Diarrhoeal disease can result in major childhood morbidity in developing countries. The endemic and epidemic picture of infectious gastroenteritis varies by country and season. Many aetiological studies have focused on combined testing for viruses and bacteria, while studies on agents and clinical features related to mixed infections are less common. Molecular methods have been shown to have improved capability in the identification of infectious agents associated with acute gastroenteritis (AGE) in children [Reference Tenover1]. The aim of this study was to investigate the aetiology and clinical features of AGE caused by multiple enteric pathogens in hospitalized paediatric patients in Chang Gung Children's Hospital in northern Taiwan. This study was approved by Institutional Review Board of Chang Gung Memorial Hospital (approval no. 96-0857B).
From October 2004 to September 2006, patients aged from 3 months to 18 years hospitalized in Chang Gung Children's Hospital (a teaching hospital and medical centre with 500 beds available for paediatric patients) with the major presentation of acute non-bloody diarrhoea (passage of watery or loose stools) within 3 days were enrolled in our study and their stool samples were collected for analysis. The demographic data, detailed disease courses, complications, and results of laboratory examinations of these patients were collected and analysed. The severity of AGE was evaluated, as previously described [Reference Vesikari2], by scoring frequency and duration of diarrhoea or vomiting, electrolyte and dehydration status, fever severity [three-point scale (points 1–3) based on the maximum temperature of fever], and the need for medical management. Complications were any extra-intestinal or uncommon presentation of AGE, including electrolyte imbalance, hypoglycaemia, convulsion, and hypotension.
The faecal specimens were collected from the patients hospitalized with AGE within 3 days after admission. Extraction of the viral nucleic acid from faecal specimens was carried out and the first-strand cDNA synthesis for RNA viruses and PCR reaction were performed according to the manufacturer's recommendations (High Pure Viral Nucleic Acid kit and First Strand cDNA synthesis kit, Roche Diagnostics GmbH, Mannheim, Germany). The PCR primer sets used for detection of rotavirus, norovirus, astrovirus, and enteric adenovirus were as previously described [Reference O'Neill3–Reference Phan5]. The PCR products were separated on 2% agarose gel, stained with ethidium bromide, and visualized using a UV box. Adobe Photoshop 6.0 (Adobe, ACD System, Arlington, TX, USA) was used to process the image. All of these faecal samples were also sent to the Clinical Microbiology Laboratory for bacterial culture of Salmonella, Shigella, and Campylobacter.
For continuous data, Student's t test was used and shown as median and interquartile range, whereas the binary data were analysed using the χ2 test. P<0·05 was considered statistically significant. All the tests were analysed using SAS system software version 8 for Windows (SAS Institute, Cary, NC, USA).
During the period of October 2004 to September 2006, a total of 303 patients [245 (80·9%) patients aged ⩽5 years] with complete medical records were enrolled and studied. There were 69 (22·8%) patients infected by multiple pathogens, including 52 multiple viral infections (17·2%) and 17 viral and bacterial co-infections (5·6%). In the solitary infection, rotavirus was the most common pathogen. Eighty patients (26·4%) were infected by rotavirus, 31 by norovirus, nine by astrovirus, 46 by enteric adenovirus, and 68 with no virus identified from the faecal specimens; 15 (4·9%) were infected by bacteria alone (Fig. 1a). The most common co-infecting viruses were rotavirus and enteric adenovirus (20/52, 38·5%), followed by rotavirus and norovirus (18/52, 34·6%) (Fig. 1b). The most common co-infecting virus and bacteria were rotavirus and Campylobacter jejuni (6/17, 35·3%), followed by rotavirus and Salmonella serogroup B (5/17, 29·4%) (Fig. 1c). As seen in Table 1, statistical analysis showed a higher incidence, higher daily frequency, and a longer duration of vomiting in patients with multiple viral infections (all P<0·0001) and rotavirus infection alone (P<0·0001, P=0·0021, P<0·0001, respectively), when compared to those with virus and bacteria co-infection; furthermore, there was a higher daily frequency of diarrhoea and a longer hospital stay in patients co-infected by virus and bacteria, compared to those with mixed viral infection (P=0·0036, P=0·0023, respectively) as well as rotavirus infection alone (P=0·0417, P<0·0001, respectively). Laboratory data showed a significantly higher C-reactive protein level and higher rate of positive occult blood in stools from patients co-infected by virus and bacteria, compared to those infected by multiple viruses (P<0·0001, P=0·0366, respectively) or by rotavirus alone (P<0·0001, P=0·0439, respectively). The summarized disease severity was not significantly different between the three groups of patients (Table 1). There was no clinical difference between patients with rotavirus infection and those with multiple viral infections (P values not shown). Dehydration-associated electrolyte imbalance or hypoglycaemia were not uncommon in patients with polymicrobial enteric infections [20/52 (38·5%)] in multiple viral infections and 5/17 (29·4%) in those co-infected by virus and bacteria]. Although there was no statistical difference in the incidence of individual complication, hypoglycaemia seemed to occur more frequently in patients with multiple viral infections (15/52, 28·8%) than in patients co-infected by virus and bacteria (2/17, 11·8%). Hypotension due to hypovolaemia was found in three patients, including two with multiple viral infections and one with viral and bacterial co-infection. Convulsion occurred in one patient with multiple viral infections while it did not occur in the patients co-infected by virus and bacteria. The overall incidence of complication appeared higher in patients with multiple viral infections (26/52, 50%) than patients co-infected by virus and bacteria (6/17, 35·3%) but without statistical significance.

Fig. 1. Aetiology of 303 paediatric patients with (a) acute gastroenteritis and those caused by (b, c) multiple enteric pathogens. Fifty-two disease episodes caused by multiple viruses are shown in panel (b) and 17 by virus and bacteria in panel (c). ADV, enteric adenovirus; ASV, astrovirus; C, Campylobacter; NV, norovirus; P, Pseudomonas aeruginosa; RV, Rotavirus; SB, Salmonella serogroup B; SD, Salmonella serogroup D.
Table 1. Clinical presentations of acute gastroenteritis caused by rotavirus, multiple viruses, and mixed virus and bacteria
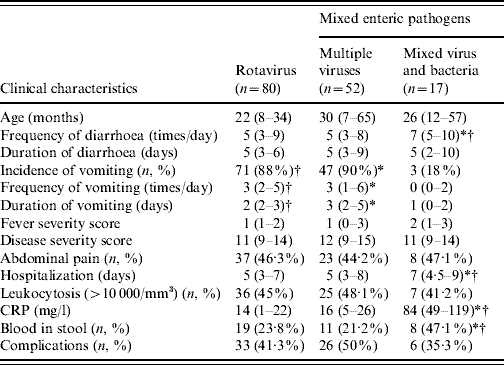
CRP, C-reactive protein.
The median values and 25% (Q1) and 75% (Q3) quartiles are given as median (Q1–Q3).
* Statistical significance in the comparison between multiple virus infection and virus and bacteria co-infection (P values shown in the text).
† Statistical significance in the comparison between rotavirus infection and virus and bacteria co-infection (P values shown in the text).
Multiple viral infections predominantly occurred in the colder seasons with 38/52 (73·1%) infections occurring in winter or early spring. Although the case number of mixed virus and bacteria infection was limited, such infection appeared to peak in winter 2004 (6, 35·3%) and summer 2005 (5, 29·4%). In the summer, rotavirus tended to be the predominant viral agent identified in patients who were co-infected with both viruses and bacteria, while in winter, rotavirus, norovirus, or enteric adenovirus have been identified as the co-infecting agent with bacteria.
The present study showed a significantly higher incidence (22·8%) of polymicrobial enteric infection in children hospitalized with AGE, compared to previous studies on the same issue. This may be due to the improvement of viral detection methods used in the study [Reference Chen6–Reference Hori9]. Rotavirus had the major role in mixed viral infections [78·8% (41/52)]. Among the infections caused concomitantly by virus and bacteria, rotavirus still played the most common role, with the majority of the events (70·6%) involving rotavirus. Campylobacter and Salmonella were the two major agents in combined virus and bacteria infections.
An earlier study showed that polymicrobial infections caused similar severity but longer lasting diarrhoea than rotavirus infection alone [Reference Uhnoo, Olding-Stenkvist and Kreuger10]. In that study, a similar disease severity was found in AGE caused by rotavirus alone, multiple viruses, and virus along with bacteria. However, in a more detailed analysis of each symptom associated with AGE, mixed viral infections resulted in higher incidence, frequency, and duration of vomiting, while the virus and bacteria co-infection manifested with more diarrhoea. These significant clinical differences might be due to the effect of overwhelming enteric bacterial infection.
Mixed virus infection, the majority of which involved rotavirus, was prevalent in the colder season. This might be related to the predominance of rotavirus infection in the winter and early spring. We also found that in summer, only rotavirus caused mixed infection with bacteria. In Taiwan, viral AGE of any cause usually peaked in colder seasons, while in summer (from June to August), the majority of the cases were caused by rotavirus [Reference Chen11]. In developed countries, viral AGE also peaked in colder seasons, although some observations indicated an increasing trend of viral AGE in the summer [Reference Hall12, Reference Koopmans and Van Asperen13]. Similarly, bacterial enteric infection occurred throughout the year in countries with a warmer climate, such as Taiwan. Thus, we see the occurrence of combined bacterial and viral infection throughout the year, while the co-infecting virus can be different from season to season.
In conclusion, AGE caused by multiple enteric pathogens are not rare in hospitalized patients in Taiwan. Rotavirus has the most important role in not only mixed viral infection but also in viral and bacterial co-infection. It is also the single most important pathogen causing AGE in young children. Effective prevention of rotavirus-associated gastroenteritis by rotavirus vaccine has been demonstrated in an overall reduction of 85–95% of cases with severe AGE of any cause [Reference Vesikari14, Reference Ruiz-Palacios15]. Immunization by rotavirus vaccine may, therefore, be a solution to the clinical and public health problem of polymicrobial enteric infections in many countries.
ACKNOWLEDGEMENTS
We are grateful to all our colleagues in the Divisions of Paediatric Gastroenterology and Paediatric Infectious Diseases of Chang Gung Children's Hospital for their help in the collection of specimens. This research was supported by grant CMRPG350391 from the Chang Gung Memorial Hospital.
DECLARATION OF INTEREST
None.




