INTRODUCTION
Influenza surveillance can provide public health officials and clinicians with early detection and situational awareness of influenza activity by determining the timing, location and degree of influenza circulation and associated diseases [Reference Thompson, Comanor and Shay1, 2]. Traditional data sources for influenza surveillance, such as positive influenza laboratory tests and medically attended influenza-related illnesses, are typically reported to public health with a delay of as much as 1–2 weeks [Reference Nsoesie3]. Consequently, public health researchers have sought novel electronic data sources that can provide timely information for rapid influenza outbreak detection [Reference Dailey, Watkins and Plant4, Reference Shaman5].
It is estimated that there are over 12 million clinic visits per year for influenza in the United States [Reference Hsieh6]. Of these, about 10–20% receive a prescription for antiviral treatment [Reference Hsieh6, Reference Havers7]. The neuraminidase inhibitors (NIs), oseltamivir and zanamavir, are currently the recommended first-line therapy for influenza [Reference Aoki8]. Monitoring NI dispensings from community pharmacy drug sales databases has emerged as a possible automated source of timely information regarding influenza [Reference Greene9].
Influenza activity in the outpatient setting tends to occur before an increased incidence of more severe disease is observed [Reference Brownstein, Kleinman and Mandl10–Reference Miller12]. Consequently, we hypothesized that changes in the weekly volume of outpatient antiviral prescriptions might precede changes in weekly counts of positive influenza tests and that NI dispensing could serve as an early indicator of epidemic influenza activity. To test our hypothesis, we assessed the timeliness, correlation, and predictive accuracy of retail pharmacy NI dispensing data in relation to laboratory-confirmed influenza activity in Quebec, Canada. Our secondary objective was to compare the above characteristics of the NI dispensing data to those of an established influenza surveillance data source, emergency department (ED) visits for influenza-like illness (ILI).
METHODS
Overview and study design
In this ecological study, we compared three weekly time series: (1) counts of NI dispensing, (2) counts of ILI ED visits, and (3) counts of positive influenza laboratory tests (as a reference measure of influenza circulation) over the 3 years 4 July 2010 to 29 June 2013, in the province of Quebec, Canada. We evaluated three key performance characteristics of NI dispensing and ILI ED time series relative to the time series of positive influenza laboratory tests: timeliness, correlation and predictive accuracy [Reference Lombardo and Buckeridge13].
Ethics approval was granted by the McGill University Faculty of Medicine Institutional Review Board.
Outcomes
Timeliness, or lead time, was assessed by measuring the lead/lag relationship of a time series (NI dispensing, or ILI ED visits) with the time series of positive influenza laboratory results. To measure the lead/lag relationship, we applied the cross-correlation function (CCF) analysis and fit Box–Jenkins transfer function models (see Data analysis section). A data source was considered timely if it demonstrated statistically significant cross-correlations at lags ⩾0, i.e. when it was lagged relative to the reference series so as to precede it. Strength of correlation (Pearson's R) [Reference Taylor14] was measured by the greatest significant cross-correlation across all lags in the CCF. Finally, predictive accuracy was estimated by fitting multivariable transfer function models to the laboratory-confirmed influenza time series. We defined the best model as the model with the lowest corrected Akaike's Information Criterion (AICc).
Sources of data
Provincial sentinel laboratory surveillance
The Institut national de santé publique du Québec performs laboratory-based surveillance for influenza year-round [15]. Aggregate weekly counts of laboratory-confirmed influenza A or B detection and the number of tests performed in participating hospitals are collated and disseminated publicly. Although the participating virology laboratories are located in hospital centres, specimens may originate from patients of any age and from various clinical settings, such as community or hospital outpatient clinics, EDs, and acute-care or long-term care inpatient wards. Nevertheless, based on published national and international guidelines for influenza testing, it is expected that most results originate from hospitalized patients or from patients at risk of severe outcomes [Reference Aoki8, Reference Harper16]. All 18 Quebec health regions are represented in the surveillance programme and the number of hospitals providing data was stable throughout the study period (44 in 2010–2011, 45 in 2011–2012, 46 in 2012–2013).
NI dispensing
Aggregate weekly counts of NI prescriptions dispensed to outpatients in Quebec retail (non-hospital) pharmacies were obtained from IMS Brogan's Canadian Weekly CompuScript proprietary drug use database. The number of pharmacies providing weekly data ranged from 1071 to 1080 (>60% of all Quebec retail pharmacies) over the study period.
