INTRODUCTION
Invasive alien species (IASs) are species introduced outside their native range by human action that go on to have significant negative impacts on the recipient environment (Jeschke et al. Reference Jeschke, Bacher, Blackburn, Dick and Essl2014; Russell & Blackburn Reference Russell and Blackburn2017), primarily on biodiversity values and ecosystem services. They differ from pest species, which might be either native or introduced, and primarily impact agricultural values, although a species can be both a pest and invasive when it impacts the entire suite of values. For those islands of the world that have only been colonized by humans recently (in the past millennium), the delineation of when human biotic disturbance and introduction of species first occurred is most clear (Delgado et al. Reference Delgado, Riera, Rodríguez, González-Moreno and Fernández-Palaciosin press), and IASs are only the subset of introduced species that have overwhelmingly negative impacts; in other words, cross a damage threshold to biodiversity values (Bartz et al. Reference Bartz, Heink and Kowarik2010).
Although there are many studies of IASs on islands, most of these have been taxon specific and focused on IAS impacts and management (e.g. Courchamp et al. Reference Courchamp, Chapuis and Pascal2003; Jones et al. Reference Jones, Holmes, Butchart, Tershy and Kappes2016; McCreless et al. Reference McCreless, Huff, Croll, Tershy and Spatz2016). Reaser and colleagues (Reference Reaser, Meyerson, Cronk, De Poorter and Eldrege2007) comprehensively reviewed the ecological and socioeconomic impacts of IASs on islands. Other studies have compared invasions on continents to islands from biological (e.g. Vitousek Reference Vitousek, Wilson and Peter1988; D'Antonio & Dudley Reference D'Antonio, Dudley, Vitousek, Loope and Adsersen1995) and macroecological perspectives (van Kleunen et al. Reference van Kleunen, Dawson, Essl, Pergl and Winter2015; Bellard et al. Reference Bellard, Cassey and Blackburn2016). In this review, we broaden the scope of previous reviews of IASs on islands by summarizing both the impacts and distribution of IASs on islands, their interactions and their management. We outline the diverse impacts IASs can have on islands, and then using a novel dataset of IAS distributions on United Nations small-island developing states (SIDSs) investigate patterns in IAS distribution across geographic regions on this subset of islands. We examine how IASs on islands interact with other major global change threats, and then discuss the roles of biosecurity, control and eradication in IAS management on islands. We conclude with future directions in IAS research and management specifically focused on islands.
IMPACTS
On islands, IASs have disproportionate impacts compared to on continents (Vitousek Reference Vitousek, Wilson and Peter1988; Bellard et al. Reference Bellard, Cassey and Blackburn2016), and native species on islands are disproportionately vulnerable to IASs compared to other threats because of attributes including a lack of behavioural, life-history and certain morphological characteristics (Vitousek Reference Vitousek, Wilson and Peter1988; Tershy et al. Reference Tershy, Shen, Newton, Holmes and Croll2015). The impacts of IASs on islands are often more readily demarcated, particularly when confounding threats are absent (e.g. Towns et al. Reference Towns, Atkinson and Daugherty2006). Historically, the focus of IAS impacts has been on biodiversity values, but vulnerability on islands to IAS impacts also extends to agriculture, economies, health and cultures. These have tended to be under-considered compared to, or considered independently of, their ecological impacts on species and ecosystems. We briefly summarize the broad forms of IAS impacts under each of these headings, but refer readers to the extensive review of IAS impacts on islands by Reaser et al. (Reference Reaser, Meyerson, Cronk, De Poorter and Eldrege2007).
Biodiversity
The small size of islands leads to smaller populations, and their isolation creates evolutionary distinctiveness (Losos & Ricklefs Reference Losos and Ricklefs2009), species impoverishment (Simberloff Reference Simberloff2000) and taxonomic disharmony (Williamson Reference Williamson1981) with the absence of some functional groups (Cushman Reference Cushman, Vitousek, Loope and Adsersen1995), which together create greater vulnerability to the impacts of IASs (Vitousek Reference Vitousek, Wilson and Peter1988; Tershy et al. Reference Tershy, Shen, Newton, Holmes and Croll2015). IASs generate negative impacts through a number of trophic and ecosystem interaction pathways. Introduced predators, such as terrestrial mammals or invertebrates, can induce strong predator–prey dynamics and rapidly cause the extirpation (i.e. local extinction) of native species populations. Introduced competitors, such as birds, reptiles and plants, can alter competition dynamics and cause reductions in the abundance, or sometimes extirpations, of native species populations. In some cases, IASs can subsidize other native and introduced species in ecosystems (Roemer et al. Reference Roemer, Donlan and Courchamp2002; Abernethy et al. Reference Abernethy, Turner, Beasley, DeVault, Pitt and Rhodes2016) and participate in novel indirect effects (Russell Reference Russell, Mulder, Anderson, Towns and Bellingham2011).
There are many examples of extinctions or extirpations of endemic animal species (land birds, reptiles, land snails and aquatic insects) caused by the introduction of predators (rats, cats, mongooses, snakes, carnivorous snails, freshwater fishes, ants and raptors). The introduction of herbivores (e.g. rabbits, feral goats, sheep, deer, pigs, horses and cattle) can impact native island vegetation as well as cause habitat loss and erosion and alter nutrient dynamics. The impacts of invasive plants are less documented and more difficult to assess (with very few if any native plant extinctions), but plant and animal invaders can contribute to alterations of both ecosystem services and dynamics (e.g. soil erosion, nutrient cycling, fire regime and water content; Fukami et al. Reference Fukami, Wardle, Bellingham, Mulder and Towns2006; Meyer Reference Meyer2014; Downey & Richardson Reference Downey and Richardson2016).
Agriculture
Agriculture on islands relies on the local environment, although the products themselves are often non-native. This production typically benefits from island isolation and the absence of pests (mainly weeds, arthropods and pathogens) found elsewhere. Indeed, many IASs are also agricultural pests, and thus their exclusion benefits both agriculture and biodiversity. The costs of IASs to agriculture in the USA have been estimated at over US$120 billion per year (Pimentel et al. Reference Pimentel, Zuniga and Morrison2005), and for invasive insects globally at over US$70 billion per year (Bradshaw et al. Reference Bradshaw, Leroy, Bellard, Roiz and Albert2016), but estimates for IAS impacts on islands are decidedly lacking (though see Mwebaze et al. Reference Mwebaze, MacLeod, Tomlinson, Barois and Rijpma2010). Island biosecurity benefits from the duality of IASs and agricultural pests, which can lead to strong border controls against IASs (e.g. Kriticos et al. Reference Kriticos, Phillips and Suckling2005), but species impoverishment on islands has been a primary driver of species introductions in the past, such as those by acclimatization societies, forestry departments, and botanical gardens (Veltman et al. Reference Veltman, Nee and Crawley1996; Hulme Reference Hulme2011). Agricultural introductions can thus be major sources of new IASs on islands (Driscoll et al. Reference Driscoll, Catford, Barney, Hulme and Martin2014), including escaped livestock and animal species being introduced for putative biocontrol without host-specificity tests (Secord & Kareiva Reference Secord and Kareiva1996; Simberloff & Stiling Reference Simberloff and Stiling1996).
Economy
Like their ecosystems, island economies tend to be less diversified (Briguglio Reference Briguglio1995). Particularly for SIDSs, there is often a reliance on only a few revenue streams, which are often tightly coupled with the terrestrial and marine environments (Pelling & Uitto Reference Pelling and Uitto2001). Disruption of these ecological environments thus has downstream impacts on island economies, in the worst cases potentially causing complete collapses of industries (Bunce et al. Reference Bunce, Mee, Rodwell and Gibb2009). More recently, tourism has become a major component of island livelihoods (Wilkinson Reference Wilkinson1987). Although much tourism on islands is generally independent of ecological values, ecotourism draws specifically from the isolation and ecological uniqueness of islands (Uyarra et al. Reference Uyarra, Cote, Gill, Tinch, Viner and Watkinson2005), and thus biotic homogenization from species introduction and invasion directly erodes trade and tourism value (Jay et al. Reference Jay, Morad and Bell2003).
Health
Due to lack of previous exposure, both human and non-human island communities are more vulnerable to introduced diseases (Daszak et al. Reference Daszak, Cunningham and Hyatt2000; Crump et al. Reference Crump, Murdoch and Baker2001). Whereas the impact of foreign diseases on human communities on islands is well recorded (Mazza et al. Reference Mazza, Tricarico, Genovesi and Gherardi2014), the effect of introduced diseases on biodiversity is less well appreciated (Young et al. Reference Young, Parker, Gilbert, Guerra and Nunn2017). However, there are striking examples of introduced diseases that are severely impacting the persistence of island species (Beever et al. Reference Beever, Waipara, Ramsfield, Dick, Horner, Goheen and Frankel2009). Furthermore, some IASs introduced to islands can be vectors for diseases, amplifying IAS impacts (e.g. introduced birds and avian malaria, rodents and leptospirosis, and mosquitoes and flaviviruses). Management of IASs is predicted to create important improvements in public health alongside biodiversity (de Wit et al. Reference de Wit, Croll, Tershy, Newton and Spatz2017).
Culture
The factors that generate unique species assemblages on islands also generate unique cultures and identities (Lionnet Reference Lionnet2011; Braje et al. Reference Braje, Leppard, Fitzpatrick and Erlandsonin press). Even today, isolation on islands attracts and promotes place identities of individuals and communities that differ from other areas (Camperio Ciani et al. Reference Camperio Ciani, Capiluppi, Veronese and Sartori2007). Islanders tend to be identified as rugged, independent and stoic (Russell et al. Reference Russell, Taylor and Aleyin press). These identities are consolidated over generations living on islands and can even become genetically embedded (Camperio Ciani & Capiluppi Reference Camperio Ciani and Capiluppi2011). Just like endemic species, these endemic island cultures are vulnerable to IASs and the ensuing biotic homogenization (Tershy et al. Reference Tershy, Shen, Newton, Holmes and Croll2015). Biological invasions can erode traditional ecological knowledge (Moller Reference Moller2009) and drive people from their traditional lands, buildings and ways of life (Lee et al. Reference Lee, Motoki, Vanderwoude, Nakamoto and Leung2015). They can be a threat to cultural monuments (e.g. the giant statues or ‘moai’ in Easter Island Rapa Nui) and to the cultural and natural integrity of United Nations Educational, Scientific and Cultural Organization (UNESCO) World Heritage Sites. However, some IASs that have been established for a long time can also adopt positive cultural value through their integration into cultures (Nuñez & Simberloff Reference Nuñez and Simberloff2005).
