INTRODUCTION
Safe water is essential to life. Globally, the lack of clean water is estimated to cause about 4% of total mortality [Reference Pruss, Kay and Fewtrell1], predominantly in the developing world. However, waterborne outbreaks also remain a source of concern in developed countries. In the USA, 20 million people are estimated to contract a waterborne infection annually [Reference Reynolds, Mena and Gerba2]. Over 4 00 000 people fell ill with cryptosporidiosis in Milwaukee in 1993 as a consequence of inadequate water filtration [Reference Mac Kenzie3]. In Canada, the Walkerton outbreak resulted in seven fatalities attributable to Escherichia coli O157:H7 infection [Reference Auld, MacIver and Klaassen4]. Waterborne outbreaks have been reported from many European countries including England and Wales [Reference Smith5], Norway [Reference Nygård6], Sweden [Reference Neringer, Andersson and Eitrem7–Reference Nygård10], Italy, Spain and Switzerland [Reference Hrudey and Hrudey11].
Finland is a large country (3 38 424 km2) with a small population (5·4 million). The number of waterworks is high (almost 2000) and most serve small communities. The majority (95%) use groundwater. However, almost half of the population are served by surface-water plants of the largest cities. Groundwater is usually not disinfected for use, while surface water is filtered and disinfected before distribution to consumers [Reference Miettinen12].
Although drinking water is generally considered safe in Finland, a number of waterborne outbreaks have occurred. Twenty-four outbreaks affecting 7700 inhabitants were spontaneously reported during the period 1980–1992 [Reference Lahti and Hiisvirta13]; since 1997 reporting of outbreaks has been mandatory, and 14 outbreaks affecting 7300 people were registered during the first 2 years [Reference Miettinen12]. The most widespread outbreak to date affected 5500 people [Reference Kuusi14], and a number of other substantial waterborne outbreaks have been reported [Reference Kukkula15–Reference Kuusi17]. The most common microbes have been norovirus [Reference Maunula, Miettinen and von Bonsdorff18] and Campylobacter [Reference Hänninen19].
We report the investigation of a serious waterborne gastroenteritis outbreak that occurred in a Finnish town in autumn 2007.
Setting
Nokia (population 30 016) is a town located in southern Finland. Over 90% of the population are served by the municipal drinking-water supply. The average daily water consumption in Nokia is 4250 m3 (year 2006). The waterworks uses groundwater, which is treated with sodium hypochlorite for disinfection and pH adjustment. Sewage is processed in one municipal waste water plant, which covers 86% of the population.
The town has a municipal health centre for primary care and a hospital for primary in-patient care and limited secondary care. Secondary and tertiary care are provided by Tampere University Hospital 20 km distant from Nokia. The Centre for Laboratory Medicine provides the municipalities with clinical laboratory services. Environmental health surveillance and monitoring of water quality is organized in collaboration with five neighbouring municipalities.
The outbreak
On Wednesday, 28 November 2007, maintenance work was conducted at the waste water plant in Nokia. The same day, technical problems were encountered in the municipal drinking-water system, and during the days following, customers complained of a bad smell and taste in their tap water. These were first misinterpreted as being caused by harmless deposits loosened from the inner walls of the pipeline and discharged during the maintenance work on 28 November.
From 30 November, environmental health and municipal waterworks officials received reports of bouts of diarrhoea and vomiting among residents, and during that same day an increase in the number of patients with gastroenteritis was observed at the health centre. Extensive water and pipeline sampling was initiated and a boil-water notice issued, and water chlorine concentration was increased.
The water contamination and its source became evident the same day. A valve in the waste-water plant connecting the drinking-water line and a waste-water effluent line had been opened during the maintenance work and inadvertently left open for 2 days. As a consequence, the drinking-water network became contaminated with sewage effluent. According to flow data, an estimated 450 m3 of waste water was mixed with the drinking water and distributed to customers, heavily contaminating household water and pipelines in southern parts of the town. The population in this area numbered over 9500.
Distribution of clean water was organized by municipal officials, the military and volunteers, and the health centre focused on treating outbreak patients, cutting back on other activities. The University Hospital prepared to open a temporary ward, which was in fact eventually not needed. Schools and day-care centres were closed from 6 to 9 December owing to teachers' and children's sick leave and the need to reduce the possible horizontal spread of gastrointestinal viruses.
After 12 days, the boil-water notice was withdrawn in the uncontaminated area. In the contaminated area, adenoviruses and noroviruses were repeatedly detected in water-supply samples despite chlorination and pipeline flushing. Finally, after extensive decontamination procedures including shock chlorination, precautionary restrictions were discontinued in the contaminated area on 18 February 2008, almost 3 months after the incident.
We conducted a comprehensive investigation to determine the magnitude and consequences of the waterborne outbreak including describing the clinical aspects, reviewing microbiological findings and estimating the disease burden using a population-based survey.
METHODS
Descriptive epidemiology
Patients visiting the municipal health centre between 28 November and 31 December 2007 because of acute gastroenteritis were registered on a line-list, including name, age, gender, address and personal identity number. The timing and range of symptoms were recorded. Prospective line-listing was initiated 6 days after the beginning of the contamination visits prior to that being added retrospectively from patient charts. The information collected from the patient register included the number of primary-care visits to the centre because of gastroenteritis as defined by the international classification of primary care (ICPC-1, WHO, Geneva) codes D01, D09, D10, D11, D25, D70, D73 or D96. The information on reported symptoms was collected from the line-list. Daily admissions to Tampere University Hospital were counted by emergency-department personnel.
Analytical epidemiology
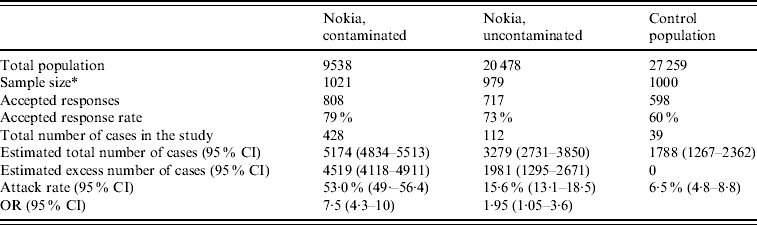
The town was divided into two study areas, contaminated (population 9538) and uncontaminated (population 20 478). The division was based on microbiological data from distribution system samples and technical modelling of water-flow directions within the network. Another municipality (population 27 259) 35 km distant from Nokia was chosen as a control population. One thousand persons for each study group were randomly selected from the population register. Groups were matched by age and gender and all age groups were included. Only one participant per household was included. The survey was conducted using postal questionnaires, the form being posted 8 weeks after the contamination had occurred. A repeat questionnaire was sent out 3 weeks later to non-responders.
Participants were asked about the timing and nature of gastrointestinal symptoms. A case was defined as a person suffering acute diarrhoea (⩾3 loose stools/day) or vomiting between 28 November 2007 and 20 January 2008. The occurrence of gastroenteritis during a 10-month period prior to the outbreak was also enquired about to ensure the comparability of the study groups.
Microbiological investigation
Faecal specimens for bacterial pathogens were not investigated from all the patients or from representative samples from consecutive cases. Instead, bacterial samples were available from cases with more severe symptoms. Specimens for detection of norovirus were collected from five randomly selected patients within the first week of the outbreak. Other viral pathogens were studied in samples from children admitted to the emergency department of the University Hospital. In order to detect enteric parasites specimens were taken from ten consecutive patients during the second week of the outbreak. Subsequently, when cases with giardiasis were detected, parasites were investigated in all symptomatic patients. Specimens were taken at the health centre or in the University Hospital.
Stool specimens were cultured for the presence of Campylobacter, Salmonella, Yersinia and Shigella spp. Norovirus was investigated using electron microscopy and PCR; rotavirus antigens were detected by enzyme immunological assay (EIA). Protozoan pathogens were investigated using microscopy of formalin-fixed samples. Subsequently, because no other protozoan pathogens were identified, only antigen detection of Giardia was used instead of microscopy. If a patient reported bloody stools, Shiga toxin-producing Escherichia coli (STEC) was examined for by culture and toxin detection (EIA). Only samples taken between 28 November and 31 December 2007 were included. For Giardia the time-period for inclusion was extended to 31 May 2008 (6 months) because of the longer incubation time and gradual case detection.
The methods used in water and pipeline analysis will be described in detail in a forthcoming paper. Briefly, samples from the drinking-water distribution system were analysed by cultivation for the presence of Campylobacter, Salmonella and Clostridium spp. PCR was utilized to analyse enteric viruses as previously described by Maunula and colleagues [Reference Maunula20]. The presence of Giardia cysts and Cryptosporidium oocysts was detected using concentration, immunomagnetic separation and epifluorescence microscopy as described in detail by Rimhanen-Finne and colleagues [Reference Rimhanen-Finne21].
Data analysis
The study areas were compared using χ2 or Kruskal–Wallis rank sum tests whenever appropriate. Attack rates were calculated as percentage of cases in a sample population. Total numbers of cases were calculated by extrapolating the age- and sex-adjusted attack rates to the total population of each area. To assess baseline rates of gastroenteritis, the attack rates from the control population were used to estimate the number of cases in the contaminated population that would have occurred in the absence of the water contamination. Estimations were made adjusting for age and sex. Confidence intervals for the total numbers were obtained using bootstrap methods [Reference Rimhanen-Finne21]. All statistical analyses were performed using R version 2.9 (or later versions) [22].
RESULTS
Healthcare visits
According to the health centre database, 1222 visits due to gastrointestinal symptoms were made between 28 November and 31 December 2007. During the busiest week, the number of patients presenting with gastrointestinal complaints was 53-fold higher than expected compared to the weekly median number of cases in a 7-month period preceding the outbreak. Children aged <10 years constituted the largest group (Figs 1, 2).
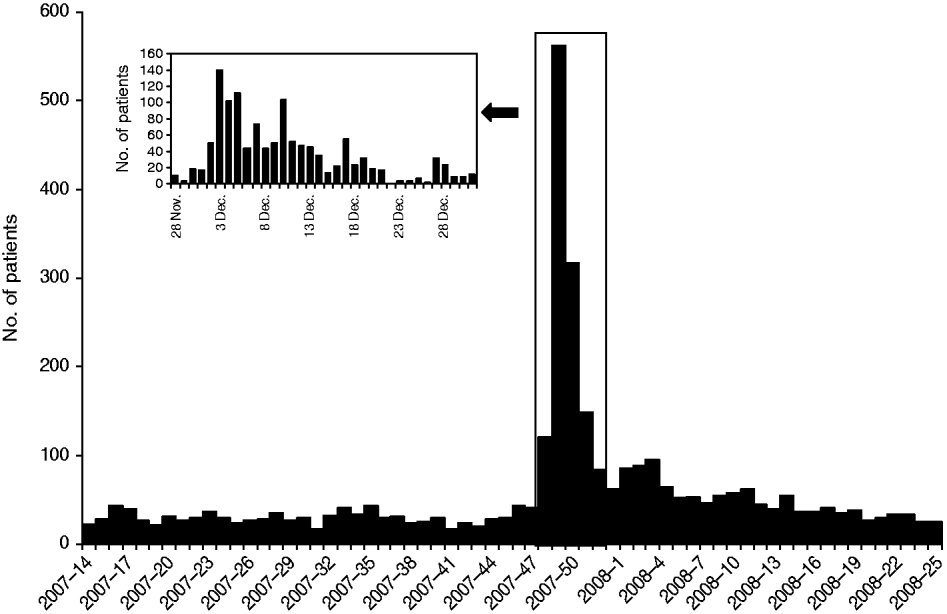
Fig. 1. Weekly number of patients attending with gastrointestinal symptoms at Nokia health centre from 1 April 2007 to 22 June 2008 (main figure). The inset represents the daily number of patients attending with gastrointestinal symptoms during the outbreak period.
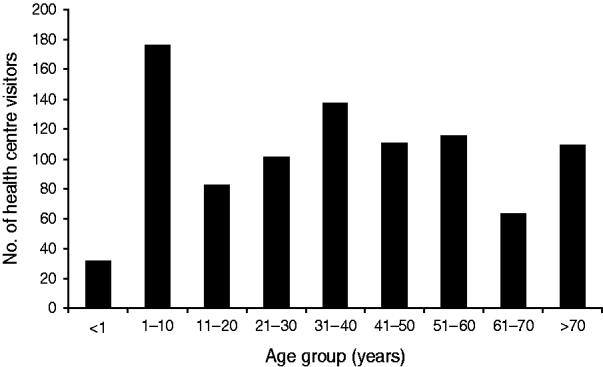
Fig. 2. Age distribution of visitors attending Nokia health centre with gastrointestinal symptoms, 28 November to 31 December 2007; data from patient register.
Line-list data were available on 1024 patients. Only one visit per person was included. The most common symptoms reported were diarrhoea (n=813, 79%), vomiting (n=601, 59%), fever (n=337, 33%) and abdominal pain (n=291, 28%). Altogether 204 visits were made to emergency rooms in Tampere University Hospital, 145 (71%) of them involving children.
To our knowledge, the outbreak did not lead directly to fatalities.
Questionnaire study
A total of 2154 participants returned the questionnaire. Thirty-one were excluded as being inadequately completed or because the respondent could not be identified; 2123 forms were finally accepted for the analysis (response rate 71%). The response rate was highest in the contaminated area and lowest in the control population. The background characteristics of the study groups showed no significant demographic differences between the study populations (Table 1). Altogether, 17% of the responders reported having gastroenteritis during the 10 months prior to the outbreak.
Table 1. Background characteristics of the three populations in the questionnaire study (only respondent data included)
Most subjects fell ill within 1 week of the contamination of the water supply (Fig. 3). The most common symptoms were diarrhoea, vomiting, nausea and abdominal pain (Fig. 4). Overall, 579 respondents met the case definition, of whom 540 (93%) were residents of Nokia. Extrapolating the result for the whole population, an estimated 8453 (95% CI 7568–9363) residents fell ill with gastroenteritis in the whole of Nokia (Table 2). The excess number of cases was 6500 (95% CI 5613–7370) in Nokia, compared to the control population. The gastroenteritis attack rate was eightfold higher in the polluted area (53·0%) and 2·4-fold higher in the unpolluted area (15·6%) compared to the control municipality (6·5%). The median duration of gastroenteritis was 3 days for cases in the polluted area, 2 days in the unpolluted area and 2 days in the control population (P<0·001). The probability of falling ill increased along with the quantity of tap water consumed in the contaminated area, but not in the uncontaminated area or in the control population.
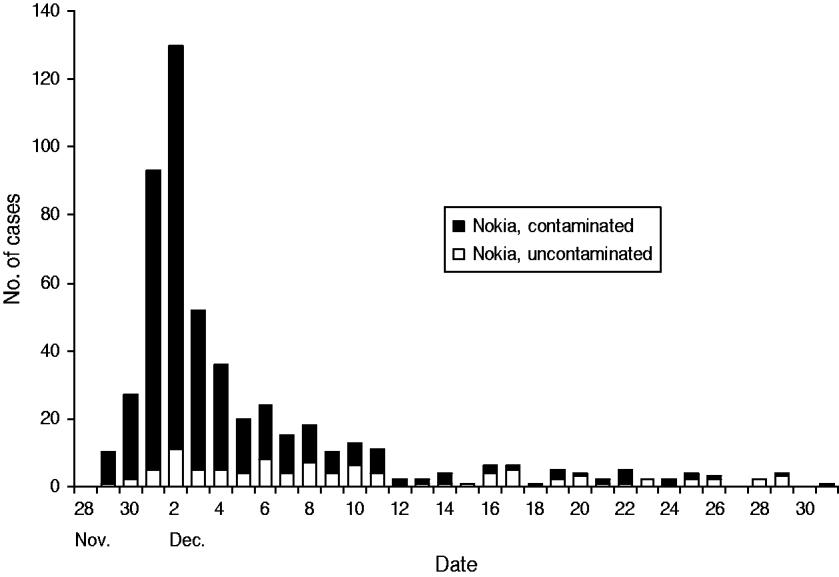
Fig. 3. The epidemic curve. Cases of acute gastroenteritis by onset of illness in two areas of Nokia, 28 November 2007 to 20 January 2008, according to the questionnaire study.
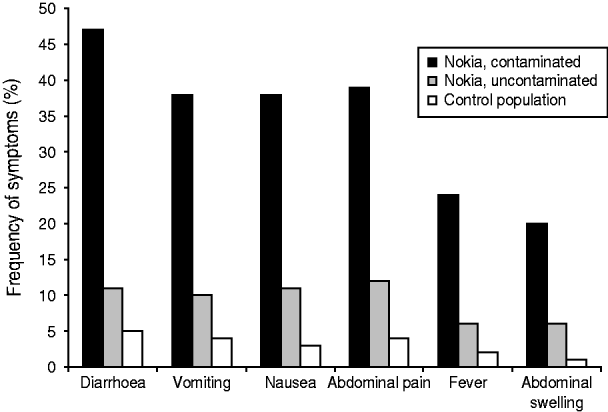
Fig. 4. Frequency of the most common symptoms in the three study populations, according to the questionnaire study.
Table 2. Background populations, response rates and main findings in the three study populations according to questionnaire data
OR, Odds ratio; CI, confidence interval.
A gastroenteritis case is defined as a person with ⩾3 loose stools/day or vomiting between 28 November 2007 and 20 January 2008.
Odds ratio: logistic regression for fulfilling the case criteria, compared to the control population.
* The original sample size was 1000 persons in both the uncontaminated and the contaminated groups. Assessment of the contaminated area was later defined according the data accumulating, and 21 persons were then moved from the uncontaminated to the contaminated group.
Microbiological findings
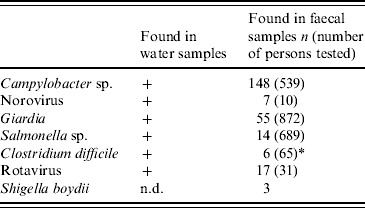
Seven enteropathogens were detected in faecal specimens (Table 3). The most frequently encountered pathogen was Campylobacter sp., with C. jejuni representing most of them, followed by Giardia. Five random samples investigated for norovirus were collected on 2 December 2007 and all were positive. Other findings included non-typhoidal salmonellas, Clostridium difficile and rotavirus. Four stool cultures grew Shigella boydii. In ten persons, two different bacterial pathogens were found in faecal specimens. Except for Shigella boydii, the same pathogens also were detected in water-distribution system samples. Ninety-eight percent (158/162) of Campylobacter- and Salmonella-positive cultures occurred within the first 3 weeks of the outbreak.
Table 3. Microbial findings in water and stool specimens
n.d., Not done.
Data represent findings from 28 November to 31 December 2007, except for Giardia, where the time period was extended to 31 May 2008. Five of the ten samples for norovirus detection were collected within 1 week after onset of the outbreak; all five were positive. Microbiology was not studied in all patients attending healthcare.
* Only culture-confirmed findings included.
DISCUSSION
This paper describes the largest reported waterborne outbreak in Finland to date. In the town of Nokia, over 8000 residents (28% of the population) were estimated to have fallen ill with gastroenteritis, 6500 of them as a consequence of water contamination. Over half of the population in the contaminated area suffered from gastroenteritis.
Although faecal contamination is a known cause of waterborne epidemics [Reference Carrique-Mas8, 23–Reference Häfliger, Hubner and Luthy26], the chain of events in Nokia was exceptional. In most circumstances, faecal contamination occurs when small amounts of sewage are mixed with clean water as a result of an obstructed or broken waste-water line or drainage from surface sources, e.g. after heavy rainfall. However, in the present case, sewage had direct entry to the distribution line through an inappropriate cross-connection. As a result, the magnitude of contamination was substantial.
The purpose and origin of the inappropriate valve was not ascertained in the investigation. It was built some decades ago, and was not prohibited during that time. After the outbreak, the national authorities required waterworks across the country to rule out the presence of similar cross-connection constructions.
Contacts with healthcare are often used to quantify the burden of illness in outbreak investigations. However, experience from previous outbreaks indicates that a large proportion of affected subjects have a self-limiting disease and do not contact healthcare providers [Reference Kuusi17]. Thus, if only health centre visitors' data are used, the burden of disease would be underestimated. In this study, a population-based survey was employed to avoid this problem. Since gastroenteritis is a common disease, a survey without proper controls might overestimate the magnitude of an outbreak if assessment of background morbidity is not done [Reference Hunter and Syed27]. To obviate this challenge, we used a control population chosen from the same region but on the opposite side of the Tampere city area. Daily contacts between these communities are likely to be minimal, so that baseline morbidity could be estimated from the control population, lending accuracy to conclusions regarding the morbidity from the water system contamination.
The gastroenteritis attack rate was high in the contaminated area, where over half of the population fell ill. This probably reflects the high concentration of pathogens in the drinking water as well as their infectivity, especially that of viruses [Reference Maunula20]. An even higher attack rate has been reported during a large waterborne norovirus outbreak in Finland [Reference Kuusi14]. In that case, however, the epidemic lasted longer and was investigated using an internet survey, making selection bias more likely.
There was also excess morbidity among residents in the uncontaminated area of the town. This can be explained by exposure to polluted water upon visiting the affected area. Another likely explanation is person-to-person transmission of certain pathogens, especially viruses. The incidence in the uncontaminated area increased later and less sharply than in the contaminated area, which would support the hypothesis of secondary spread. Furthermore, what is referred to here as the affected area is an estimate, which was grounded on a thorough judgement of available network and microbiological data. However, it may not be completely precise.
The duration of illness was longer in those living in the affected area. Bacterial infections were possibly more common in residents in the contaminated area, while in the uncontaminated area secondary spread of viral infections may have been more common. Another reason may be the quantity of exposure. Those living in the affected area were probably more extensively exposed to contaminated water than others, who perhaps ingested contaminated water only occasionally. Furthermore, those most intensively exposed may have been infected with several pathogens, either simultaneously or consecutively.
The frequency of previous episodes of gastroenteritis during the 10 months preceding the outbreak was 17% in the whole study population without differences between study groups. This is in good agreement with the yearly incidence of gastroenteritis (19·4/100 person-years) in a British cohort [Reference Wheeler28].
The survey was conducted as soon as possible after the outbreak. Since the outbreak took place shortly before the Christmas and New Year holiday season, it still took over 2 months before the survey could be launched. This conceivably resulted in some degree of recall bias. In addition, this outbreak gained wide coverage in the public media, which may have caused over-reporting of symptoms. The response rate of those who fell ill during the outbreak may have been higher, which might lead to overestimation of the disease burden. Nevertheless, the overall response rates in all study areas were relatively good, making significant bias unlikely.
The spectrum of pathogens in both water and patient samples was wide, consistent with the mechanism of contamination [Reference Gallay24, Reference Vestergaard25]. This distinguishes the outbreak from most Finnish waterborne epidemics, where usually only Campylobacter and/or norovirus are seen. Although samples were not systematically studied in all symptomatic patients, almost 200 Campylobacter-associated cases were confirmed in this outbreak, which is about 200 times more than the mean monthly number (n=1) for Campylobacter cases during the 24 pre-outbreak months in Nokia (National Infectious Disease Register). Only a few samples were collected to detect norovirus in the early days of the epidemic, but all (5/5) were positive. Despite the small number of confirmed cases, we regard norovirus as a major pathogen in this outbreak. A number of faecal specimens collected for other purposes were later analysed for enteric viruses, and almost a third of them contained norovirus [Reference Maunula20]. Astroviruses, adenoviruses, rotaviruses and enteroviruses were also detected. In another study connected to this outbreak and covering children attending the University Hospital, 40% (20/50) of samples yielded norovirus. In the same study, a variety of other viral pathogens were also detected, and a notable proportion of patients yielded mixed viral findings in their stools [Reference Räsänen29]. In addition to Campylobacter and norovirus, Giardia was regarded as a major pathogen. Although Giardia has been isolated from natural sources in Finland [Reference Horman30], domestic infections are uncommon.
Drinking-water contamination was considered to be the obvious source of this outbreak, and this study provides strong evidence to support the assumption. Most subjects fell ill shortly after the valve was opened and microbes found in stools were in accord with findings in pipeline samples. Finally, the incidence of the cases was highest in the contaminated area.
Regardless of the severity of the event, the clinical consequences were milder than feared. Most of those afflicted in Nokia suffered self-limiting gastroenteritis and no permanent damage to their health is anticipated. To our knowledge, there were no obvious fatal outcomes. However, Contributory influences in the case of deaths mainly for other reasons cannot be excluded. If waterborne outbreaks cause fatalities, these are most often caused by STEC O157:H7. Although O157:H7 was present in Finland during the 1990s, it is nowadays uncommon [Reference Kuusi, Siitonen, Eklund, Iivonen, Kela, Kuusi, Lyytikäinen and Ruutu31] and was not detected in this outbreak. Hepatitis A virus (HAV) is another severe infection associated with waterborne exposure. HAV is highly uncommon in the Finnish population, but sporadic cases do occur. HAV was not found in water samples during this outbreak, and no hepatitis cases were detected. Clostridium difficile was identified, but the hypervirulent ribotype O27 was absent (S. Kotila et al., unpublished observation).
This study demonstrates the value of a population-based controlled survey in estimating disease burden in outbreaks when illnesses are mild or moderate. In this particular case, the survey showed that the actual number of those affected was almost seven times higher than the number of people seeking medical care. Furthermore, the use of a control population affords greater accuracy when assessing excess morbidity connected to an outbreak.
APPENDIX. The Pirkanmaa Waterborne Outbreak Study Group
Pekka Collin1,2, Markku Korpela1,2, Anna-Leena Kuusela1,2, Sami Mustajoki1,2, Heikki Oksa1,2, Sirpa Räsänen1, Terhi Uotila2 and Tina Katto2.
(1 University of Tampere, 2 Tampere University Hospital).
ACKNOWLEDGEMENTS
This study was financially supported by the Competitive Research Funding of Tampere University Hospital (Grants 9E129 and 9L002). We thank Anneli Keinonen and Eero Vuori for providing register data, Sakari Jokiranta for parasite investigation and Helena Kovari for arranging the line-listing. We also thank the staff of the laboratories who analysed water and pipeline samples: Savo-Karjalan Ympäristötutkimus Oy, Finnish Food Safety Authority Evira: Veterinary Bacteriology Research Unit, laboratory services in the Hospital District of Helsinki and Uusimaa (HUSLAB), Kokemäenjoen vesistön vesiensuojeluyhdistys ry, Labtium Ltd and the laboratories of the National Institute for Health and Welfare.
DECLARATION OF INTEREST
None.