ED visits for ILI
Aggregate weekly counts of visits for ILI to a Quebec ED were obtained from the Daily Report on the Situation in Emergency Departments and Hospitals database. Each acute-care hospital in Quebec is required to report the daily number of patients registering with the ED who present with the chief complaint of ILI, defined as fever and cough, as well as the total number of patients registering with the ED for any reason.
Data analysis
Analyses were performed using R version 2.14 (www.r-project.org) and SAS v. 9.3 (SAS Institute Inc., USA).
CCF: analysis of timeliness and correlation
The CCF is a frequently used measure of the association between two time series; it quantifies their correlation over a range of time lags [Reference Dailey, Watkins and Plant4, Reference Chan11, Reference Bowie and Prothero17]. However, the empirical CCF (estimated from the raw data) is highly prone to bias when one or both data series exhibit temporal autocorrelation. Because of the autocorrelation within each individual series, the empirical CCF of two unrelated time series can display significantly high but spurious correlations. Moreover, estimates may be confounded by the effect of seasonal covariates [Reference Bowie and Prothero17–Reference Fisman19]. Furthermore, long-scale phenomena, such as seasonality, tend to overwhelm the CCF, obscuring short-scale fluctuations that are more relevant to the surveillance of influenza outbreaks [Reference Bloom, Buckeridge and Cheng20]. To remove the effects of autocorrelation on the CCF, we first filtered (i.e. ‘pre-whitened’) the NI dispensing data by fitting an autoregressive integrated moving average (ARIMA) model [Reference Helfenstein18, Reference Bloom, Buckeridge and Cheng20, 21]. That model was then applied to the laboratory surveillance reference time series, and the CCF of the two residual series was estimated [22]. From this CCF, we identified the lags at which the maximum and earliest statistically significant correlations occurred. A two-sided P value < 0·05 was considered statistically significant. We also used this approach to estimate the CCF of the ILI series with the laboratory-confirmed series. We also performed the CCF analyses separately for each of the three study seasons to evaluate if our results would be consistent across influenza outbreaks.
Transfer function models
We assessed the value of NI dispensing data for the prediction of influenza activity using ARIMA modelling [Reference Helfenstein18, 21, Reference Gilca23]. Bivariate and multivariate Box–Jenkins transfer function models were developed to describe the relationship between the pre-whitened output series (laboratory surveillance data) and each of the pre-whitened input series (NI dispensing and ILI ED visits). Only positive lags with a significant cross-correlation were considered. Log-transformations of the series were considered to meet model assumptions. The AICc of the ARIMA model of the output series was used as a yardstick when comparing the informative potential of different transfer function models. An input series was considered to be a useful predictor if its inclusion lowered the AICc.
Sensitivity analyses
To assess if our results were robust to the use of a proportion as opposed to counts, we re-ran our CCF and prediction model analyses using the weekly proportion of positive influenza tests (weekly count of positive laboratory influenza tests/weekly count of influenza tests performed) as the reference time series and the weekly proportion of ILI ED visits (weekly count of ILI ED visits/weekly count of total ED visits) instead of ILI ED counts. No denominator for NI dispensing was available in our dataset.
RESULTS
NI dispensing
There were 21 066 NI prescriptions dispensed during the study period (5550 in 2010–2011, 3347 in 2011–2012, 12169 in 2012–2013). Of these dispensings, 20 999 (99·7%) were for oseltamivir.
Description of the 2010–2011, 2011–2012 and 2012–2013 influenza seasons
The influenza types and subtypes circulating in Quebec during each season of the study period are described in Table 1. The 2010–2011 and 2012–2013 influenza seasons were both characterized by a predominance of A(H3N2) (87% and 78% of strains, respectively). However, the intensity of the 2012–2013 epidemic was marked, with peak weekly counts that were more than twice as high as in 2010–2011, and four times greater than during the relatively mild 2011–2012 season (Fig. 1). In 2011–2012, the outbreak was briefer and peaked later (March 2013), with concomitant circulation of both influenza A and B in roughly equal proportions (45% and 55%, respectively).
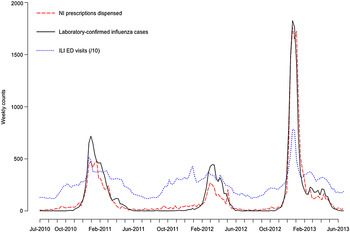
Fig. 1. Time-series plots of the weekly counts of neuraminidase inhibitor (NI) prescriptions dispensed, acute-care hospital emergency-department (ED) visits for influenza-like illness (ILI), and laboratory-confirmed cases of influenza in Quebec, Canada, 2010–2013.
Table 1. Circulating influenza strains, by season, from 2010 to 2013 in Quebec, Canada

* Estimated percentage of total circulating strains for that year, based on genetic and antigenic characterization of a sample of provincial surveillance viral isolates from throughout the season.
† Strains not included in that year's trivalent seasonal influenza vaccine.
Bold font indicates the predominant circulating strain for that season.
Description of the NI and ILI series in relation to the influenza series
The NI series closely tracked the laboratory influenza series. The ILI ED series also exhibited a similar seasonality; however, there was a considerable volume of ILI presenting to Quebec EDs year-round, even when little or no influenza was circulating. Moreover, in 2011–2012, the peak in weekly ILI ED visits occurred in early January, before the seasonal influenza epidemic began.
CCF: correlation and timeliness (Table 2)
In the overall analysis, the CCFs for the NI series with the laboratory series demonstrated that NI dispensing temporally coincided with (maximal correlation at lag 0) and was strongly correlated with (correlation of 0·68) laboratory-confirmed influenza activity. Assessing the CCF of these two series separately for each of the three seasons, this result was consistently observed over the entire study, with maximal correlations of ⩾0·5 occurring at lag 0 and the earliest significant correlation at lag 1 in each individual season. The magnitude of the peak correlation with influenza activity was stronger for the NI series than the ILI series overall (0·68 vs. 0·50) and in two of the three seasons (0·50 vs. 0·67 in 2010–2011, 0·54 vs. 0·33 in 2011–2012, 0·73 vs. 0·61 in 2012–2013). The timeliness of the ILI series showed mild variability in the year-to-year analysis, with peak correlations and earliest significant correlations occurring at either lags 0 or 1.
Table 2. Correlation coefficients in the cross-correlation functions between the time series of neuraminidase inhibitor prescription dispensing, acute-care hospital emergency-department visits for influenza-like illness, and a common reference time series of laboratory-confirmed influenza cases in Quebec, Canada, 2010–2013

NI, Neuraminidase inhibitor; ILI, influenza-like illness; ED, emergency department.
Prediction models
Using NI dispensing or ILI ED visits as input series in separate single-input Box–Jenkins transfer function models improved the fit of a predictive model for weekly counts of laboratory-confirmed influenza cases (Table 3). In a multivariable model, both series were significant predictors of influenza counts. Including NI dispensing data at a lag of 2 weeks in this model optimized fit.
Table 3. Fitted univariate ARIMA model, and bivariate and multivariable Box–Jenkins transfer function models for the prediction of weekly cases of laboratory-confirmed influenza infection

ARIMA, Autoregressive integrated moving average; AICc, Akaike's Information Criterion corrected; n.a., not applicable; NI, neuraminidase inhibitor; ILI, influenza-like illness; ED, emergency department.
* Where p represents the order of the autoregressive term, d represents the order of differencing, and q represents the order of the moving average term.
† The positive lag (i.e. lead time, when the input series is lagged to precede the reference indicator output series) with a statistically significant cross-correlation that minimized the AICc, and optimized model fit.
‡ The P value of the coefficient for the input series.
Sensitivity analyses
Repeating the analyses using the proportion of positive influenza tests as the reference time series and the proportion of ILI ED visits instead of ILI ED counts did not affect the magnitude or the direction of the associations that we observed.
DISCUSSION
We found that NI dispensing from retail pharmacies was timely and strongly correlated with laboratory-confirmed influenza activity during the same week over three non-pandemic seasons. This association was observed after filtering to correct for autocorrelation, and is therefore a feature of the short-scale relationship between the two data streams [Reference Bloom, Buckeridge and Cheng20]. NI dispensings were also a significant predictor of laboratory-confirmed influenza activity in a multivariable model. The model's estimated predictive potential was maximal when the log-transformed NI time series was lagged to precede the log-transformed laboratory surveillance data by 2 weeks.
Traditionally, systems for monitoring influenza activity have relied on reports from diagnostic virology laboratories as their primary source of information. Such laboratory surveillance data are highly specific: all cases reported are confirmed influenza infections. However, in Quebec, as in many jurisdictions [Reference Chretien24, Reference Sugawara25], sentinel laboratories must first manually submit weekly data to a web portal and there is typically a delay of 1–2 weeks before the results are published. Because all prescriptions in Quebec are subject to electronic adjudication at the time of dispensing, the monitoring of NI sales represents a potentially feasible, less laborious, inexpensive, and automated surveillance method.
Previous studies in Quebec and elsewhere have shown that ambulatory care or ED-based syndromic surveillance data for acute respiratory illness or ILI can provide earlier signals for seasonal influenza outbreaks compared to measures of more severe disease, such as hospitalizations or mortality due to pneumonia and influenza [Reference Brownstein, Kleinman and Mandl10–Reference Miller12]. Because community pharmacy NI dispensing represents milder infections treated as outpatients, we hypothesized that the NI data would lead the laboratory surveillance series, as the latter is primarily representative of patients hospitalized with severe disease [26]. Conversely, if clinicians’ knowledge of current levels of influenza circulation, based on laboratory surveillance reports, very strongly influences antiviral treatment, NI dispensing would lag behind this reference indicator. In our CCF analysis, however, the maximal cross-correlation between the NI and laboratory surveillance data was at lag 0 and we also observed a significant cross-correlation at a lead time of 1 week. Moreover, when included in a regression model, the greatest predictive value of the NI series was with a lead time of 2 weeks. Taken together, our observations suggest that surveillance of NI prescriptions could provide an early indication of epidemic influenza activity. Moreover, given that NI dispensing data are collected automatically, it should be possible to conduct surveillance using these data with little or no reporting delay.
Earlier studies have suggested that prescription drug dispensing might offer timely information regarding influenza activity, without necessarily quantifying a lead-lag relationship to an established reference time series. Using a space–time permutation scan statistic, Greene et al. compared the performance of 10 types of electronic clinical data, including antiviral dispensing, for the detection of clusters of illness related to influenza during the 2007–2008 season in Northern California [Reference Greene27]. Antiviral dispensing provided the earliest signal for one of the clusters, detected two of the four events, and produced no false alarms. During the second wave of the 2009 pandemic in Ontario, Canada, Aramini et al. found that A(H1N1) counts were associated with NI prescriptions in a Poisson regression analysis; statistical significance was greatest when the series were not lagged (P < 0·001) [Reference Aramini, Muchall and Pollari28].
To date, assessments of the correlation between antiviral prescribing and influenza activity, have not accounted for autocorrelation and seasonality. Therefore, these estimates are likely to be biased. Furthermore, in contrast to our study, none of the prior work on NI dispensing compared it to more than one traditional data source, which is necessary to understand its usefulness in context with currently used methods. In Japan, local and national-level dispensing data demonstrated empirical correlations (R) of >0·95 with ILI sentinel surveillance data [Reference Sugawara25, Reference Yoshida29]. In the national study, empirical correlations during the 2009 A(H1N1) pandemic and the following season (2010–2011) were very similar (R = 0·992 and 0·972, respectively) [Reference Sugawara25]. However, there was modest variability between prefectures and the lowest regional correlation was 0·689. In the United States Vaccine Safety Datalink Project, differences were observed between eight different medical care organizations. The empirical correlations of weekly antiviral dispensings with the proportion of tests positive for influenza ranged from 0·34 to 0·72 [Reference Greene9].
As with all forms of syndromic surveillance, antiviral dispensings cannot be as specific an indicator of influenza activity as laboratory data. Several factors may contribute to ‘false-positives’, i.e. occurrences of NI dispensing that do not represent an incident case of influenza infection. First, while we expect that the majority of prescriptions were for treatment of acute illness, our data did not allow us to assess indication. Prophylaxis has been estimated to be the indication for <10% of antiviral dispensing in the 2000–2010 Vaccine Safety Datalink Project study [Reference Greene9]. That proportion is probably even smaller in our Quebec data; since the 2009 A(H1N1) pandemic, early treatment is preferred over prophylaxis due to concerns regarding emergence of drug resistance [Reference Aoki8, Reference Baz30]. Although personal stockpiling of antivirals is discouraged [Reference Brett and Zuger31], evidence of such activity has been reported when spikes in NI sales coincided with media coverage of highly pathogenic H5N1 influenza, but not with other markers of influenza activity [Reference Ortiz32]. Significant amounts of stockpiling might therefore trigger a false alarm for an influenza outbreak. However, if the monitoring of NI dispensing is performed as part of a multistream surveillance programme, it offers the opportunity for public health officials to recognize inappropriate prescribing and intervene to reduce the risk of lack of availability of treatment for those that need it most [Reference Brett and Zuger31, Reference van den Wijngaard33].
