Introduction
The genus Retrophyllum C.N. Page was erected by Page (Reference Page1989 [‘1988’]) for a small group of species in the family Podocarpaceae. This group has been recognised as distinct ever since Bertrand (Reference Bertrand1874) used the name Podocarpus sect. Polypodiopsis C.E.Bertr. for two species that were then known as Podocarpus vitiensis Seem. and P. minor (Carrière) Parl. A similar concept was followed by Wasscher (Reference Wasscher1941), Buchholz & Gray (Reference Buchholz and Gray1948) and Gray (Reference Gray1962), among others. This sectional name appeared to be based on an earlier name, Polypodiopsis Carrière, but that name, and the name Polypodiopsis muelleri Carrière, were proposed for rejection by Mill & Weston (Reference Mill and Weston2004) on the grounds that the names are ambiguous and could refer to a species of a genus of Proteaceae, such as Beauprea Brongn. & Gris. These two nomenclatural proposals were subsequently approved (Barrie, Reference Barrie2006). By the time of Gray (Reference Gray1962), Podocarpus sect. Polypodiopsis comprised six named species, P. rospigliosii Pilg. having been described by Pilger (Reference Pilger1923), P. comptonii J.Buchholz and P. palustris J.Buchholz by Buchholz (Reference Buchholz1949) and P. filicifolius N.E.Gray by Gray (Reference Gray1962). Shortly afterwards, de Laubenfels (Reference de Laubenfels1968) published a short note pointing out that the material Gray (Reference Gray1962) had used to describe her new species Podocarpus filicifolius was a mixture of juvenile foliage of what he regarded as Podocarpus vitiensis Seem. and detached fruits of what he called P. blumei Endl. [= Nageia wallichiana (C.Presl) Kuntze], and therefore he regarded Podocarpus filicifolius as a synonym of P. vitiensis [= Retrophyllum vitiense].
More recently, authors have realised that Podocarpus L'Hér. ex Pers. as conceived by Buchholz & Gray (Reference Buchholz and Gray1948) and earlier workers is heterogeneous, and have split it into numerous smaller genera. De Laubenfels (Reference de Laubenfels1969) erected the genus Decussocarpus de Laub., with three sections: Decussocarpus sect. Decussocarpus corresponded to Podocarpus sect. Polypodiopsis, D. sect. Dammaroides (Bennett) de Laub. to P. sect. Nageia (Gaertn.) Endl. and D. sect. Afrocarpus (J. Buchholz & N.E.Gray) de Laub. to P. sect. Afrocarpus J.Buchholz. Decussocarpus sect. Decussocarpus had four species: D. vitiensis (Seem.) de Laub., D. minor (Carrière) de Laub. and D. comptonii (J.Buchholz) de Laub. in the South Pacific, and D. rospigliosii (Pilg.) de Laub. in tropical South America. To these was subsequently added Decussocarpus piresii Silba, a close ally of D. rospigliosii that was rather unsatisfactorily described from Brazil (Silba, Reference Silba1983). Later, on account of a change in the rules of the International Code of Botanical Nomenclature, the generic name Nageia Gaertn. had to be adopted for Decussocarpus, and de Laubenfels (Reference de Laubenfels1987) then referred to the three sections as Nageia sect. Nageia (= Decussocarpus sect. Dammaroides), N. sect. Polypodiopsis (C.E.Bertr.) de Laub. (= D. sect. Decussocarpus) and N. sect. Afrocarpus (J.Buchholz & N.E.Gray) de Laub. (= D. sect. Afrocarpus). De Laubenfels (Reference de Laubenfels1987) accepted five species in Nageia sect. Polypodiopsis, namely Nageia vitiensis (Seem.) O. Kuntze, N. comptonii (J.Buchholz) de Laub., N. minor Carr., N. rospigliosii (Pilg.) de Laub. and N. piresii (Silba) de Laub. In both that paper and his earlier one, he regarded Podocarpus filicifolius as a synonym of Nageia/Decussocarpus vitiensis and Podocarpus palustris as a synonym of N./D. minor.
Page (Reference Page1989) decided that the three sections of Nageia as delimited by de Laubenfels (Reference de Laubenfels1987) were each worthy of independent generic status. For Nageia sect. Polypodiopsis, he had to propose the name Retrophyllum C.N. Page, because Decussocarpus de Laub. had been declared illegitimate and was (and still is) unavailable for use. Retrophyllum as delimited by Page (Reference Page1989) had five species, corresponding to those recognised under Nageia by de Laubenfels (Reference de Laubenfels1987): Retrophyllum vitiensis (Seem.) C.N. Page on Fiji (the New Guinea etc. distribution was not accounted for), R. comptonii (J.Buchholz) C.N. Page and R. minus (Carrière) C.N. Page (by Page mis-spelled ‘minor’) on New Caledonia, and ‘at least two’ – R. rospigliosii (Pilg.) C.N. Page and R. piresii (Silba) C.N. Page – in tropical South America. Once again, Page (Reference Page1989) included Podocarpus palustris under Retrophyllum minus, but unlike de Laubenfels (Reference de Laubenfels1969, Reference de Laubenfels1987) he did not account for Podocarpus filicifolius nor cite Gray's Reference Gray1962 paper in his bibliography. The possibility that there might be more than two species of Retrophyllum in South America was repeated in Page's later treatment of Podocarpaceae for The Families and Genera of Vascular Plants (Page, Reference Page, Kubitzki, Kramer and Green1990), but the reason for this opinion was unexplained in both works.
As well as these five extant taxa, two Australasian fossils have been assigned to Retrophyllum as well as an undescribed fossil species from Argentina that is known from leafy branches bearing attached pollen cones (Wilf, Reference Wilf2012; Wilf et al., Reference Wilf, Escapa and Cúneo2012, Reference Wilf, Escapa, Cúneo, Kooyman, Johnson and Iglesias2014; Merkhofer et al., Reference Merkhofer, Wilf, Haas, Kooyman, Sack, Scoffoni and Cúneo2015). A fourth fossil species may also belong to Retrophyllum, although the necessary combination has not yet been made. The Miocene fossil Retrophyllum vulcanense M.Pole is known from St. Bathans and Mata Creek in Otago, New Zealand (Pole, Reference Pole1992, Reference Pole1997; Hill & Brodribb, Reference Hill and Brodribb1999; Pole, Reference Pole2007), while R. australe R.S.Hill & Merrifield from West Dale in Western Australia is slightly older, of Middle Eocene or Oligocene age (Hill & Merrifield, Reference Hill and Merrifield1993; Hill & Brodribb, Reference Hill and Brodribb1999). Both these appear to be unequivocal members of Retrophyllum. However, on account of some morphological differences, Decussocarpus maslinensis D.T.Blackburn from South Australia (Blackburn, Reference Blackburn1981) was later transferred to the extinct genus Willungia R.S.Hill & M.Pole as W. maslinensis (D.T.Blackburn) R.S.Hill & M.Pole ex R.R.Mill & R.S.Hill (Hill & Pole, Reference Hill and Pole1992; Mill & Hill, Reference Mill and Hill2004). Rather more contentious is Decussocarpus araucoensis (E.W.Berry) D.R.Greenw., an Eocene fossil from Coronel, Chile (Berry, Reference Berry1922; Greenwood, Reference Greenwood1987). This may well also belong to Retrophyllum but, unlike the other fossil species, it has not yet been compared with similar, Retrophyllum-like fossils that were described by Hill & