Introduction
The discovery of antimicrobials was one of the most important medical advancements crucial to global health [Reference Laxminarayan1]. Antimicrobial resistance (AMR), partly related to the overuse of antimicrobial drugs, is now a common global health problem [Reference Laxminarayan2, 3]. Infections that were once easily cured with antimicrobial medications are now becoming more difficult, and in some cases impossible to treat effectively, resulting in premature mortality [4]. It was estimated that AMR could lead to 10 million deaths a year in 2050, globally [Reference O'Neill5]. Information regarding the relative increased in inpatient mortality due to AMR is useful to inform and motivate both change in clinical practice and policy decision making. Consequently, the purpose of this study was to estimate the relative increased in inpatient mortality from antimicrobial resistant nosocomial infections (AMR NI) in Thailand.
Methods
A retrospective cohort study was conducted at Ramathibodi Hospital, Bangkok, a 1400-bed university hospital in Thailand, between 1 January 2008 and 31 December 2012. The population of this study were patients admitted to critical areas, namely, medical intensive care unit (MICU), intermediate medical care unit (IMCU), surgical intensive care unit (SICU), cardiovascular–thoracic surgical intensive care unit, and coronary care unit (CCU) who subsequently contracted NI in either critical areas or other wards. The patients in this cohort were identified by the infection control unit of Ramathibodi Hospital using the surveillance definitions of the United States Centers for Disease Control and Prevention (US-CDC) [Reference Horan, Andrus and Dudeck6]. There was no randomisation process of sample selection. Patients were categorised based on the exposure of interest including AMR and non-AMR infections in order to estimate the relative increased in inpatient mortality of AMR to non-AMR [Reference Cosgrove7, Reference de Kraker8]. Patients who acquired infections caused by bacteria that were resistant to at least three classes of antimicrobial agents (multidrug-resistant, MDR) bacteria were allocated to the AMR group. Those who acquired sensitive bacteria were allocated to the non-AMR group. Patients with community-acquired infections and colonisation cases (as defined by infection prior to 2 days post-admission) were excluded. The five most common types of bacterial infections included in this study were Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Staphylococcus aureus [9–Reference Kritsotakis11]. The three most common infection sites within the NI surveillance system were studied. These include pneumonia (PNI), urinary tract infection (UTI) and bloodstream infection (BSI) [9–Reference Kritsotakis11]. A Charlson co-morbidity index score (CCI score) was computed and assigned to each patient in order to adjust for his/her comorbidities and the severity of baseline diseases [Reference Charlson and Carmeli12]. We censored the Cox model at hospital discharge. The survival time from date of infection until hospital discharge was captured by a time variable. The specimen collection date on which the symptoms were firstly noticed by the physician was chosen to represent the onset of NI [Reference Horan, Andrus and Dudeck6]. The event of interest was inpatient mortality. A patient who died was treated as a failure case while a patient who was discharged alive was treated as a censored case. The hazard ratio of mortality was estimated using multivariate Cox proportional hazard model with STATA 15.0 controlling for a number of potential confounders. The certain covariates associated with inpatient mortality at P < 0.10 from the univariate analysis [13] were considered for backwards stepwise elimination method along with clinical considerations to construct the multivariate Cox proportional hazard model. The univariate analysis of survival function, the association among covariates, the nested model and the proportion hazard assumption (or Schoenfeld residuals) were tested [13]; see Supplementary Material for details (Supplementary Material is available on the Cambridge Core website).
The prevalence of AMR NI reported in published national surveillance [Reference Phumart14] was used as a factor for estimating the number of AMR NI cases in a year. Then the expected annual number of deaths from AMR NI, overall and categorised by type of bacteria, was the product of the number of AMR NI cases in a year multiplied by the hazard ratios of mortality from the Cox model. The associated variance and the confidence bounds were computed to report the uncertainty of the extrapolated nationwide annual relative increased in inpatient mortality, see Supplementary Material for details (Supplementary Material is available on the Cambridge Core website).
This study protocol has been reviewed and approved by the Committee on Human Rights Related to Research Involving Human Subjects of Faculty of Medicine Ramathibodi Hospital, Mahidol University. The Institutional Review Board number for this study is ID 10-56-41. Data were anonymous, kept confidential and not linked to individuals.
Hospital settings
At the time of study, the hospital had nine intensive care units covering medical, surgical and paediatric populations, as well as general inpatient care units. There were 10 infection control nurses and one physician running the infection prevention and control based on the US-CDC surveillance strategies. Infection prevention measures were mainly those recommended by the US-CDC, the Society for Healthcare Epidemiology of America and WHO. The infection prevention and control programme has been performing prospective NI surveillance focusing on ICU patients continuously for decades.
Results
Descriptive analysis
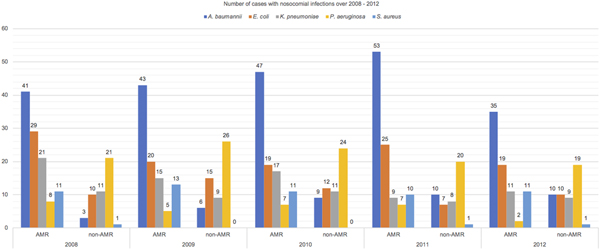
Study results were reported following STROBE guideline for an observational study [Reference Vandenbroucke15]. There were 523 patients included in this cohort. Majority of NI during the study period (73.80%) were caused by the aforementioned five species of MDR organisms. Table 1 reports the distribution of patients’ characteristics. There was an almost equal mix of males and females and most of them were elderly. Higher proportion of patients with AMR infections than those with non-AMR infections (77% vs. 58%) in the MICU, IMCU and CCU were observed. The median length of hospital stay for patients in the AMR group was longer than those in the non-AMR group. The patients in both groups were different in the distribution of CCI score. Fifty per cent of patients acquired UTI, 30% acquired PNI, 15% acquired BSI, while the remaining patients acquired multiple site of infections. Among 523 critical care inpatients with NI, 201/386 (52%) patients with AMR and 53/137 (39%) with sensitive infections died. Figure 1 reports the number of cases with NI over 2008–2012. NI caused by A. baumannii, E. coli, K. pneumoniae and S. aureus had a greater proportion of AMR than non-AMR infections but P. aeruginosa had a smaller proportion of AMR than non-AMR infections.

Fig. 1. Number of cases with nosocomial infections over 2008–2012.
Table 1. Descriptive analysis, n (%) or median (IQR)

a Multiple conditions.
Survival analysis
Hazard ratios of inpatient mortality and 95% confidence bounds for covariates in the Cox proportional hazard regression model were reported in Table 2. Patients in the AMR group had a greater chance to die by 32% relative to those patients in the non-AMR group. There was no significant increase in the hazard of inpatient mortality related to AMR, for all tested bacteria. Hazard ratios were estimated to be 1.92 (95% CI 0.10–35.52), 1.25 (95% CI 0.08–20.29), 1.60 (95% CI 0.13–19.10) and 1.84 (95% CI 0.04–95.58) for A. baumannii, E. coli, P. aeruginosa and S. aureus, respectively. The other covariates adjusting for the Cox model comprised of sex, admitted ward, CCI score, site of infection, number of NI episode and the interaction effects between CCI score and site of infection.
Table 2. Multivariate cox proportional hazard regression model

Nationwide annual relative increased in-hospital mortality from AMR NI in Thailand
The estimated nationwide annual relative increased in inpatient mortality from AMR NI in Thailand was reported in Table 3. This study estimated that around 111 295 AMR NI cases occurred in several types of hospitals in Thailand and 48 258 deaths in 2012. Resistant A. baumannii was the most common cause of death. About 60 000 cases with resistant A. baumannii infections accounted for almost 40 000 deaths in that year.
Table 3. Annual relative increased in-hospital mortality from AMR NI

Discussions
We found that almost three-quarters of all patients with NI acquired resistant bacterial infections over the study period. The number of cases with AMR NI in our study was consistent with findings in a recent study in Thailand [Reference Lim16]. The distribution of the type of bacterial infections was also in accordance with the national antibiogram data provided by the National Antimicrobial Resistance Surveillance Center Thailand (NARST) [17]. The relative increased in inpatient mortality from AMR NI was estimated using a survival analysis controlling for confounding factors [Reference Shurland18–Reference Huang21], including CCI score in order to adjust patients’ clinical baseline as other recent studies had done [Reference Pena22–Reference Nguyen24]. Compared with patients in the non-AMR group, the mortality chances for patients in the AMR group with A. baumannii, E. coli, P. aeruginosa and S. aureus were 92%, 25%, 60% and 84% higher, respectively. Because resistant E. coli in our study was ESBL-producing organisms which can be treated by widely available carbapenem antimicrobials, mortality of infection caused by this bacteria remained low. Another explanation is that the most common site of E. coli infection was UTI, which generally is not significantly associated with fatal outcome. On the other hand, A. baumannii mostly causes infection of the respiratory system which tends to carry higher mortality background. Only a few antimicrobial agents such as colistin, a nephrotoxic agent and tigecycline, a bacteriostatic agent with unpromising efficacy in the literature, are available for treatment. As a result, mortality associated with A. baumannii infection in our study was high. There were several studies that reported the mortality chances of these bacterial infections in Thailand and worldwide. Lim et al. published in 2016 [Reference Lim16] estimated the mortality attributable to multidrug resistance in hospital-acquired infection in Thailand for A. baumannii, E. coli, P. aeruginosa and S. aureus as 70%, 19%, 65% and 44%, respectively. Yang et al. published in 2013 [Reference Yang23], a retrospective 10-year study was conducted at a 2900-bed teaching hospital located in Northern Taiwan, reported crude 14-day mortality rates of A. baumannii in hospital-acquired PNI and ventilator-associated PNI were 43.8% and 31.3%, respectively. Huang et al. published in 2012 [Reference Huang21], a retrospective cohort study was conducted in Taipei Veterans General Hospital, Taiwan, estimated odd ratio of mortality caused by carbapenem-resistant A. baumannii bacteraemia was 1.03 (95% CI 0.48–2.20) relative to carbapenem-susceptible A. baumannii bacteraemia. Abernethy et al. published in 2015 [Reference Abernethy25], a large national study in England, reported a 30-day all-cause mortality rate of E. coli was 18.2% (95% CI 17.8–18.7%). Schreiber et al. published in 2011 [Reference Schreiber, Chan and Shorr19] reported the mortality rate in patients with bacteraemia complicated S. aureus PNI was 39% compared with non-bacteria. The meta-analysis of Cosgrove et al. published in 2003 [Reference Cosgrove26], enrolled studies between January 1980 and December 2000 reported a significant increase in odd ratio (1.93; 95% CI 1.54–2.42; P < 0.001) of mortality associated with methicillin-resistant S. aureus bacteraemia compared with methicillin-susceptible S. aureus bacteraemia. Our study estimated higher expected hazard ratios of mortality for AMR NI relative to non-AMR NI than other studies. Overall, mortality of AMR NI in our study was not statistically significant different from those of non-AMR. It might be due to the fact that these patients were all critically ill which posed them at risk of dying. Furthermore, with relatively more available resources for treatment as compared with other non-teaching hospitals, mortality of AMR NI might be lowered as shown by some studies that effective antibiotics played an important role in improving outcomes of these infections [Reference Wang27, Reference Gutiérrez-Gutiérrez28].
This study estimated that the annual relative increased in inpatient mortality in Thailand would be 48 258 cases out of 111 295 (over 50%) cases with AMR NI. The study of Lim et al. published in 2016 [Reference Lim16] estimated the burden of MDR bacteria in Thailand to be 19 122 of 45 209 (43%) deaths in 2010. The preliminary study of health and economic impact of AMR in Thailand published in 2012 [Reference Phumart14] found 38 481 additional deaths of 87 481 (43%) AMR cases in 2010. However, our annual number of deaths is higher than those reported by others. We identified the annual number of deaths as the relative difference hazard ratio of mortality for the AMR and non-AMR groups based on the Cox model fitted with individual-level data of our study samples. To the best of our knowledge, this is the most updated data collected from patients in Thailand. The conditional mortality on having AMR was derived using the hazard ratio of mortality provided by the Cox model. Kritsotakis et al. in 2017 [Reference Kritsotakis11] also used the multivariate Cox proportional hazard model to estimate hazard of death between patients with hospital-acquired infections and uninfected patients in terms of adjusted hazard ratios and associated 95% confidence bounds. Even though, Lim et al. in 2016 [Reference Lim16] used a 30-day all-cause mortality rate while the preliminary study in 2012 [Reference Phumart14] used a decision tree model. Moreover, our study took place in the critical care units of a large tertiary hospital. Most of the patients in our study had more severe illnesses than other hospital types. Consequently, the samples used may not necessarily be representative of the whole NI population in Thailand. This may result in an overestimate of AMR burden estimation for all hospitalised patients in the country. However, to the best of our knowledge, hospital-acquired infection surveillance in most hospitals in Thailand now focus on the ICU population according to targeted surveillance strategies.
Limitations
There are four main study limitations comprising the selection of an appropriate comparator, the lack of adjustment for acute severity of illness parameters, the potential statistical challenges to the estimation and the lack of accounting for the competing risk of being discharged in the multivariate Cox proportional hazard regression model. First, we have taken the more conservative approach of assuming that resistant infections are replacing susceptible ones so that we used the susceptible ones as comparators. The majority of studies to date have compared outcomes in patients infected with the resistant strain of an organism with patients infected with the susceptible strain of the same organism [Reference Cosgrove7]. We did not have patients with no infection as comparator to deal with the competing risk of infection (susceptible and resistant, which might differ) and death. Engemann et al. in 2003 [Reference Engemann29] compared outcomes among patients with resistant infections to uninfected control subjects selected on the basis of specific criteria which assesses the burden of having a resistant infection rather than no infection. The latter type of comparison results in a much higher estimate of adverse events attributable to resistance [Reference Cosgrove7]. Second, acute severity of illness scores and their use in predicting outcomes have gained considerable interest for predicting ICU and in-hospital mortality [Reference Johnson, Kramer and Clifford30]. The data on the physiologic measurements for severity of illness score calculation were incomplete. The results of this study might overestimate the impact of AMR infection due to the lack of adjustment for acute severity of illness parameters. Third, the AMR status was treated as an independent variable in the multivariate Cox proportional hazard regression model. We did not address the endogeneity of AMR status. It is possible that variables correlated with AMR status may also be related to death. In the absence of controls for potential endogeneity bias, the estimated impact of AMR status on relative increased in inpatient mortality may be overestimated. Additionally, statistical power may not have been reached due to limited sample size for certain susceptibility group. Finally, in-hospital mortality was used, and therefore using Cox proportional hazards methods on such an outcome may have issues – such as not accounting for the competing risk of being discharged and overall mortality wherein mortality after discharge is included.
Summary
AMR has become a major public health threat and is spreading worldwide. Infectious diseases are the second main cause of death in low- to middle-income countries. This study sought to estimate the relative increased in inpatient mortality from AMR NI in Thailand to non-AMR NI using a multivariate Cox proportional hazard regression model. The Cox modelling was controlled for nine potential confounders while the AMR group has an elevated risk of inpatient mortality by 32%, it is not significant. In the absence of AMR bacteria, this study estimated that annually there would be 111 295 fewer AMR cases and 48 258 fewer deaths. The findings will assist in informing policy decision makers in their enactment of Thailand's National Strategic Plan on Antimicrobial Resistance for 2017–2021.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818003436.
Author ORCIDs
K. Malathum 0000-0002-3186-638X
Acknowledgement
This publication fulfils a part of the degree programme of Doctor of Philosophy (Pharmacy Administration), Faculty of Graduate Studies, Mahidol University. We are grateful to the Ramathibodi Hospital for supporting and facilitating the study. We would like to express our appreciation to Associate Professor Yoel Lubell, University of Oxford and Mahidol Oxford Tropical Medicine Research Unit for reviewing this manuscript.
Financial support
This work was supported by the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0276/2551).
Conflict of interest
None.