DISTRIBUTION
With the rapid increase in global trade over the past few centuries has come the concomitant increase in transportation of IASs (Westphal et al. Reference Westphal, Browne, MacKinnon and Noble2008). IASs are now distributed across the planet and although only a subset of IASs are globally distributed, many more IASs are regionally and locally significant (Courchamp et al. Reference Courchamp, Fournier, Bellard, Bertelsmeier, Bonnaud, Jeschke and Russell2017). Islands have tended to accumulate higher numbers of IASs per unit of land area (e.g. van Kleunen et al. Reference van Kleunen, Dawson, Essl, Pergl and Winter2015), which was historically interpreted as a vulnerability of islands to IAS colonization, in addition to their known vulnerability to IAS impacts (D'Antonio & Dudley Reference D'Antonio, Dudley, Vitousek, Loope and Adsersen1995). This colonization vulnerability has been ascribed to the impoverishment of native biota on islands (taxonomic disharmony, a lack of certain functional groups and ‘vacant niches’) and of evolution in long isolation from continental influences and pressures (e.g. strongly top-down predation, grazing mammals and diseases) not found on islands (Vitousek Reference Vitousek, Wilson and Peter1988). However, IASs have also been disproportionately introduced to islands (‘propagule pressure’) in an effort to enrich island biotas, and so islands may not be any more vulnerable to invasion than any other site (Jeschke & Genovesi Reference Jeschke and Genovesi2010). Thus, it is now generally considered that the greater number of IASs on islands per unit of area reflects higher propagule pressure to islands compared to continents (Denslow Reference Denslow2003). Although biological invasions of islands increase species richness relative to the extinctions caused by IASs and other threats, globally biological invasions are reducing biodiversity, as few species are introduced widely, while many more unique native species go extinct (Sax & Gaines Reference Sax and Gaines2003).
Small-island developing states
Expert-verified lists of IASs on SIDSs are available from the Global Register of Introduced and Invasive Species (GRIIS) version 2.1. We use these data to analyse the occurrence, status and impact (sensu Latombe et al. Reference Latombe, Pyšek, Jeschke, Blackburn and Bacherin press) of terrestrial and aquatic IASs across four SIDS geographic regions comprising 33 self-governing, wholly island nations located in the Atlantic (n = 2), Caribbean (n = 13), Indian (n = 4) and Pacific (n = 14) regions. We exclude Bahrain in the Arabian Sea and Singapore in the South China Sea (sole geographical representatives in their region and connected to the mainland), along with continental (Belize, Guinea-Bissau, Guyana and Suriname) and sub-island (East Timor) SIDSs.
Our analysis excludes island territories and dependencies of continental nations that are not SIDSs (see also Keppel et al. Reference Keppel, Morrison, Watling, Tuiwawa and Rounds2012), and we caution that patterns of IAS introduction to and distribution on these islands with closer continental trade and governance links may differ (Costello et al. Reference Costello, Springborn, McAusland and Solow2007). In the GRIIS, invasive species are defined as those that are known somewhere in the world to have impacts on biodiversity, ecosystems and the services they provide. We analyse records of all IASs in SIDSs regardless of whether impact has yet been observed on an island.
Across our 33 SIDSs, we documented 8668 presence records for 2034 potential IASs, comprising 76% plants, 23% animals and the remaining 1% fungi, chromists, viruses, bacteria and protozoa. Invaded environments were 83–88% terrestrial and 9–14% aquatic (variation arising from IASs present in both habitats), with the final 3% being host specific. Evidence of invasiveness was documented for just over half (53%) of these species on at least one SIDS (for the other half it was not specified rather than necessarily absent). For 5% of IAS records in SIDSs, the origin was unknown and the species classified as cryptogenic (i.e. potentially in native range).
Patterns
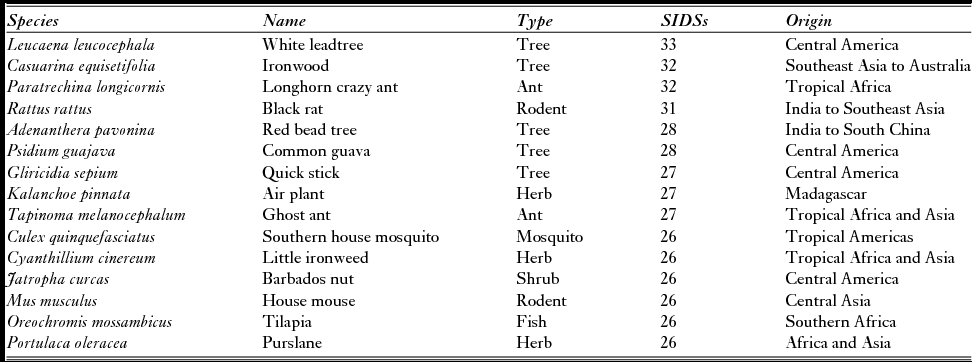
Just under half (45%) of IASs were only recorded in one SIDS, and only 53 IASs (2.6% of the IASs in SIDSs) were recorded in all four regions, a similar nested pattern others have found for IASs on tropical islands (Traveset et al. Reference Traveset, Kueffer and Daehler2014). The prevalence of IASs in SIDSs differed significantly among regions, with IASs more likely to be present in multiple SIDSs in the Pacific Ocean (Fig. 1). The top 15 IASs, present in 26 or more SIDSs around the world, comprised a mix of plants, ants, rodents, a mosquito and a fish, and reflected that SIDSs exclusively fall in tropical regions (Table 1). Notably, plants make up over half (60%) and are the only intentional introductions. Importantly, although these plants and ants are the most widely distributed, they are not necessarily the most damaging. This emphasizes the importance of ongoing vigilance and biosecurity to prevent the spread of other damaging IASs to islands, but perhaps also that the spread of the most damaging IASs has been purposefully limited.

Figure 1 Percentage of IASs by percentage of SIDSs by region (source: Global Register of Introduced and Invasive Species). IAS = invasive alien species; SIDS = small-island developing state.
Table 1 Fifteen of the invasive alien species most globally prevalent on islands (source: Global Register of Introduced and Invasive Species). SIDS = small-island developing state.
The SIDS with the most IASs was Cuba (n = 682), while that with the fewest was Sao Tome and Principe (n = 46), although we caution that the latter almost certainly reflects a lack of documentation effort. We investigated the relationship between IAS richness in SIDSs with their population size (median 197,541, range 1190–11,179,995), total land area (median 751 km2, range 21–462,840 km2) and coastline (median 403 km, range 24–6112 km), stratifying by region. All variables were log10 transformed to correct for right-skew. Population size, area and coastline of SIDSs are all tightly correlated, so we performed a linear regression of log10 IAS richness against the interaction of SIDS region and the absolute values of the first axis of a principal components analysis combining log10 values of all three variables. The first principal component captured 88% of the variability in SIDS population size, area and coastline. There was a strong positive relationship between IAS richness and the combination of SIDS population size, area and coastline (p = 0.006). Pacific Ocean SIDSs had significantly more IASs than other SIDS regions (p = 0.046), reflecting more IASs present at smaller SIDS sizes (Fig. 2). In the Pacific and Indian Oceans, SIDSs tended to have more IASs at smaller sizes but accumulated them more slowly, while in the Caribbean and the Atlantic Ocean, SIDSs tended to have fewer IASs at smaller sizes but accumulated them more rapidly (Fig. 2). We suspect that these trends reflect variability in the number of large islands within each SIDS, for which we do not have data. Analysis of the explanatory variables independently suggested that human population size had the stronger effect among them on IAS richness, as others have found for alien plant and bird species richness on islands (Blackburn et al. Reference Blackburn, Delean, Pyšek and Cassey2016), where trade and social rather than biogeographical factors have stronger impacts on IAS richness (van Kleunen et al. Reference van Kleunen, Dawson, Essl, Pergl and Winter2015).

Figure 2 Number of IASs in small-island developing states against a principal component axis combining small-island developing state population size, area and coastline (source: worlddata.info). Regional lines of best fit are shown. Diamonds = Pacific Ocean; circles = Caribbean Sea; triangles = Indian Ocean; squares = Atlantic Ocean. IAS = invasive alien species.
Indicators
Patterns in IASs on islands over space and time should be quantified by indicators to allow robust assessment of trends and interventions (Latombe et al. Reference Latombe, Pyšek, Jeschke, Blackburn and Bacherin press). Trends in indicators of pressure (e.g. number of IASs), state (e.g. IAS impacts) and response (e.g. international agreements and national policy adoption) have all been applied to IASs (McGeoch et al. Reference McGeoch, Butchart, Spear, Marais and Kleynhans2010). The number and accumulation of IASs (i.e. indicator pressure) in SIDSs in our analysis differed significantly by region. Other studies have shown that impacts of IASs (i.e. indicator state) are greater on islands than continents (Reaser et al. Reference Reaser, Meyerson, Cronk, De Poorter and Eldrege2007), but at the same time eradications of IASs (i.e. indicator response) from islands create meaningful reversals in native species declines (Jones et al. Reference Jones, Holmes, Butchart, Tershy and Kappes2016; Russell et al. Reference Russell, Cole, Zuël and Rocamora2016). Globally, the rate of alien species introductions has increased substantially over the past 200 years, and continues to do so, although with notable exceptions for mammals and fishes and in places such as New Zealand where stricter biosecurity is enforced (Seebens et al. Reference Seebens, Blackburn, Dyer, Genovesi and Hulme2017). However, globally consistent information is patchy for robust indicators on distribution (McGeoch et al. Reference McGeoch, Butchart, Spear, Marais and Kleynhans2010) and impact (Bellard & Jeschke Reference Bellard and Jeschke2016). Ultimately, better information for developing and monitoring indicators of IASs on islands will allow improved prioritization of IAS management on islands (McGeoch et al. Reference McGeoch, Genovesi, Bellingham, Costello, McGrannachan and Sheppard2016).
Invasion pathways comprise the vectors for transportation and the routes travelled (Essl et al. Reference Essl, Bacher, Blackburn, Booy and Brundu2015). The pathways for IASs arriving at islands are a subset of those for IASs in general, where long-distance transportation by marine vessel and aircraft naturally dominates (Hulme Reference Hulme2009). This makes the dispersal of IASs to islands different in rate and type from both continents and natural colonizations (Wilson et al. Reference Wilson, García-Díaz, Cassey, Richardson, Pyšek and Blackburn2016). Due to delays in reporting, new IASs on islands can take over a decade to enter records (Seebens et al. Reference Seebens, Blackburn, Dyer, Genovesi and Hulme2017), and an invasion debt is created by biological lag effects for IASs that have already arrived but are not yet established or expanded on islands (Essl et al. Reference Essl, Dullinger, Rabitsch, Hulme and Hülber2011). In the future, the vectors and pathways of IAS introduction to islands will continue to evolve (Hulme et al. Reference Hulme, Bacher, Kenis, Klotz and Kühn2008) and interact with other global change factors, such as climate change (Mainka & Howard Reference Mainka and Howard2010).
