INTRODUCTION
Clostridium difficile (C. diff) is a spore-forming, Gram-positive anaerobic microorganism that produces enterotoxins A and B, and generally colonizes the gastrointestinal tract after an alteration in the normal gut flora [Reference Cohen1]. Clostridium difficile infection (CDI) is a common cause of antibiotic-associated diarrhoea, accounting for 20–30% of all antibiotic-associated diarrhoea episodes [Reference Cohen1, 2]. A CDI diagnosis is confirmed via stool test to identify the presence of C. diff or its toxins [Reference Cohen1, 2].
Nosocomial CDI (N-CDI) is considered the most common cause of infectious diarrhoea in the healthcare setting [Reference Cohen1, 2]. Known risk factors for N-CDI include antibiotic use, advanced age, length of stay in healthcare facilities, reception of chemotherapy agents, gastrointestinal surgery/manipulation, and the use of proton pump inhibitors (PPIs) or histamine-2A receptor antagonist (H2RA) [Reference Cohen1–Reference Loo3]. While CDI predominantly occurs in hospitalized patients, healthy persons without a history of antibiotic use, recent hospitalization, and other common CDI risk factors are increasingly being diagnosed in the ambulatory setting [2–4]. Since 2001, the incidence of community-associated CDI (CA-CDI) and N-CDI have increased [2, 4, 5]. A 2010 population-based surveillance conducted by the Centers for Disease Control and Prevention (CDC) investigated the prevalence in eight areas of the USA and reported that CA-CDI cases accounted for about 32% of all CDI cases [Reference Lessa6].
Since CDI is not a reportable condition in the USA [Reference Lessa6], the peer-reviewed literature on CDI is typically from non-US sources [Reference Loo7, Reference Noren8]. The few US-based studies are retrospective evaluations that primarily investigated the differences in incidences and CDI risk factors between CDI diagnosed in the inpatient and outpatient settings [Reference Kuntz9–Reference Chitnis15]. Most studies of CDI were either conducted in targeted populations, did not include CDIs acquired in non-hospital healthcare facilities [e.g. skilled nursing facilities (SNFs), long-term care facilities (LTCFs)], only included patients treated in the inpatient setting, or did not assess specifically the risk differences between CA-CDI and N-CDI [Reference Noren8–Reference Chitnis15].
Considering the increasing incidence of CDI and its associated costs and risks (e.g. CDI-related hospitalizations, colectomies, etc.), further investigation is needed to understand how patients are being exposed to C. diff spores, especially in the community setting. The lack of information on risk factors common to both CA-CDI and N-CDI suggests that there may be risk factors associated with CA-CDI that are not associated with N-CDI. The purpose of this study was to assess risk factors associated with CDI and to assess and contrast CDI risk factors and clinical outcomes in patients with N-CDI and CA-CDI treated with an anti-CDI agent in the ambulatory setting.
METHODS
Study design and setting
This is a retrospective, data-only cohort study of patients diagnosed with CDI any time between 1 April 2005 and 30 April 2011. Patients were followed from CDI diagnosis until 180 days after diagnosis, death, or Kaiser Permanente Colorado (KPCO) membership termination, whichever came first. This study was conducted at KPCO, an integrated healthcare delivery system with over 530 000 members seen at 25 ambulatory clinics in Colorado. The majority of services at KPCO are provided by Colorado Permanente Medical Group (CPMG) salaried physicians in system-owned outpatient facilities. In addition, CPMG physicians attend at two contracted hospitals and their associated emergency departments (EDs), 67 LTCFs and 51 assisted living (ALF) facilities and seven SNFs. KPCO has its own centralized, ambulatory laboratory where stool samples are processed for C. diff toxin B. In 2010, about 12% of Colorado's health insurance-covered lives were KPCO members.
KPCO utilizes an electronic medical record (EMR) (Health Connect®, Epic Systems, USA) where coded and free-text medical, pharmacy, laboratory, death, etc. information from health-system owned, contracted, and other facilities are recorded. In addition, KPCO accounts for deaths of members who terminated membership via the US Social Security Administration, state death registries, and death-records.com. Furthermore, outpatient (e.g. Medicare Part B-covered services) and inpatient (e.g. Medicare Part A-covered services) health services not performed at KPCO-owned and -contracted facilities are captured from billing claims. Information from the study was obtained from queries of the KPCO administrative and claims databases.
