INTRODUCTION
Egypt is considered the largest poultry producer in the Arab world and produces about 23% of the total poultry production [Reference Freiji1]. Since 1964, poultry production in Egypt has grown substantially with growth rate of 301·2% in the 1990s worth Egyptian pounds/Livre Egyptienne (LE) 17–18 billion (US$ 3–3·2 billion) [Reference Hosny2]. Local poultry meat production was sufficient to satisfy home consumption and up to 2 million birds were exported to the Arab countries annually [Reference Taha3]. The size of the labour force involved in poultry production was about 1·5 million permanent workers and one million temporary workers, representing about 6% of Egypt's 23·7 million labour force and more than 15% of the agricultural work force [Reference Freiji1, Reference El Nagar and Ibrahim4]. The structure of the poultry industry in Egypt consists of two main divisions: (1) commercial enterprises estimated in 2006 to be 850 million birds [Reference Abdelwhab and Hafez5] and expected to be 1·444 billion birds by 2010 [Reference El Nagar and Ibrahim4] and (2) 250 million household poultry [Reference Abdelwhab and Hafez5] kept by 8·1 million householders representing 4–5 million families out of a population of 82 million [Reference Meleigy6]. The increase in the number of poultry houses and other associated establishments has occurred randomly and irrationally without definite long-term planning. The Food and Agriculture Organization (FAO) classified poultry production, based on implementation of biosecurity measures, into four sectors: sectors 1 and 2 include the integrated commercial companies, grandparent, parent and layer farms where biosecurity measures are usually enforced. Sector 3 includes non-regulated, non-registered small- to medium-scale commercial activities while sector 4 contains backyard rural, in-house, and rooftop-raised poultry. In Egypt, household poultry production is in close contact with commercial farms of sectors 3 and 4, due to the presence of poultry in the same buildings as people, or by temporary workers in commercial farms keeping their own household birds at home and/or the selling of unused feed, feeders and hoppers from commercial farms to rural family poultry producers [Reference Freiji1, Reference Aly, Arafa and Hassan7].
Egypt was the second African country, after Nigeria, to declare the infection of poultry with highly pathogenic avian influenza (HPAI) H5N1 on 16 February 2006 [Reference Aly, Arafa and Hassan7]. The early control strategy was based on: stamping out infected birds, implementation of quarantine measures and restriction of movement. However, the disease spread rapidly and widely throughout the country within a short period. Therefore, Egypt changed its control strategy to mainly mass vaccination, surveillance and pre-emptive culling of infected birds to combat the disease. Vaccination of backyard birds using inactivated H5 vaccines was provided by the government free of charge while commercial companies adopted their own vaccination practices with widely varying standards [Reference Hafez8]. Several types of inactivated H5N1 and H5N2 vaccines were supplied by a number of vaccine manufacturers and used in the field [Reference Abdelwhab and Hafez5]. Different types of surveillance programmes, namely active, passive and targeted surveillance were established to elucidate the spread of H5N1 usually in poultry sectors but also rarely in other animals. Surveillance highlighted continuous and extensive circulation of the virus despite the control efforts [Reference Aly, Arafa and Hassan7–Reference Arafa10]. Culling of infected birds, if done, is selective and the post-culling procedures are implemented slowly in suboptimal conditions which increase the chances of the virus spreading to nearby birds and humans without actual control of the disease. Here we summarize more than 4 years' experience in surveillance, diagnosis, and control activities mobilized to confront H5N1 virus in Egypt and discuss the major challenges hampering the containment of the disease.
EPIDEMIOLOGY OF H5N1 IN EGYPT
Infection of domestic poultry
Commercial poultry
Chickens are the most common species of the commercial poultry sector in Egypt; however, turkeys, ducks, geese and quail farms are not uncommon. Widespread prevalence of the virus in the commercial sector in the first wave of the disease in 2006 was observed where 766 commercial chicken farms (366 broiler, 332 layer, 67 broiler breeder, one grandparent), 31 turkey, 22 duck and one quail farms were officially reported to be infected with HPAI H5N1 by the National Laboratory for Veterinary Quality Control on Poultry Production (NLQP) [Reference Aly, Arafa and Hassan7]. Shortly after adoption of the vaccination strategy, the incidence of the disease decreased, where one, five, 15 and 14 positive commercial grandparent, breeder, layer, and broiler farms out of 3610 (0·97%) examined farms in 2007, respectively were reported. In 2008, examination of 8682 commercial farms revealed existence of the virus in 10 layer and 17 broiler farms of sector 3 [Reference Hafez8]. Moreover, out of 22 024 examined commercial poultry farms in 2009 there were one, two, eight and 10 positive layer breeder, broiler breeder, layer and broiler farms, respectively and two positive duck farms [Reference Hafez8, Reference Kilany11] (M. M. Aly et al., unpublished data). The virus has been recently detected from internal egg contents of layer and breeder chickens which shared 99% identity with recent human H5N1 viruses [Reference Kilany11] (E. M. Abdelwhab et al., unpublished data).
Backyard birds
In 2006, 204 positive backyard flocks were reported; 76, 19, four and two outbreaks reported in rural chickens, ducks, turkeys and geese, respectively, in addition to 103 outbreaks in mixed rural birds [Reference Aly, Arafa and Hassan7]. Furthermore, the virus was detected in 246/816 (30%), 89/1723 (5·2%) and 151/1435 (10·5%) backyard flocks tested in 2007, 2008 and 2009, respectively [Reference Hafez8] (M. M. Aly et al., unpublished data). The virus was more prevalent in mixed waterfowls and chickens than turkeys. In contrast, there was no evidence for infection of 193 exposed pigeons in early 2006–2007 although the samples were taken from dead or clinically ill pigeons and others from the vicinity of infected poultry [Reference Aly12].
Live-bird markets (LBM)
Before 2009, LBM in Egypt were considered as a missing link in the epidemiology of H5N1 virus. From January to April 2009, the national laboratory in cooperation with FAO examined 573 LBM and 71 of them tested positive for avian influenza subtype H5N1 [Reference Abdelwhab9]. By the end of the year; examination of 944 LBM from throughout Egypt revealed 108 H5N1-positive LBM in 2009 and the highest prevalence (76%) was detected in those LBM that sold waterfowl (M. M. Aly et al., unpublished data). Taken together, these data revealed the continuous circulation of H5N1 virus in different poultry sectors in Egypt and emphasized the role of waterfowl in backyard birds and/or LBM as a reservoir for the virus [Reference Abdelwhab9].
Infection of wild birds
The location of Egypt in the Black Sea–Mediterranean and the East Africa–West Asia flyways of migratory birds (Fig. 1) increases the chances of transmission of influenza viruses to and from Egypt, Africa, Europe and Asia. Isolation and identification of HPAI H5N1 from wild ducks in Egypt, namely common teals, was reported in December, 2005 (3 months before the outbreak in poultry) by Saad et al. [Reference Saad13]. The detected virus showed 99·4% sequence identity of the HA gene with the parent virus detected in the first wave of the outbreaks in domestic poultry in Egypt. This suggests wild birds as a source of introduction of H5N1 into Egypt; however, other routes could not be ruled out. The risk of multiple introductions of other H5N1 viruses through wild birds necessitated permanent surveillance in more than 30 wetlands (Fig. 1) in Egypt by the Ministry of Agriculture (NLQP) and Ministry of Environment in cooperation with the Naval American Research Unit 3 (NAMRU-3). However, HPAI H5N1 has not been detected in migratory birds since February 2006 by any of the surveillance projects described.

Fig. 1. Flyways of migratory birds and location of the major wetlands in Egypt (adapted from [Reference Abdelwhab9]). Black-grey areas refer to the location of the major wetlands of migratory birds. Dotted lines refer to the migratory birds' flyways.
Infection of zoo and feral birds
During the first introduction of H5N1 virus into Egypt, birds in the main zoo (Giza zoo) on 18 February 2006, exhibited symptoms confirmed to be caused by HPAI H5N1 virus. About 167 samples collected from different species were examined. The virus was more prevalent in Galliformes especially turkeys and peacocks; Anseriformes (Grocer duck, wild duck, geese), Phoenicopteridae (Greater flamingo), Passeriformes (sky sparrow, crow), and Ciconiiformes (cattle egret) were less affected while Columbiformes (wild pigeons), Struthioniformes (ostrich, emu) and Anatidae (swans) were not infected [Reference Arafa14, Reference Mady15]. Furthermore, local feral birds (e.g. crows, egrets, ibis, wild pigeons, sparrows, doves, Upupa epops, etc.) have free access to culled bird carcasses and/or litter infected or contaminated with HPAI H5N1 virus (Fig. 2). The virus was identified in faecal samples collected in February 2006 from egrets roosting besides Giza zoo and from crows in 2006 and 2007 in two different provinces in Egypt (M. D. Saad et al., unpublished data). Therefore, the role of feral birds in the spread of HPAI H5N1 should be addressed.

Fig. 2. Risk factors that could influence the epidemiology of H5N1 virus in Egypt. (a) Free access of egret and feral birds to disposed H5N1-infected birds and/or contaminated litter. (b) Free access of dogs during burning of culled birds infected with H5N1 virus. (c) Rooftop birds as a common poultry rearing system in villages and suburban areas.
Infection of mammals
Close contact between infected birds, particularly backyard birds, and different animal species in Egypt is common. Paucity of epidemiological data to substantiate the susceptibility, route of infection and the potential role of these animals in spread and or transmission of H5N1 in Egypt remains ambiguous. The virus was not detected in swab samples collected from sheep and goats (M. M. Aly et al., unpublished data), eight black tigers in the zoo [Reference Arafa14] as well as pigs [Reference El-Sayed16]. However, 4·6% of 240 serum samples collected from asymptomatic pigs in close contact with probably infected birds was reported which was higher than the infection rate of pigs in China and Vietnam [Reference El-Sayed16]. Similarly, 1 week after an outbreak of H5N1 in poultry, HPAI H5N1 virus was isolated from three donkeys and a high exposure rate (27/105, 25·7%), was identified by HI testing in naturally exposed donkeys suffering from respiratory illness [Reference Abdel-Moneim, Abdel-Ghany and Shany17]. These reports indicate the extensive circulation of H5N1 in and among animals due to the long-term and extensive exposure of infected birds to other susceptible species. It should be noted that dead birds infected with H5N1 are frequently eaten by stray dogs (Fig. 2), cats and sometimes fish. Thus, surveillance activities must include all animals or at least domesticated animals that have daily contact with the infected birds.
Human infection
The first case of human infection with H5N1 avian influenza virus in Egypt was confirmed on 20 March 2006. From March 2006 to March 2009, the Egyptian Ministry of Health reported a total of 6355 suspected cases of H5N1 infection [Reference Kandeel18]. According to the WHO report on 20 November 2010, of 112 laboratory-confirmed cases in Egypt, 36 were fatal. All confirmed clinical cases, except for two, were linked to poultry probably infected with H5N1 virus when an infection of 107 cases was linked to backyard birds either through contact with or involvement in the slaughter and defeathering of backyard birds, mostly 1 week prior to the onset of illness [19]. Only two infected persons were working in commercial poultry farms in 2006 and they both recovered; one case was a female chicken vendor [19].
Women were exposed to backyard birds for longer and more extensive periods than men. Therefore infection of females was 67/112 (60%) of whom 30 (45%) died compared to 45/112 (40%) infected males, of whom only six (13%) died up to 20 November 2010. The disease was reported in different age groups, from 12 months to 75 years [19]. However, in 2006, infection in adults aged >18 years (66·6%) was higher than in children aged <6 years (16·7%) or middle aged individuals (16·7%). However, the number of infected children increased (31/39, 79%) in contrast to adults (6/39, 15%) in 2009 and the mortality rate decreased from 56% in 2006 to 10% in 2009 [Reference Abdelwhab20]. It remains unclear why more children have been infected since late 2008, although epidemiological and statistical analyses correlate human infections with close contact, i.e. playing with (for children) or slaughtering and/or defeathering of (for adults) backyard birds [Reference Kandeel18, Reference Abdelwhab20, Reference Tseng21]. Hence, increased exposure rate of children to infected birds, immune status of toddlers, rapid identification and prompt anticipatory treatment with oseltamivir should be considered [Reference Kandeel18, Reference Abdelwhab20]. There was no evidence of H5N1 infection in the infected children's parents/caregivers which might suggest that the virus is not easily transmissible in humans in Egypt [Reference Schroedl22]; however, limited human-to-human transmission was claimed in a number of family clusters [Reference Dudley23, Reference Fasina, Ifende and Ajibade24]. Furthermore, infection of pre-school children was linked to a less virulent virus clade with different genetic markers which could facilitate adaptation of the virus to humans [Reference Abdelwhab20].
Spatial and seasonal pattern of the disease
Egypt has 29 provinces, 17 of these provinces are located in Lower Egypt (LE), of which 11 are in the Nile delta, and 12 provinces located in Upper Egypt (UE) (Fig. 3). The Nile delta is ~40 000 km2 where more than half of Egypt's 82 million people live and large numbers of poultry are raised, traded and consumed. Lack of geographical barriers or borders between most of the Egyptian provinces resulted in the appearance of Egypt as a small village or one epidemiological unit. Incidence of HPAI H5N1 virus in LE was higher than UE from 2006 to 2009 in commercial farms, backyards and humans (Fig. 3) and the outbreaks were concentrated mostly in the Nile delta [Reference Aly, Arafa and Hassan7, Reference Hafez8]. In 2009, 83%, 50·3% and 27·3% of commercial farms, backyards, and LBM, respectively, reported positive in the highly populated Nile delta (Aly et al., unpublished data). Similarly, 66% of human infections were reported in LE and about 64% of the total human infections occurred in the Nile delta (Fig. 3). Concentrated and diverse poultry production, in the Nile delta region with rapid and random movement could be the main reason for the establishment of the virus in that region. However, further in-depth investigation of climatic, ecological and social studies should be conducted to define the conditions that favour the perpetuation and circulation of the virus in this region [Reference Abdelwhab9].

Fig. 3. Geographical distribution of cumulative H5N1 infections in (a) commercial poultry farms, (b) backyard birds, and (c) humans in Egypt from 2006 to 2009.
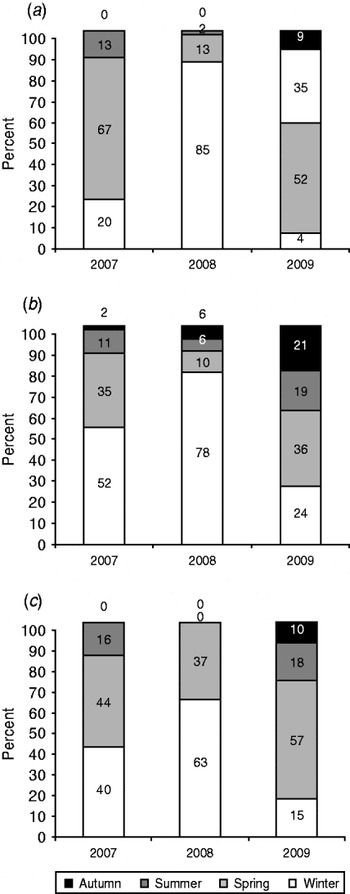
Association of H5N1 infection with winter months was observed in Egypt during 2006–2008 [Reference Aly, Arafa and Hassan7, Reference Hafez8]. The incidence decreased throughout the summer and autumn seasons when the temperature increased. However, in 2009, circulation of the virus all year around has been reported in both commercial poultry and backyard birds (Fig. 4) [Reference Aly, Arafa and Hassan7, Reference Hafez8]. The data demonstrated that in Egypt in 2009 the epidemiology of HPAI in birds had changed over time with outbreaks especially in backyard birds, now occurring in the warmer months of the year (summer and spring). Similar changes in the seasonal pattern were observed in human infections where infected human cases in 2006 were detected in the spring (77·9%), winter (16·6%), autumn (5·6%) with no cases reported in summer. In contrast, human infections in Egypt in 2009 revealed circulation of the virus all year around with incidence rates of 57·4% in spring, 17·9% in summer, 15·4% in winter and 10·3% in autumn (Fig. 4) [19].

Fig. 4. Seasonal pattern of H5N1 in (a) commercial poultry, (b) backyard birds, and (c) humans in Egypt in 2007–2009.
CHALLENGES FOR CONTROL OF H5N1 IN EGYPT
Impact of the disease
From October 2005 to February 2006 many rumours circulated about the emergence of the disease, which affected poultry consumption. Decreased demand for poultry and poultry products led to a significant decline in price. Losses experienced by the Egyptian economy between October 2005 and February 2006 amounted to LE 3 billion (about US$ 0·5 billion) [Reference Hosny2, Reference El Nagar and Ibrahim4]. The first wave of the disease in 2006 resulted in the culling of more than 30 million birds with 250 000 workers losing their jobs nationwide due to the drastic decrease in poultry production, as well as by the closure of feed mills and some retail and marketing operations [Reference El Nagar and Ibrahim4]. International trade restrictions and cessation of poultry export caused significant economic effects. The poultry industry was estimated to have lost more than US$ 1 billion [Reference Meleigy6]. Infection and subsequent culling of pure national valuable genetic lines and breeds were some of the important negative consequences of the disease. Demand for vaccination and drugs increased by 11·6% (for further details see [Reference Hosny2, Reference El Nagar and Ibrahim4]). Suboptimal vaccination strategies, constant emergence of new infections and culling of poultry resulted in waste of resources and enhanced antigenic drift of the virus in both poultry and mammals.
Genetic and antigenic changes of the virus
It is widely accepted that the highly error-prone replication of influenza viruses and viral genome reassortment are common features of AIV which facilitate the fitness of the virus to stay one step ahead of its host [Reference Suarez25]. Increased evolutionary rate of AIV might be accelerated either by the immune pressure (due to prior immunization or natural infection) exerted on the replicating viruses in different hosts and/or jumping of the virus from one species to another [Reference Yassine26]. In Egypt, both immune pressure exerted by the extensive vaccination and/or continuous inter-species and intra-species transmission of the virus are driving factors for genetic and antigenic drift of H5N1 which constitutes a major challenge for control of the disease. Evolution of HPAI H5N1 in Egypt since 2006 generated two major diversified sublineages; the first sublineage contains all immune escape mutants from vaccinated birds and the second sublineage contains the recent human isolates from 2009 to 2010 and most of the viruses detected in backyard birds [Reference Abdelwhab20]. In general, it is concluded that significant mutations in the virus haemagglutinin were eventually established (1) in the immunogenic epitopes corresponding sites permitting the field variants to evade the immune response of vaccinated birds and in turn decrease the efficacy of the currently used vaccines. (2) Several mutations were fixed in the real-time reverse transcriptase–polymerase chain reaction (RT–qPCR) primer specific sites of the H5 gene [Reference Slomka27] and to a lesser extent in the conserved M gene [Reference Spackman28] resulting in false-negative results (for more details see [Reference Abdelwhab29, Reference Abdelwhab30]). (3) In addition, a deletion in the receptor-binding domain which could facilitate the inter-species and intra-species transmission, emergence of less virulent virus in humans and affecting the sensitivity of serological tests was observed. (4) Last, but not least, several synonymous and non-synonymous mutations were recorded in the proteolytic cleavage site of the H5 gene without adverse effect on pathogenicity [Reference Arafa10, Reference Abdelwhab20, Reference Abdel-Moneim31–Reference Kilany33]. Oseltamivir (Tamiflu®)-resistant marker (N294S) in the viral neuraminidase was reported in two viruses isolated from a suspected family cluster in 2007 in Egypt [Reference Earhart34] and amantadine-resistant markers in the M2 gene were also observed in two chicken isolates (E. M. Abdelwhab et al., unpublished data). However, these mutations were not fixed and are rarely seen.
Vaccine and vaccination
Vaccination update
Approximately 1·3 billion doses of different H5 vaccines, of which none contained Egyptian field strains, were used until January 2009 [Reference Hafez8]. The homology of the H5 gene of the currently used H5N2-based vaccines and H5N1 reverse genetically modified vaccines share 78% and 94%, respectively, with the currently circulating viruses [Reference Abdelwhab and Hafez5]. Insufficient efficacy of these vaccines in protecting chickens and turkeys after experimental infection with the newly emerging variant HPAI H5N1 strains in Egypt has been recently demonstrated [Reference Kilany33, Reference Kim35] (E. M. Abdelwhab, unpublished data). In contrast, optimum protection under experimental conditions was achieved by several inactivated tissue culture and/or oil-adjuvanted vaccines prepared from the Egyptian field strains [Reference Bahgat36] (E. M. Abdelwhab, unpublished data). Novel vaccines generated by reverse genetics from the current Egyptian immune escape mutants are being considered for licensing. However, regular updates of the vaccinal strains in the face of antigenic drift of the H5N1 virus are needed annually or every 2 years to optimize the efficacy of these vaccines against the newly emerging variants [Reference Suarez25].
Vaccination coverage
The statutory national vaccination programme of backyard birds, also covering small farms with up to 500 birds, was provided by the government to local communities free of charge and conducted door-to-door twice a year [Reference Peyre37]. However, there was no post-vaccination monitoring activity for household poultry. A recent study highlighted that blanket vaccination of household poultry covered only 1–50% with conspicuously limited impact on the incidence of infection [Reference Rijks and ElMasry38]. Therefore, vaccination of backyard birds is no longer provided nor supervised by the government. On the other hand, the commercial poultry producers apply their mass vaccination programme which is usually of highly variable standards (different vaccines, frequency, dose, route, age, etc.) and the Egyptian general organization of veterinary services monitors only <6·5% of vaccinated poultry in the commercial sectors [Reference Hafez8, Reference Peyre37]. Furthermore, vaccination crews could facilitate the spread of the virus from one place to another due to inadequate application of the biosecurity precautions during vaccination [Reference Peyre37].
Vaccine control
Measures to control the marketing of A1 vaccines in Egypt are inadequate. Due to industry pressure, the first introduction of the vaccine was implemented, with government approval, without adequate efficacy and potency testing prior to the vaccine's release onto the market [Reference Abdelwhab and Hafez5, Reference Peyre37]. Moreover, there are no definite regulations for distribution and storage of the vaccines and accredited programmes supervised by the national institutions for commercial farms in contrast to those defined for backyard birds. Uniform regional vaccination within the commercial sector could be helpful in hindering transmission of the virus between farms, backyard birds and humans in the same region, particularly in the Nile delta [Reference Abdelwhab and Hafez5].
Compartmentalization
As a forward step to stop vaccination of poultry, compartmentalization was planned as an exit strategy from the vaccination policy. Three grandparent and three parent companies with a total of 26 unvaccinated farms successfully passed the preliminary selection criteria of the World Organization of Animal Health (OIE) for compartmentalization. A total of 24 648 swab and serum samples tested negative for avian influenza. These farms are now supposed to be able to export their poultry in the near future [Reference Abdelwhab and Hafez5].
Poultry industry infrastructure
In general, poultry production in Egypt, as mentioned earlier, is divided into four sectors based on implementation of biosecurity measures; however, many farms in sectors 3 and 4 are not registered with the official authorities which hinders the monitoring and early recognition of infections and allows silent and wide spread of the virus. Reforming of the poultry industry infrastructure in Egypt is a fundamental approach to the control of HPAI.
Backyard birds
Although the majority of householders keep mainly ducks and chickens together, nevertheless rearing of geese, turkeys and pigeons in close contact with other animals and humans in the same household is a common practice in Egypt [Reference El Nagar and Ibrahim4]. Some years ago, the government encouraged this production sector by small loans and marketing facilities. Up to the end of the 1970s, rural poultry production was an important source of Egypt's poultry meat and eggs. Rural poultry production prior to the HPAI crisis was estimated to be about 10% of the market share of the meat production sector and 30% of the egg market. Backyard birds produced 22% of chicken meat, 64% of ducks, 34% of turkeys, and almost all geese and pigeons [Reference Taha39]. Flock size can range from 10–20 birds up to a few hundred [Reference Hosny2]. It is estimated that backyard birds are mostly reared in primitive cages, rooftops, or as scavengers with virtually no biosecurity (Fig. 2). They move or graze through streets, roads or fields. These birds are in close contact with either local feral birds and/or wild migratory birds [Reference El Nagar and Ibrahim4, Reference Abdelwhab and Hafez5].
The attitude of the backyard birds' householders hinders cooperation with vaccination committees. In some cases they refuse the vaccine and hide their birds without vaccination or they may vaccinate some birds and leave others unvaccinated. Moreover, backyard waterfowl in Egypt are considered a potential reservoir of the virus and a mixing vessel for selection of variants to infect humans [Reference Abdelwhab20] or break through the immune system, and cause infection in vaccinated birds [Reference Hafez8]. But under village conditions it is not practical to separate the different species and such a suggestion will complicate the control efforts [Reference Aly, Arafa and Hassan7].
Marketing system
Due to insufficient capacity of slaughterhouses, lack of marketing infrastructure and cultural preference for consumption of freshly slaughtered birds the poultry meat trade in Egypt depends mainly on LBM. Two types of LBM in Egypt exist; retail shops and traditional LBM where minimal, if any veterinary supervision or food safety standards are implemented. Moreover, slaughtering, defeathering and evisceration of birds are usually conducted in the markets which increases the risk of human infections. Multiple bird species of several ages with variant ecological niches and from different localities are usually present inside one market. Therefore, surveillance of LBM indicated broad circulation of the virus in poultry populations nationwide. Changing the consumer preference from live birds to frozen meat will require great efforts and time. However, since 1 July 2010, Egypt has enforced the ban on selling live poultry nationwide, which had been in place since May 2009, although only across five governorates.
Under the new legislation, only licensed slaughterhouses with a resident veterinarian are allowed to handle live poultry. Vigorous control efforts to stop smuggling of live poultry, increasing the capacity of slaughterhouses (current capacity <30% of poultry production), tracing the source of birds in markets and financial support for regular monitoring of LBM will remain a significant challenge to the control of H5N1 in Egypt [Reference Abdelwhab9].
Biosecurity
It is well known that biosecurity is the first line of defence against infectious diseases. The inadequate biosecurity standards in the Egyptian poultry farms particularly in sector 3 which produces more than 75% of broilers in Egypt played a significant role in the rapid spread of H5N1 infection in early 2006. In contrast, farms with strict biosecurity measures (grandparent, integrated companies and some parent farms) were less affected by HPAI infection. Different approaches to enforce biosecurity measures and reform the poultry farms in sector 3 (2000–50 000 bird capacity) are currently tested by the Egyptian government in cooperation with the FAO. Registration of poultry farms and establishment of a geographical information system (GIS) to precisely define the location of poultry farms in Egypt is in progress.
Laboratory capacity
From 2006 to mid-2009, Egypt initiated a national laboratory for veterinary quality control on poultry production (NLQP) for all surveillance activities in poultry nationwide with total maximum capacity of 600 samples daily. Currently, three (seven are planned) satellite-accredited laboratories which will increase the capacity of NLQP and facilitate broad regional inspection have been established. International cooperation with the OIE reference laboratories is well defined through twinning programmes and different scientific projects. Moreover, establishment of biosafety level-3 (BSL3) laboratory and animal facilities are in progress.
Improvement of diagnostics
Increased specificity and sensitivity of diagnostics is of great importance. A number of mismatches in the current circulating viruses and the primers and probes that are oligonucleotide-specific for H5 [Reference Slomka27] and M gene [Reference Spackman28] segments have been established in recent Egyptian H5N1 viruses [Reference Abdelwhab29, Reference Arafa40]. This produced false-negative results when examined by the corresponding RT–qPCR. Updated RT–qPCR primers containing degenerative bases [Reference Abdelwhab29] and/or newly developed multiplex assays for simultaneous detection and discrimination between different sublineages circulating in Egypt have been successfully developed [Reference Abdelwhab30], and are more sensitive than the generic assays.
Antigens used in haemagglutination inhibition (HI) test for routine monitoring of the post-vaccination immune response are usually supplied by the company producing the vaccines. However, recent studies have shown that serum antibodies produced by these vaccines did not have any cross-reaction against HI antigens prepared from the field strains [Reference Hafez8]. Similarly, serum obtained from vaccinated birds with experimental vaccines prepared from the Egyptian variant strains did not react with other H5-based antigens of vaccine strains in Egypt (E. M. Abdelwhab et al., unpublished data; W. H. Kilany et al., unpublished data). Therefore, the use of field antigens based on temporal and/or geographical bases for serological evaluation of the current H5 vaccines against possible infection with field strains has been proposed instead of the homologous antigens of the vaccines. Furthermore, monoclonal antibody-based ELISA from Asian H5N1 virus as well as rapid chromatographic strips failed to detect the new Egyptian variants but not the parent virus isolated in 2006 in Egypt (T. C. Harder et al., unpublished data).
Compensation policy
Compensation payments for culling of poultry in 2006 in Egypt were estimated to be US$ 29 375 000 [Reference Albrechtsen41]. However, this compensation approach during the first wave of 2006 was not comparable either with the actual production costs or with the type and age of the birds. This resulted in less cooperation from the farmers [Reference Abdelwhab and Hafez5]. Currently, there is no compensation for culling of poultry and logistical support for depopulation of infected birds is paid for by the farmers. This resulted in decreased notification, if any, of the outbreaks and the subsequent inconsistent stamping out of infected flocks discovered accidentally in the active surveillance will lead to endless circulation of the virus [Reference Aly, Arafa and Hassan7, Reference Hafez8]. The FAO has supported the Egyptian government in developing a market-value compensation policy to support culling of infected birds and managing the post-depopulation requirement [Reference Albrechtsen41].
Public awareness campaign
Public communications information strategy is considered one of the basic measures to minimize the socioeconomic impact and to limit widespread transmission of the virus. Immediately after emergence of the disease in 2006, extensive awareness programmes targeting various high-risk groups and stakeholders (poultry industry personnel, veterinarians, para-veterinarians, military and interior ministries, social associations, the media and religious leaders) were implemented to deal with panic reactions, and regulate and enforce the laws. Later, door-to-door awareness programmes were conducted successfully by volunteer women, particularly in villages and suburban areas. However, communication and education in Egypt is inconsistent and sustainability must exist in order for policy and legislation to be effective.
CONCLUSION
Effective control measures should be encouraged to control HPAI virus in poultry in Egypt to mitigate the possibility of the emergence of a new pandemic. Continuous inter-species and intra-species circulation of the virus in and among different host species and subsequent transmission to humans is feasible. There is increasing evidence that stable lineages of H5N1 viruses are being established in chickens and humans in Egypt. De-novo changes in the virus, host spectrum, genetic alterations, evolution, seasonal pattern and geographical distribution should be carefully monitored. For the foreseeable future, unless integration of multifaceted strategies and global collaboration are available, the likelihood of H5N1 persistence in Egypt will compromise the poultry industry, endanger public health and pose a serious pandemic threat. The endemic situation of H5N1 in Egypt is overwhelming the control efforts and more international cooperation is greatly needed.
ACKNOWLEDGEMENTS
The authors thank Professor Mona M. Aly and staff members of the National Laboratory for Quality Control of Poultry Production (NLQP), Egypt, especially Dr Walid H. Kilany, for cooperation and providing the information necessary to complete this work. The authors are also grateful for Professor Y. M. Saif, Head of Food Animal Health Research Program, The Ohio State University, USA for revision of the manuscript.
DECLARATION OF INTEREST
None.