INTERACTIONS
Globally across continents and islands, IASs rank highly as a threat to biodiversity (Maxwell et al. Reference Maxwell, Fuller, Brooks and Watson2016). On islands alone, IASs currently rank even higher as a threat to threatened (Bellard et al. Reference Bellard, Cassey and Blackburn2016) and critically endangered species (Tershy et al. Reference Tershy, Shen, Newton, Holmes and Croll2015), and were the greatest threat to extinct species (Tershy et al. Reference Tershy, Shen, Newton, Holmes and Croll2015). However, compared to other threats, the collective impacts of IASs are often subtler, overlooked and harder to quantify and unambiguously assign (Courchamp et al. Reference Courchamp, Fournier, Bellard, Bertelsmeier, Bonnaud, Jeschke and Russell2017), leading conservation managers to often focus on other threats, even if those are more intractable to solve. We discuss the interactions of IASs with the other global change threats identified by Maxwell et al. (Reference Maxwell, Fuller, Brooks and Watson2016).
Over-exploitation
Over-exploitation of species, through logging, hunting, fishing and plant gathering, continues to be the primary threat to biodiversity (Maxwell et al. Reference Maxwell, Fuller, Brooks and Watson2016). Islands have a long history of over-exploitation (Atkinson et al. Reference Atkinson, Coomber, Passmore, Greenhill and Kushnick2016), with Easter Island being a stark example of the ecological collapse that can follow (Brander & Taylor Reference Brander and Taylor1998). IASs both interact with and benefit from over-exploitation of native species. On islands, over-harvesting of plants and animals by humans has been augmented by predation or herbivory by IASs, leading to rapid species extinctions (Cheke & Hume Reference Cheke and Hume2008) and community regime shifts (Walker & Meyers Reference Walker and Meyers2004). Subsequently, modified habitats then provide vacant niche opportunities and decreased resilience to further biological invasions (Shea & Chesson Reference Shea and Chesson2002).
Agricultural activity
Agriculture on islands has always relied on the introduction of favoured non-native species. Many of these species that were introduced for agriculture have themselves gone on to become pests and IASs (Driscoll et al. Reference Driscoll, Catford, Barney, Hulme and Martin2014), and the agricultural pathway itself is a major vector for inadvertent new IAS introductions (e.g. hitchhikers). Agricultural intensification on islands has also led to widespread habitat clearance, which further encourages new invasions. Biocontrol has often been a preferred means for controlling IASs, historically for vertebrates and still today for plants and invertebrates (Messing & Wright Reference Messing and Wright2006). However, biocontrol should only be done if the consequences are extremely well understood (Simberloff Reference Simberloff2006). IAS management on islands must therefore necessarily incorporate habitat management and restoration in order to rebuild resilience to invasion. Heterogeneous landscapes must also be strategically managed for IASs in order to minimize connectivity (Perry et al. Reference Perry, Moloney and Etheringtonin press) and promote native biodiversity across multiple land uses.
Urban development
The increasing trend towards urbanization focuses human activities into more densely populated areas. Whereas to some extent this mitigates habitat loss in more natural areas, it creates urban ‘deserts’, and urbanization typically impacts productive rural ecosystems (Martinuzzi et al. Reference Martinuzzi, Gould and Gonzalez2007). This inevitably reduces native species richness, and indeed all species richness in highly urbanized areas, but in suburban areas it increases introduced species richness (McKinney Reference McKinney2008). Densely populated cities have long been hubs for IAS transportation (Hulme Reference Hulme2009), and urbanized populations tend to associate nature firstly with introduced species (Shapiro et al. Reference Shapiro, Peterson, Stevenson, Frew and Langerhansin press). Trade in pets and ornamental plants is focused around urban areas, which are source points for IASs into surrounding landscapes (Carrete & Tella Reference Carrete and Tella2008; Hulme Reference Hulme2015), and air and sea ports of urban areas on islands are the primary entry points for IAS incursions.
Climate change
Although climate change ranks low among current threats to biodiversity (Maxwell et al. Reference Maxwell, Fuller, Brooks and Watson2016), the magnitude of this threat is forecast to grow (Bellard et al. Reference Bellard, Bertelsmeier, Leadley, Thuiller and Courchamp2012), and islands will also be disproportionately vulnerable to the predicted impacts of climate change, such as sea level rise and coastal inundation (Mimura Reference Mimura1999). Predicting climate change impacts on islands is challenging, as larger-scale weather systems interact with island geography (Caujape-Castells et al. Reference Caujape-Castells, Tye, Crawford, Santos-Guerra and Sakai2010). The threats of IASs and climate change are predicted generally to interact positively (Bellard et al. Reference Bellard, Thuiller, Leroy, Genovesi, Bakkenes and Courchamp2013). Five consequence of climate change for invasive species include: (1) altered transport and introduction mechanisms; (2) establishment of new invasive species; (3) altered impact of existing invasive species; (4) altered distribution of existing invasive species; and (5) altered effectiveness of control strategies (Hellmann et al. Reference Hellmann, Byers, Bierwagen and Dukes2008). IASs are adaptable colonists with a broader range of tolerances to environmental variation and disturbance, and although climate change may not alter the rate of IAS introduction, it is likely to improve establishment rates (Hulme Reference Hulmein press). Climate change resilience and IAS management should be considered simultaneously in island conservation planning (Courchamp et al. Reference Courchamp, Hoffmann, Russell, Leclerc and Bellard2014); however, because island nations have very little control over global climate change, it may be most worthwhile then investing in IAS mitigation through control and eradication programmes (Jones et al. Reference Jones, Holmes, Butchart, Tershy and Kappes2016), which will generate additional resilience in native species populations and ecosystems to climate change threats.
Community engagement
As we demonstrated for SIDSs, the number of IASs on islands is directly correlated with island area and human population size (Kueffer et al. Reference Kueffer, Daehler, Torres-Santana, Lavergne and Meyer2010, Blackburn et al. Reference Blackburn, Delean, Pyšek and Cassey2016). Even on uninhabited islands, IASs originated from human agency, and their management depends on human intervention. The management of IASs on islands, or indeed anywhere, must therefore be considered as part of a broader exercise of engagement within a coupled socio-ecological system (Crowley et al. Reference Crowley, Hinchliffe and McDonald2017; Schmitz et al. Reference Schmitz, Arnaiz-Schmitz, Herrero-Jáuregui, Díaz, Matos and Pinedain press). Conflicts of interest can arise in IAS management when species that are considered invasive by one sector of society are considered a resource by another (Russell Reference Russell2014). Important examples include game animals, pets and ornamental plants. Where appropriate recourse is not given to managing such conflicts, broader opposition to IAS management can occur because of opposition to methods, sensitivity around species with domestic analogues and low awareness of impacts (Witmer & Fuller Reference Witmer and Fuller2011). People living on islands tend to have tighter-knit communities and senses of identity, and IAS management must take into account their broader values and, in particular, an inclusive approach to IAS decision-making and management (Russell et al. Reference Russell, Taylor and Aleyin press). Ultimately, these social aspects determine whether eradication of an IAS in an island is an option and which methods are acceptable (Oppel et al. Reference Oppel, Beaven, Bolton, Vickery and Bodey2011; Glen et al. Reference Glen, Atkinson, Campbell, Hagen and Holmes2013).
MANAGEMENT
Biological invasions occur along a series of stages (Blackburn et al. Reference Blackburn, Pyšek, Bacher, Carlton and Duncan2011; though see also Colautti & MacIssac Reference Colautti and MacIsaac2004), and at each stage the suite of available management responses in order to mitigate, reduce or eliminate IAS impacts differs. Costs and opportunities for managing IASs increase rapidly as invasion progresses. Because of their clearly demarcated borders and limited areas, management responses to IASs can be more decisive on islands (Simberloff Reference Simberloff2001). Managers must have processes: to prevent IAS arrival with biosecurity, to manage the urgency associated with recent colonists and early eradication, for ongoing adaptive management, and for control strategies. The two most important objectives at any stage of an invasion are likely to be reducing population size and ‘rolling back’ the distribution of the invader.
Biosecurity
Prevention of biological invasions is by far the most cost-effective management strategy (Leung et al. Reference Leung, Lodge, Finnoff, Shogren, Lewis and Lamberti2002; Timmins & Braithwaite Reference Timmins, Braithwaite, Veitch and Clout2002; Hulme Reference Hulme2006). Biosecurity comprises the strategy, efforts and planning to minimize the likelihood of invasive species transport, arrival or establishment (Meyerson & Reaser Reference Meyerson and Reaser2002, Reference Meyerson and Reaser2003). Optimal biosecurity and surveillance must consider the relative risks and the financial costs of differing strategies in order to implement the optimal biosecurity strategy (Hauser & McCarthy Reference Hauser and McCarthy2009; Rout et al. Reference Rout, Moore, Possingham and McCarthy2011). Biosecurity initiatives are generally classified as quarantine, surveillance and response actions. Quarantine involves isolation of propagules and potential vectors at specified locations to facilitate screening and treatment, and may occur at departure points, during transport or following arrival at an island. Surveillance consists of the actions taken to monitor for propagules during transport and following arrival, both within and outside of quarantine. Once there is a quarantine breach or surveillance detection, a contingency response is launched. A contingency response is a calculated exercise to eliminate the incursion. It must be made immediately and with the same intensity as an eradication campaign. Before an incursion actually occurs, it is important that the capacity and planning for a contingency response are established. In all cases biosecurity is an ongoing action with associated costs in the face of unrelenting propagule pressure.
Biosecurity is particularly important on islands where although the probability of an invasion occurring can be quite low, the conservation value is greatest and so the consequences of any such invasion can be comparatively great. Uninvaded islands typically have the highest conservation value and should always be a priority for IAS management. Islands predispose themselves to successful biosecurity as they typically have fewer entry points (e.g. air and sea ports) through which all traffic must pass. Island biosecurity historically focused on protecting agricultural values, but the same processes have readily been adapted to protecting biodiversity values. Successful biosecurity also requires appropriate consideration of policies, governance and broader economics (Cook et al. Reference Cook, Liu, Murphy and Lonsdale2010; Heikkilä Reference Heikkilä2011; Richardson Reference Richardson and Richardson2011). The interaction between biosecurity initiatives and the invasion process can be conceptually represented (Fig. 3). The earlier in the invasion process that a propagule is intercepted, the greater the probability of invasion prevention, often at a reduced cost.
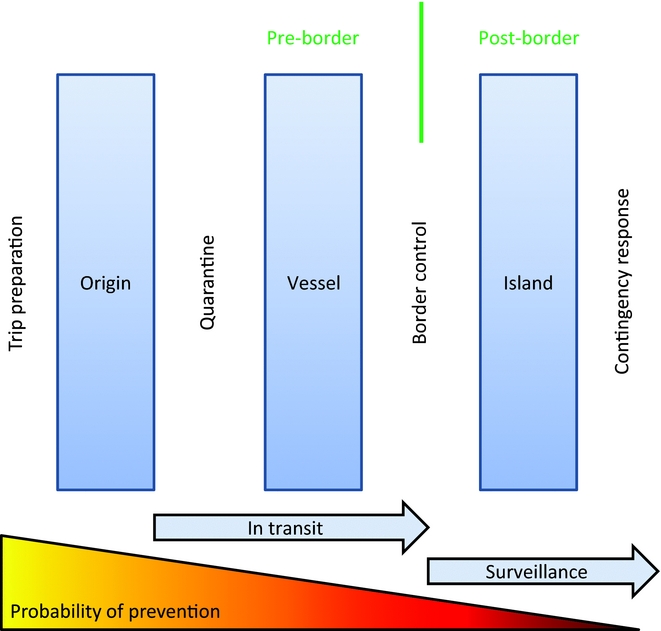
Figure 3 Biosecurity for intercepting invasive alien species on islands (modified with permission from Broome Reference Broome, Witmer, Pitt and Fagerstone2007, following Blackburn et al. Reference Blackburn, Pyšek, Bacher, Carlton and Duncan2011).
Control
For most IASs, once they have become established on an island (i.e. are self-sustaining and breeding), elimination is no longer feasible and management must move towards control and mitigation (Rejmánek & Pitcairn Reference Rejmánek, Pitcairn, Veitch and Clout2002). Plants, invertebrates and vertebrates have all been eradicated as incursions during the establishment phase of biological invasions (Glen et al. Reference Glen, Atkinson, Campbell, Hagen and Holmes2013). If eradication of the species is possible, it is most likely to be successful at this time, and for some taxa such as insects, eradication is generally only possible during this phase (Liebhold et al. Reference Liebhold, Berec, Brockerhoff, Epanchin-Niell and Hastings2016). Reducing the population size of an IAS during establishment increases the likelihood of it falling below Allee thresholds, thereby facilitating eradication by preventing secure establishment (Leibhold & Bascompte Reference Liebhold and Bascompte2003).
Significant IAS management can take place at the sub-island level at intensively managed sites, particularly where local suppression to zero density may be achieved (we reserve the term ‘eradication’ for an island-wide removal programme following IAS establishment). Such sites are important for protecting values until such time as island-wide eradication becomes feasible, and management strategies at these sites can mimic those for entire islands (e.g. ‘mainland islands’ in New Zealand; Saunders & Norton Reference Saunders and Norton2001). IAS management on islands typically relies on a few standard taxa-specific methods, such as manual, mechanical, chemical and biological control (Rocamora & Henrietta Reference Rocamora and Henriette2016). Classical biological control, by introducing host-specific natural enemies, constitutes both a control option, especially for widespread invasive plants and arthropods, and a tool to partially restore invaded natural systems (Van Driesche et al. Reference Van Driesche, Pratt, Center, Rayamajhi, Tipping, Van Driesche, Simberloff, Blossey, Causton, Hoddle, Marks, Heinz, Wagner and Warner2016). However, the development of new control tools, particularly advances in synthetic biology (Piaggio et al. Reference Piaggio, Segelbacher, Seddon, Alphey and Bennett2017), may create novel opportunities for IAS control and eradications (Campbell et al. Reference Campbell, Beek, Eason, Glen and Godwin2015).
Eradication
Eradication is best attempted when the population growth rate is low (i.e. the invasion curve is flat). Eradication is therefore likely to be most successful either early in the invasion, just following arrival, or else once the population has stabilized at a local carrying capacity. However, any reduction in population size at carrying capacity will prompt an increase in the population growth rate. Six criteria are considered necessary for eradication: (1) removal exceeds rate of increase at all densities; (2) immigration is prevented; (3) all reproductive individuals can be put at risk by the eradication technique; (4) individuals can be detected at low densities; (5) the benefits of the project outweigh the costs; and (6) the project is socio-politically acceptable (Bomford & O'Brien Reference Bomford and O'Brien1995). Eradication at carrying capacity must therefore be a decisive action, and is unlikely to be achieved as an outcome of ongoing control, and thus we treat it differently from control at carrying capacity (Bomford & O'Brien Reference Bomford and O'Brien1995). Eradication at any stage may be possible, given sufficient investment to ensure that the rate of removal greatly exceeds the population growth rate, and preferably should occur as early as possible in the invasion pathway, where success rates are typically higher and overall costs lower (Rejmánek & Pitcairn Reference Rejmánek, Pitcairn, Veitch and Clout2002; Glen et al. Reference Glen, Atkinson, Campbell, Hagen and Holmes2013). However, eradication of invasive vascular plants is always challenging when they have long-lasting seedbanks (Meyer Reference Meyer2014; Panetta Reference Panetta2015).
Permanent eradication of IASs is often more cost effective (Pascal et al. Reference Pascal, Lorvelec, Bretagnolle and Culioli2008) and ethical (Russell et al. Reference Russell, Cole, Zuël and Rocamora2016) than control over very long time horizons. However, the number of IAS taxa for which island-wide eradication is currently possible is limited. Eradication feasibility is currently high for invasive mammals on islands with sizes on the scale of thousands to tens of thousands of hectares, and can be achieved at carrying capacity. Mammal eradications have focused on the most widespread species including invasive rodents (Howald et al. Reference Howald, Donlan, Galván, Russell and Parkes2007), feral cats (Campbell et al. Reference Campbell, Harper, Algar, Hanson, Keitt, Robinson, Veitch, Clout and Towns2011) and ungulates (Campbell & Donlan Reference Campbell and Donlan2005). From the Database of Island Invasive Species Eradications (DIISE), over 1200 eradication attempts have been made on over 800 islands worldwide, with a success rate of c. 85%. The majority of these eradication attempts (60%) have been in the Pacific Ocean (with half of these being in New Zealand), but only 10% have been in SIDSs (Fig. 4). This bias reflects the history of expertise in invasive mammal eradications (Russell & Broome Reference Russell and Broome2016), the resources required for whole-island invasive mammal eradication (Russell & Holmes Reference Russell and Holmes2015) and the role that greater inhabitation on SIDSs plays in eradication feasibility (Oppel et al. Reference Oppel, Beaven, Bolton, Vickery and Bodey2011). Greater investment in IAS eradication from SIDSs is warranted alongside other conservation interventions (Dahl Reference Dahlin press).
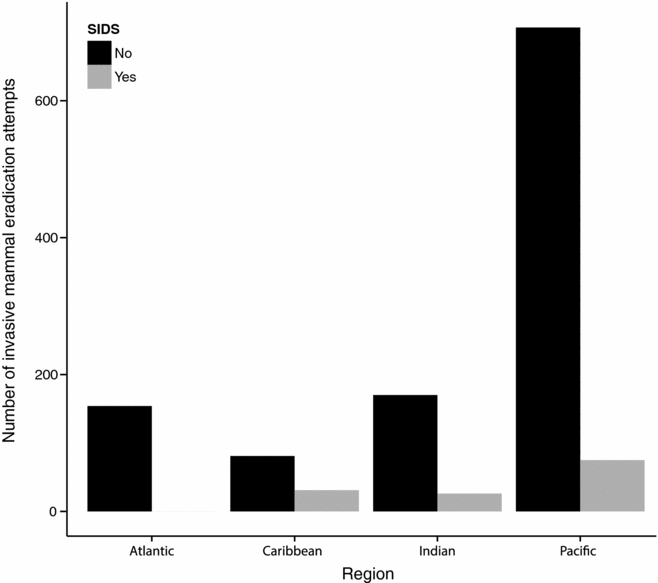
Figure 4 Invasive mammal eradication attempts (including successful, successful but reinvaded, failed, in progress or to be confirmed) comparing SIDSs to all other islands, where events are classified as good or satisfactory data quality and the whole island was treated. Data accessed February 2017 (source: Database of Island Invasive Species Eradications). SIDS = small-island developing state.
In comparison, invasive bird eradication feasibility is currently limited to islands with sizes on the scale of hundreds of hectares for volant birds, but is only limited to islands with sizes of tens of thousands of hectares for non-volant birds. For reptiles and amphibians, only early interception has proved successful (Kraus Reference Kraus2009). Plant eradications are almost entirely restricted to the sub-island level, with sizes on the scale of tens to hundreds of hectares (Rejmánek & Pitcairn Reference Rejmánek, Pitcairn, Veitch and Clout2002), and as for animal eradications depend on the attributes of the target species (Panetta Reference Panetta2015), require a strong commitment to completion (Buddenhagen & Tye Reference Buddenhagen and Tye2015) and where once again early interception is critical (Mack & Lonsdale Reference Mack, Lonsdale, Veitch and Clout2002). Invasive invertebrate eradication feasibility varies greatly depending on the biology of the taxa involved and the tools available. Arthropods have been successfully eradicated over areas at the scale of tens of thousands of hectares (Tobin et al. Reference Tobin, Kean, Suckling, McCullough, Herms and Stringer2014), and with new technique developments (Boser et al. Reference Boser, Hanna, Holway, Faulkner and Naughtonin press), some advances have recently been made for eradicating invasive ants in areas at the scale of tens of hectares (Hoffman et al. Reference Hoffmann, Luque, Bellard, Holmes and Donlan2016), but for other insects there remains an urgency to discover and develop appropriate tools for any reliable detection and control, let alone eradication (Brockerhoff et al. Reference Brockerhoff, Liebhold, Richardson and Suckling2010).
CONCLUSIONS
IASs are disproportionately prevalent on islands, where they also generate disproportionate impacts compared to continental areas. They have played a major role in structuring modern ecological communities on islands. The impacts of IASs on islands are forecast to only increase with time, and today and for the foreseeable future, IASs are likely to be the strongest drivers of plant and animal population declines and extinctions on islands, but the identification and scope of IAS impacts on islands should be broadened to include non-biodiversity impacts. Indicators for IASs on islands, including their occurrence, status and impact, should all be collected and monitored over time. Conceptualizing the biological invasion process in a population biology framework better equips managers to respond to IASs on islands, including implementing successful biosecurity, control and eradication interventions. The impact and distribution of IASs on islands will continue to grow and interact with other global change threats, and climate change is likely to have particularly pernicious impacts on the biodiversity impacts of IASs. Because inhabited islands are tightly coupled socio-ecological systems, more work is required in order to understand how to work with island communities on IAS management, and stronger collaborations among island countries and territories may contribute to that effort.
ACKNOWLEDGEMENTS
Thanks to the editor Nick Polunin for inviting this contribution to the special issue and his patience and proof-reading during its preparation. Thanks to Steffen Oppel and two anonymous reviewers for their positive and thorough comments. James Russell was funded by Rutherford Discovery Fellowship grant RDF-UOA1404. The Global Register of Introduced and Invasive Species (GRIIS) has been developed with co-funding from the European Union through the Secretariat of the Convention on Biological Diversity (CBD) within the framework of the Global Invasive Alien Species Information Partnership (GIASIP). The GIASIP has come together to assist Parties to the CBD, and others, implement Article 8(h) and Target 9 of the Aichi Biodiversity Targets: “By 2020, invasive alien species and pathways are identified and prioritized, priority species are controlled and eradicated, and measures are in place to manage pathways to prevent introduction and establishment.”