Outpatient antiviral treatment of influenza is rarely based on laboratory testing, in large part because test results are not available during the patient encounter. It has been estimated that only 3–6% of outpatients treated with antivirals in the United States were tested for influenza [Reference Greene9]. NIs are therefore being prescribed empirically, based on a clinical syndrome [Reference Aoki8]. However, other respiratory viruses that may temporally co-circulate with influenza, such as respiratory syncytial virus, also frequently cause ILI, especially outside of peak periods of influenza activity [Reference Zambon34]. Accordingly, influenza virus was the cause of ILI in 652/1501 (43%) participants that presented to a Canadian community-based sentinel clinic surveillance system in 2012–2013 [Reference Skowronski35]. Although we did not measure the impact of co-circulating respiratory viruses, our NI dispensing series was clearly more specific than our ILI ED data and trends in NI dispensing and laboratory surveillance data were very closely associated, even after correcting for seasonality. We believe that prescribers’ prior knowledge of circulating levels of influenza contributes to, but cannot fully account for, this phenomenon. Laboratory surveillance results take >1 week before being published and our NI data coincided with or led laboratory surveillance by 1–2 weeks. Fiejté et al. [Reference Fiejté36] reported that, during the second wave of the 2009 A(H1N1) pandemic, in Utrecht, The Netherlands, among patients prescribed oseltamivir in the community setting, those in whom the decision to treat was in accordance with national guidelines more frequently started their course of therapy compared to those in whom the treatment decision was deemed inappropriate (97·4% vs. 55·9%, P < 0·001). Thus, it appears that patients’ behaviour after the medical visit affects NI dispensing counts and that those with more severe symptoms or those more likely to be infected with influenza may also be more likely to complete their prescription.
One limitation of our study is that the data did not allow for age-stratified analyses. Patients’ age influences influenza transmission dynamics and, consequently, data timeliness. Studies using laboratory-based [Reference Schanzer, Vachon and Pelletier37] and syndromic surveillance [Reference Brownstein, Kleinman and Mandl10, Reference Chan11] have demonstrated that data from children offer the earliest lead times. Identifying the age groups that provide the timeliest data might further improve the utility of monitoring antiviral dispensings. We were also unable to determine if NI data timeliness and correlation with influenza activity were consistent geographically across Quebec. Therefore, though our data were collected province-wide, results may not be valid for all regions. Another caveat is that NI dispensing data may not perform similarly during a pandemic. Moreover, indications for NI treatment and NI prescription rates vary significantly across countries [Reference Kramarz38]. Consequently, our results may not be applicable in jurisdictions without similar access to prescription drugs. In addition, because we did not have weekly data on circulating influenza A subtypes, we could not evaluate their specific effect using our model. Further, estimating true influenza incidence from laboratory or ILI surveillance remains a challenge [Reference Thompson, Comanor and Shay1, Reference Gilca23, Reference Thompson39]. Therefore, any reference indicator used to assess NI dispensing will be imperfect and results may vary based on the choice of comparator. Finally, there are no data available on the proportion of patients with influenza that receive antiviral treatment in the outpatient setting in Quebec or Canada, nor do we know the factors associated with Canadian physicians’ decisions to prescribe influenza antivirals.
We took several measures to ensure the validity of our results. First, we pre-whitened the time series prior to estimating correlations between them, removing autocorrelation and thereby reducing the possibility of biased measures of association [Reference Bowie and Prothero17]. Further, by filtering the effect of seasonality, we focused on the short-scale features of the lead-lag relationships to produce estimates applicable to the detection of rapidly evolving influenza outbreaks [Reference Bloom, Buckeridge and Cheng20]. Furthermore, the choice of ARIMA methodology for building prediction models is well suited for shorter-term forecasting, as it places greater weight upon recent past values [21]. Finally, year-by-year analyses demonstrated that our estimates were stable to variations in epidemic season timing, duration, overall severity, peak intensity and antigenic characterization of the predominant strains.
In summary, the correlation, timeliness and predictive ability of NI dispensing data in relation to laboratory-confirmed influenza activity suggests that this readily available data stream could act as a leading indicator for outbreak detection. Monitoring NI dispensing, especially in parallel to traditional sources of surveillance data, should increase public health practitioners’ situational awareness of influenza activity, thereby facilitating timely interventions and resource management.
ACKNOWLEDGEMENTS
We thank Patricia Fontela, MD, PhD for thoughtful review of this manuscript; Hugues Charest, PhD and Michel Couillard, PhD for kindly providing the laboratory surveillance data; Monique Douville-Fradet, MD, MHSc for kindly providing RQSUCH data; IMS Brogan for generously providing the antiviral dispensing data.
No funding sources were used for this study.
DECLARATION OF INTEREST
G.D.S. has received research funds from GlaxoSmithKline and Sanofi-Pasteur for unrelated studies. The remaining authors do not have a commercial or other association that might pose a conflict of interest.







