across regions, the data may be limited to certain populations and may not be applicable to all geographies. We attempted to make the structure and the variables included in the model transparent so that interested partners could substitute their own probabilities and costs where they are known and differ from the literature. Lack of studies examining treatment in a sufficient number of children limit this analysis to adult populations. The definition of early treatment among published studies was inconsistent. It is inconclusive as to whether the difference between 4 symptomatic days and 7 symptomatic days (two different definitions of early treatment) is significant in affecting patient outcomes. Finally, certain important outcomes were ignored, such as the successful treatment of a viral infection.
Although survival rates were high in the empirical treatment study regardless of treatment, receiving antibiotics early actually resulted in a slightly lower effectiveness. However, the cost per patient with doxycycline was lower than conventional treatment, perhaps because early antibiotic treatment resulted in 9% fewer hospitalizations (and therefore fewer complications). The majority of patients treated empirically were classified in the FUO complication, which cost less than renal failure, respiratory failure, or MOF. Most patients treated conventionally were classified as having renal failure, which is more costly than FUO.
Prophylaxis with doxycycline showed a treatment benefit and significant cost savings. It did not prevent disease, but it did decrease hospitalization and mortality rates and improved health outcomes, as previously reported. Although the severity of disease in the prophylactic studies was less than empirical treatment studies among the control groups (lower attack rate, fewer hospitalized), nevertheless, the decision model showed a clear advantage to prophylaxis for leptospirosis for either antibiotic in both the United States and in Barbados. Further studies need to be conducted to evaluate the effectiveness of other antibiotics for prophylactic use to prevent leptospirosis.
The discrepancy between our findings for prophylaxis and treatment within 4–7 days of symptom onset indicated that there may be a narrow window of time after infection for initiating therapy, but it has thus far been impossible to predict the evolution of severe disease. If early treatment is to prevent severe leptospirosis, but not the infection itself, then early diagnosis becomes the key factor in administering early treatment. The recent development and availability of real-time PCR assays for leptospirosis [Reference Smythe58, Reference Levett59] may make empirical therapy more of a reality at symptom onset. This study should be revisited when such information becomes available. An appropriate and prompt diagnosis would also prevent some of the costs associated with misdiagnoses and with the co-occurrence of leptospirosis during outbreaks of dengue or other illnesses with similar syndromes. The clear treatment and cost advantage to prophylaxis shown in this study is significant and supports the practice of prophylaxis in endemic areas. Antibiotic prophylaxis in high-risk communities could decrease the burden of leptospirosis on these communities and have a significant public health impact.
ACKNOWLEDGEMENTS
Sources of funding used to assist in the preparation of this manuscript include Dr Flowers' PhRMA Health Outcomes Research grant and the Emory Faculty Development Award. We also acknowledge Dr C. N. Edwards for kindly providing Bayview Hospital data for use in the cost-effectiveness analysis.
DECLARATION OF INTEREST
None.