Ethical standards
This study was reviewed and all aspects were approved by the Kaiser Permanente Colorado Institutional Review Board on 17 October 2012. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Study population
This study included all patients identified during the study period with a positive ambulatory C. diff toxin B gene (tcdB) laboratory measure from a medical office encounter and/or a primary discharge diagnosis of CDI (ICD-9 code 008.45) recorded during an ED visit or LTCF, SNF, ALF, or inpatient stay. The Xpert® C. difficile PCR assay (Cepheid, USA) was utilized by the KPCO central laboratory to detect C. diff toxin B gene. Briefly, a loose stool is collected from a patient with suspected CDI in a sterile, airtight, screw-capped specimen container with no preservative. The specimen is refrigerated until delivery to the central laboratory. Frozen or formed stools are rejected by the laboratory. According to the manufacturer's instructions, duplicate testing is not recommended or necessary due to the sensitivity of PCR. Sensitivity, specificity, positive predictive value, and negative predictive value for Xpert ® C. difficile are 97·3%, 97·9%, 90·0%, and 99·5%, respectively [Reference Dalpke16]. For study purposes, a positive, ambulatory CDI laboratory value was considered a CDI diagnosis.
As no laboratory data were available from ED visits or LTCF, SNF, ALF, or inpatient stays, the sample was limited to patients with a primary discharge diagnosis of CDI to increase the probability of true CDI cases. Only patients with a primary discharge diagnosis were included as the CDI ICD-9 code has been found to have limited sensitivity [Reference Chan17, Reference Scheurer18] and other positions can represent documentation of a history of CDI [Reference Dubberke19]. All included patients were adults (i.e. ⩾18 years) and a KPCO member at time of CDI diagnosis with continuous KPCO membership in the 180 days prior to CDI diagnosis. Included patients had no prior positive CDI laboratory measure or diagnosis code recorded during the 180 days prior to index CDI diagnosis. In addition, all included patients were required to have purchased an anti-CDI agent (i.e. metronidazole, vancomycin, fidoxamicin, rifaximin) from a KPCO pharmacy within 7 days of diagnosis.
All patients were assessed objectively to determine the setting where CDI was contracted using the following algorithm (Fig. 1):
-
(1) If a patient was diagnosed with CDI during an ED visit, SNF, ALF, or LTCF stay, or hospital admission, his/her medical chart was reviewed. If there was specific notation (e.g. symptoms present on admission, C. diff laboratory measure at admission was positive) in the medical chart by a physician of CDI being present upon admission/presentation, the patient was categorized as a patient with CA-CDI. Otherwise, the patient was categorized as a patient with N-CDI. Medical chart review included all available pertinent information that was recorded before, after, and at the time of CDI diagnosis.
-
(2) If a patient was diagnosed with CDI during a medical office encounter (i.e. positive C. diff toxin B laboratory measure) and identified to have had a SNF, ALF, or LTCF stay, ED visit, or hospital admission within 4 weeks prior to the day of diagnosis, the patient's medical chart was reviewed. If there was specific notation in the chart by a physician of CDI being present upon admission/presentation, the patient was categorized as a patient with CA-CDI. Otherwise, the patient was categorized as a patient with N-CDI.
-
(3) If a patient was diagnosed in the ambulatory setting and had no SNF, ALF, or LTCF stay, ED visit, or hospital admission within 4 weeks prior to the day of diagnosis, the patient was categorized as patient with CA-CDI.
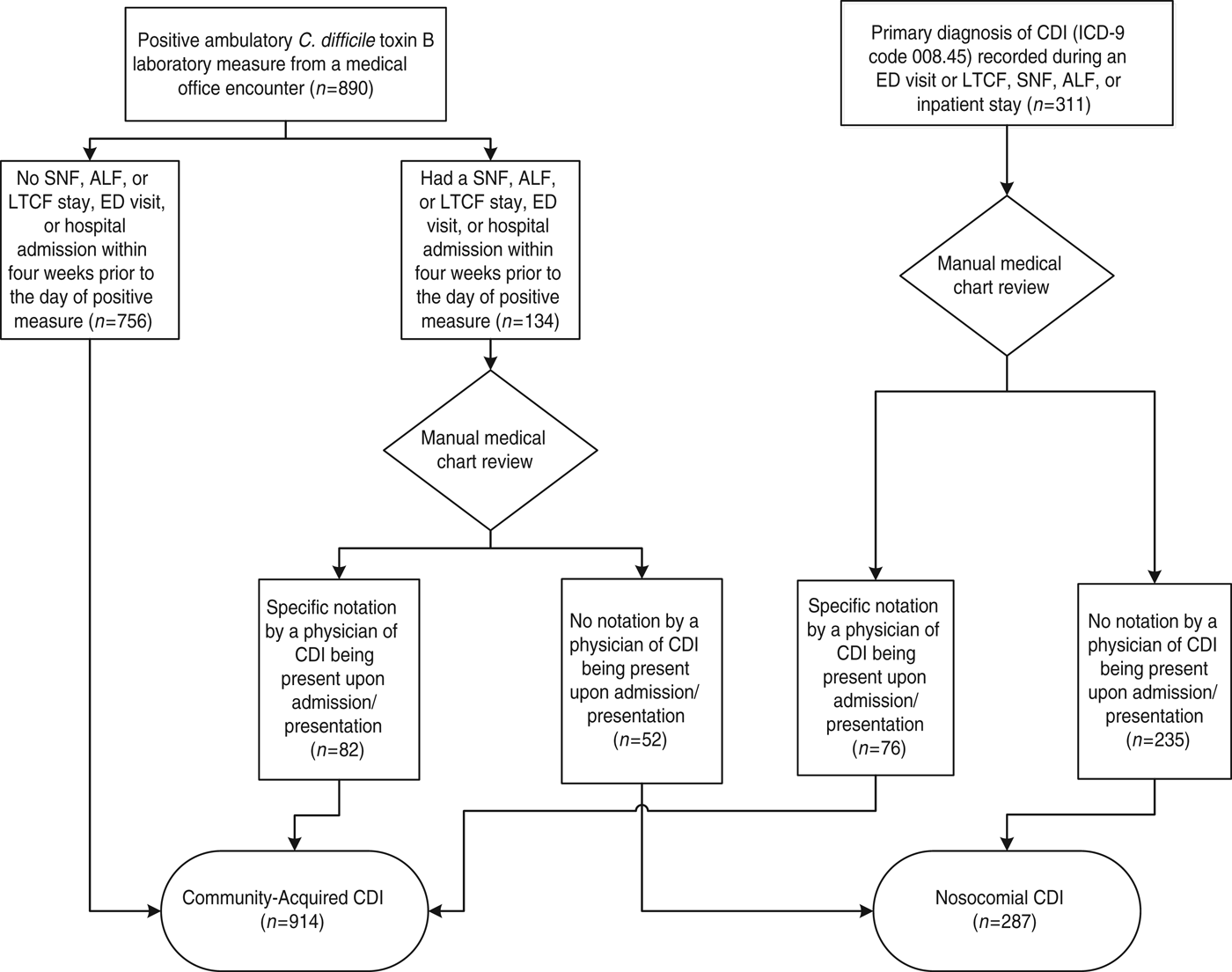
Fig. 1. Patient assignment to community-acquired or nosocomial Clostridium difficile infection (CDI) group. ALF, Assisted living facility; ED, emergency department; LTCF, long-term care facility; SNF, skilled nursing facility.
Study outcomes
The primary outcome was an assessment and comparison of clinical, demographic, and sociodemographic risk factors between patients with CA-CDI and N-CDI. Eligible patients were treated for CDI in an ambulatory setting. Secondary outcomes included comparisons of rates of CDI recurrence, death, and CDI-related death in the 180 days after diagnosis and hospitalization/re-hospitalization in the 21 days after diagnosis between patients with CA-CDI and N-CDI. Types of ambulatory CDI treatment were also compared. Furthermore, an analysis of the factors independently associated with CA-CDI was conducted.
Data collection
The study cohort was identified through queries of KPCO's electronic administrative and claims databases, including the EMR. Manual medical record reviews were conducted to determine objectively the source (i.e. community-acquired vs. nosocomial) of CDI.
Information on medical office C. diff laboratory measurements and results were obtained from the KPCO laboratory database. Information on ED visits, SNF, ALF, and LTCF stays, and hospital admissions were obtained from the KPCO claims database. Information on patients' date of birth, sex, residence community, household members, race, ethnicity, prescription drug benefit, health plan type, and Medicaid status at the time of CDI diagnosis was obtained from the KPCO membership database. If any of this information was missing, a patient's medical record was manually reviewed to obtain the information. Information on diagnosed patient comorbidities (e.g. digestive disorders, gastroenteritis), medical office visits, and peripartum status in the 180 days prior to CDI diagnosis was obtained from the KPCO EMR database using pre-defined ICD-9 codes (codes available from the corresponding author upon request). Information on prescription medication purchases and administrations in the 180 days prior to and after CDI diagnosis were obtained from the KPCO pharmacy database using pre-defined Generic Product Identifier codes (codes available upon request). Information on patient deaths in the 180 days after CDI diagnosis was obtained from the KPCO death registry.
Data analysis
The ages of patients and household members age were calculated as of CDI diagnosis date. Household members were categorized in the following age groups <1, ⩾1 to <4, and ⩾4 to ⩽10 years. Race was categorized as white, unknown/unreported, and other. A chronic disease score (CDS), a validated measure of a patient's burden of chronic illness, was calculated using ambulatory prescription medication purchases in the 180 days prior to CDI diagnosis [Reference Clark20, Reference Von, Wagner and Saunders21]. The CDS ranges in values from 0 to 36 with increasing values indicating a higher burden of chronic illness. The length of antibiotic use, where applicable, prior to CDI diagnosis was calculated by summing the antibiotic days supplied in the 180 days prior to CDI diagnosis. The count of ambulatory medical office visits in the 180 days prior to CDI diagnosis was summed. Health plan types were categorized as health maintenance organization, deductible/co-insurance, and other. Patient communities were categorized as rural, suburban, and urban. C. diff recurrence was determined by assessing for an ambulatory purchase of another anti-CDI agent in the 180 days after the purchase of the index anti-CDI agent. C. diff-related death was assessed by a cause of death with an ICD-10 code of A04.7 (‘Other bacterial intestinal infections: Enterocolitis due to Clostridium difficile’).
All analyses were performed comparing the CA-CDI group to the N-CDI group. Patient characteristics were reported as mean [±standard deviation (s.d.)] or median [with interquartile range (IQR)], as applicable, for interval-level variables and percentages for categorical variables. Wilcoxon rank-sum tests and t tests, as applicable, and χ 2 tests of association or Fisher's exact tests, where applicable, were used to assess differences between groups for interval-level and categorical variables, respectively. To identify factors associated with CA-CDI, all factors with a P < 0·2 in the bivariate analyses and a prevalence of ⩾10 patients were entered into a multivariate, logistic regression model. Factors in the model were assessed for multicollinearity (i.e. ρ>0·3); however, no multicollinearity was detected. Two-way factor interactions were constructed and tested for statistical significance. No interactions were statistically significant and, thus, none were included in the final model. All analyses were performed using SAS v. 9.2 (SAS Software Inc., USA).
RESULTS
A total of 1201 patients were diagnosed with CDI during the study period. Of these, 890 (74·2%) were diagnosed in a medical office. Of the final study cohort, 914 (76·1%) and 287 (23·9%) were identified with CA-CDI and N-CDI, respectively. Patients with CA-CDI were more likely to be younger (P < 0·001), female (P = 0·002), and had a lower mean CDS (P < 0·001) (Table 1). Patients with N-CDI were more likely to have had a diagnosis of peritonitis (P = 0·008), digestive disorder (P < 0·001), vascular insufficiency (P = 0·045), and/or intestinal obstruction (P < 0·001). There was a numerically higher percentage of patients with N-CDI (61·0%) than patients with CA-CDI (54·6%) who made an ambulatory purchase of an antibiotic prior to CDI diagnosis but the difference did not reach statistical significance (P = 0·057). However, among patients who made an ambulatory purchase of an antibiotic prior to CDI diagnosis, patients with CA-CDI were more likely to have purchased a penicillin antibiotic (P < 0·001) while patients with N-CDI were more likely to have purchased a fluoroquinolone antibiotic (P < 0·001).
Table 1. Baseline patient characteristics by source of exposure
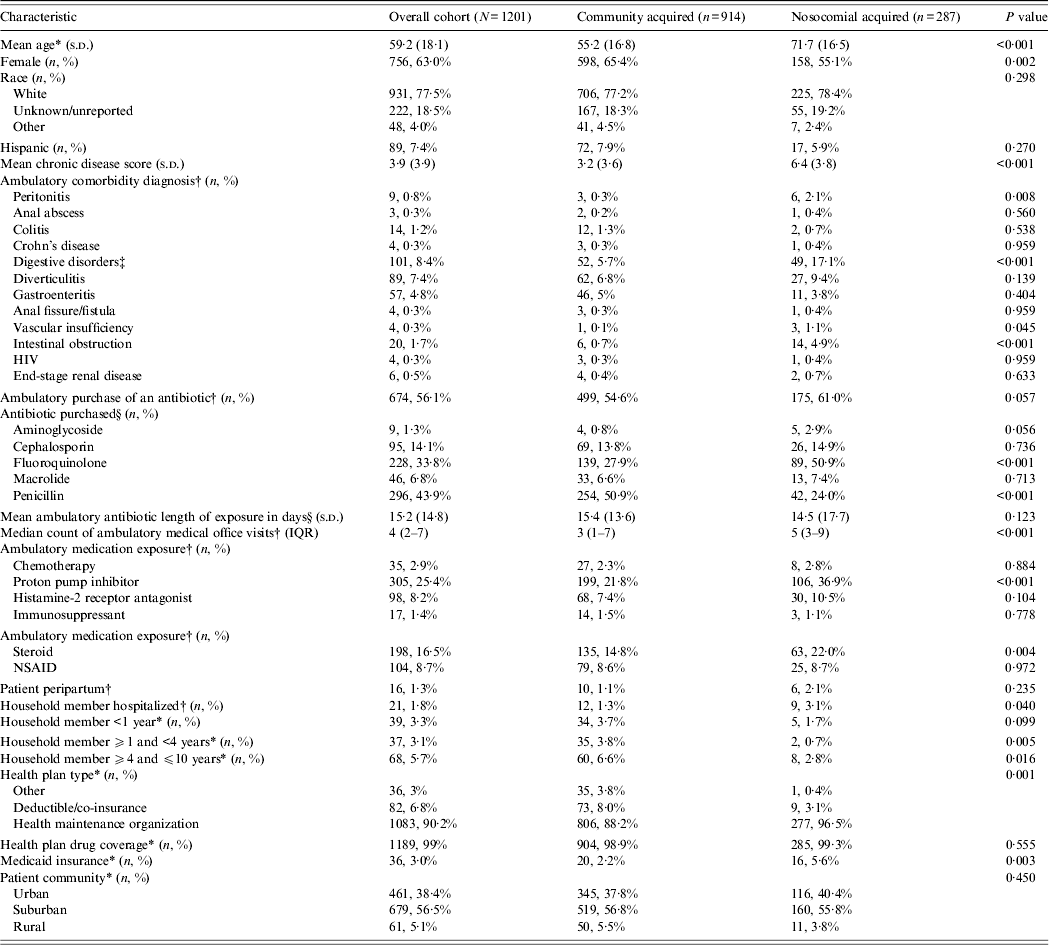
IQR, Interquartile range; NSAID, non-steroidal anti-inflammatory drugs.
* As of Clostridium difficile infection (CDI) diagnosis date.
† In the 180 days prior to CDI diagnosis date.
‡ Includes ICD-9 codes 530.xx, 536.xx, 564.xx.
§ Among patients who received an antibiotic in the 180 days prior to CDI diagnosis date (n = 674).
Patients with N-CDI were more likely to have purchased a PPI (P < 0·001) and/or steroidal agent (P = 0·004) and had a household member who had been hospitalized (P = 0·004) within the 6 months prior to the patient's CDI diagnosis. In addition, patients with N-CDI had a higher count of ambulatory medical office visits in the 180 days prior to CDI diagnosis (P < 0·001) with an equivalent percentage in each group (~9%) having had no office visits (P > 0·05). Patients with CA-CDI were more likely to have had a household member who was aged ⩾1 and <4 years (P = 0·005) and/or ⩾4 and ⩽10 years (P = 0·016) as of the date of the patient's CDI diagnosis. There were significant differences across the health plan types between the two groups (P = 0·001). In addition, patients with N-CDI were more likely to have had Medicaid insurance (P = 0·003).
Patients with N-CDI were more likely to have had recurrent CDI (P = 0·043) and been hospitalized within the 21 days after CDI diagnosis (P = 0·011) (Table 2). Of patients who were receiving an antibiotic prior to their CDI diagnosis, patients with N-CDI were more likely to continue their pre-CDI antibiotic treatment (P = 0·042). Patients with CA-CDI were more likely to receive metronidazole treatment for CDI (P = 0·041). Patients with N-CDI were more likely to die from any cause within 6 months of their CDI diagnosis (P < 0·001). However, when examining causes of death, relatively few patients died from CDI and there were equivalent proportions of CDI-related deaths between the groups (P > 0·05).
Table 2. Outcomes by source of exposure
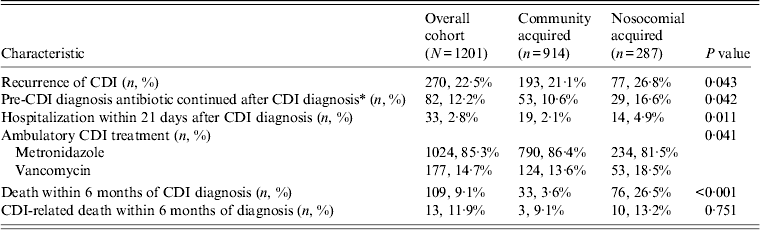
CDI, Clostridium difficile infection.
* Among patients who received an antibiotic in the 180 days prior to CDI diagnosis date (n = 674).
In the multivariate analysis, increasing age [odds ratio (OR) 0·95, 95% confidence interval (CI) 0·94–0·96], increasing CDS (OR 0·85, 95% CI 0·81–0·89), diagnoses of a digestive disorder (OR 0·46, 95% CI 0·28–0·76) and an intestinal obstruction (OR 0·21, 95% CI 0·07–0·61), and having had a household member hospitalized prior to CDI diagnosis (OR 0·11, 95% CI 0·03–0·39) were associated independently with a decreased likelihood of contracting CA-CDI (Table 3). No factors were identified that were associated independently with an increased likelihood of contracting CA-CDI.
Table 3. Factors assessed for independent association with community-acquired CDI infection
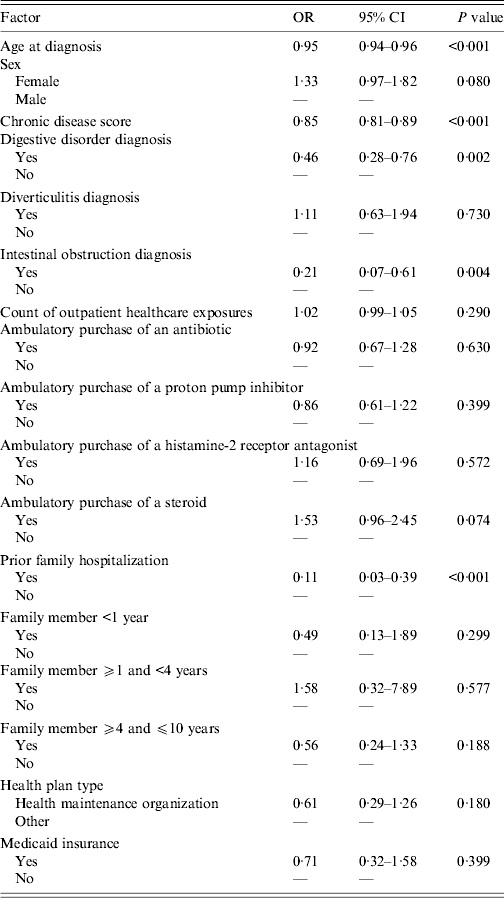
OR, Odds ratio; CI, confidence interval.
C statistic = 0·817.
DISCUSSION
In this retrospective analysis of over 1200 patients diagnosed with CDI in hospitals, medical offices, SNFs, ALFs, and LTCFs who were treated with an ambulatory anti-CDI agent, we assessed numerous patient characteristics and CDI-related risk factors and compared them between community-associated and nosocomial CDI in order to provide clinicians with a more comprehensive understanding of CA-CDI risks and treatments. We systematically examined patients' records in order to determine more accurately the setting where CDI was acquired. Our multivariate analysis identified that increasing age and chronic disease burden, digestive disorders, intestinal obstructions, and a recent hospitalization of a household member are factors independently associated with a reduced likelihood of CA-CDI. Given that CDI predominantly has been thought to be a nosocomial infection, much of the existing research and literature reasonably revolves around N-CDI [Reference Cohen1, 2]. However, with the incidence of CA-CDI increasing, it is important to understand the potential sources of exposure to C. diff in the community [Reference Leffler and Lamont22].
Our study is unique in that we identified a substantially higher percentage of patients with CA-CDI (76%) vs. N-CDI (24%) in our patient population. Other studies that have investigated CA-CDI and N-CDI reported CA-CDI percentages of around ⩽50% [Reference Lessa6, Reference Kuntz9, Reference Khanna11–Reference Kutty13]. Our higher percentage of CA-CDI patients may be related to the methodology used to identify CA-CDI where we used both electronic database queries and manual chart reviews in our identification process while other studies relied solely on electronic database queries [Reference Lessa6, Reference Kuntz9, Reference Khanna11–Reference Kutty13]. In addition, we relied solely on a primary discharge diagnosis of CDI for patients diagnosed in the ED, LTCF, SNF, ALF, or inpatient setting; thus, limiting our sample to patients with a high probability of an actual CDI [Reference Chan17–Reference Dubberke19]. Nevertheless, the high percentage of CA-CDI patients we identified suggests that CA-CDI is a growing threat to community health.
Our results confirm that populations not typically considered at high risk for CDI are contracting CDI in the community setting [2–4]. While the majority (56%) of our patients with CDI had been exposed to antibiotic therapy in the 180 days prior to their CDI diagnosis date, only 21% of the patients unexposed to an antibiotic were exposed to a PPI or H2RA. Overall, our antibiotic, PPI, and H2RA exposure rates in patients with CA-CDI were similar to those reported by Chitnis and colleagues (i.e. antibiotic 64%, PPI 28%, H2RA 9%) [Reference Chitnis15]. Together, these results suggest that seemingly healthy patients are contracting CDI; including younger patients and those without histories of recent antibiotic or acid-suppressing medication use and hospitalization, and diagnosis of a gastrointestinal condition.
In addition, only small proportions of our patients with CDI had been exposed to chemotherapeutic, immunosuppressant, steroidal, or non-steroidal anti-inflammatory medications. Furthermore, even though CA-CDI patients were more likely to have had a household member aged between 1 and 10 years, relatively few of our patients with CDI, overall, were exposed to household children aged ⩽10 years. Kuntz and colleagues also found similar results where patients with CA-CDI did not have risk factors normally associated with CDI infections [Reference Kuntz9]. Their CA-CDI patients also were relatively young and 17% of their patients did not have any previously identified risk factors [Reference Kuntz9].
As C. diff spores often may be found in water and retail foods [Reference Gould and Limbago23], there may be ‘hotspots’ in the community where infection is more prominent. We found that only a small percentage (5%) of our patients with CDI were from rural communities, thus suggesting that exposure to farm animals, such as cows, horses, and dogs that have been linked to CDI [Reference Gould and Limbago23], was limited in our study population. However, most of our patients with CDI had been exposed to some ambulatory medical care in the 180 days prior to CDI diagnosis suggesting that such exposure may predispose patients to CDI [Reference Chitnis15]. It has been reported that at least 50% of hospitalized patients are colonized with C. diff as asymptomatic carriers [Reference Cohen1]. Thus, it is possible that CDI may be due in part to exposure to household members who were previously hospitalized and shedding spores after hospital discharge. However, we identified very few patients with CDI (~2%) who were exposed to a household member who had been hospitalized.
In our bivariate analysis, patients with N-CDI tended to be older, had a higher CDS, and had been diagnosed with a digestive disorder, vascular insufficiency, peritonitis and/or an intestinal obstruction. In addition, we found that patients with N-CDI were more likely to have had a CDI recurrence, hospitalization after CDI diagnosis, and died after CDI diagnosis. These findings suggest that patients with N-CDI carried a higher burden of chronic disease and had a more complex clinical scenario. Our study supports other investigations that found CA-CDI to be less severe and more commonly found in females and younger patients [Reference Kuntz9, Reference Khanna11]. Patients with N-CDI were more likely to have purchased a fluoroquinolone antibiotic within the 180 days prior to their CDI diagnosis. In comparison to most penicillin antibiotics, fluoroquinolone antibiotics provide more broad-spectrum antibacterial coverage; thus, possibly increasing susceptibility to CDI.
In our multivariate analysis, we identified that for each additional year of age there was a 5% reduced likelihood of CA-CDI. In addition, each 1-point increase in CDS value conferred a 15% reduced likelihood of contracting CA-CDI. Further, we identified that patients with a diagnosis of digestive order had a 54%, and patients with an intestinal obstruction had a 79%, reduced likelihood of contracting CA-CDI. Similar to our findings, Khanna and colleagues in their small, retrospective analysis of a CDI registry in Minnesota reported that patients with CA-CDI were more likely to have been younger and had a lower comorbidity burden than patients with N-CDI [Reference Khanna11]. Conversely, Khanna and colleagues reported that exposure to an acid-suppression medication was associated with N-CDI while we did not find this association [Reference Khanna11]. Our results may have differed from Khanna and colleagues as they combined all acid-suppressive medications while we looked at PPIs and H2RAs independently [Reference Khanna11]. Despite the numerous factors that we examined, we identified few factors that were independently associated with CA-CDI indicating that CA-CDI and N-CDI are occurring in similar populations. Kuntz and colleagues in their investigation of outpatient- vs. inpatient-diagnosed CDI similarly identified few factors that differentiated the diagnosis setting [Reference Kuntz12].
In comparison to previous studies, our study evaluated a large cohort that included patients objectively diagnosed with CDI in both community and healthcare facilities, including hospitals, SNFs, ALFs, and LTCFs. We examined numerous clinical and sociodemographic factors including patients' geographical locations and household members' characteristics. Our study objectively reviewed CDI cases to enable us to categorize more accurately patients according to the setting in which C. diff was most likely acquired. However, our study did have limitations. We were unable to identify specific C. diff strains infecting our patients as our laboratory did not perform such analyses. Therefore we were unable to compare strains in N-CDI vs. CA-CDI. While we utilized all patients with a positive C. diff ambulatory laboratory value, we only examined patients with a primary discharge diagnosis of CDI from the ED, LTCF, SNF, ALF, and inpatient settings. This may have limited our sample size of patients with N-CDI but provided a higher probability of identification of patients with an actual CDI from these settings. Our ability to detect factors independently associated with CA-CDI that had a low prevalence (e.g. Medicaid status) in our sample was restricted. The possibility of false-negative/false-positive laboratory results for CDI exists; however, the PCR test used in our health system had robust sensitivity, specificity, positive predictive value, and negative predictive value. As this was a retrospective evaluation, we did not interview any study patients and, thus, were unable to examine food and animal exposures as potential risk factors. Nevertheless, we did use geographical data to identify rural (i.e. as a proxy for farm animal) exposure. In addition, this study was conducted for one health plan in one geographical region. Other health plans/health systems may find different exposure patterns. Furthermore, we were unable to examine over-the-counter (OTC) medication use (e.g. antacids) but we did examine prescription acid-suppression medication use and patient diagnoses for digestive disorders that included dyspepsia and oesophageal reflux where an OTC medication may be recommended.
CONCLUSIONS
With an increasing prevalence of CA-CDI in the USA, there is a heightened need for the investigation of CA-CDI-related issues. Our study of over 1200 patients with CDI examined numerous possible risk factors of CA-CDI vs. N-CDI. We found that more patients contracted CA-CDI than N-CDI and that the patient group most at risk for CA-CDI was young, healthy, and had few traditional risk factors, such as gastrointestinal disorders, compared to patients with N-CDI. It is important to identify risk factors so that clinicians can identify patients at risk for CA-CDI and work with them to prevent CA-CDI and its associated costs and complications (e.g. CDI recurrence, hospitalization, colectomies). As we identified few factors independently associated with CA-CDI, additional research is needed with larger populations of patients with CA-CDI and N-CDI and access to both unconventional and conventional exposure data to ascertain factors most relevant to CA-CDI.
ACKNOWLEDGEMENTS
The authors acknowledge Aubrey E. Jones for her contribution to the editing of the manuscript for clarity and purpose. This study was funded by the Kaiser Permanente Colorado Pharmacy Department.
DECLARATION OF INTEREST
None.







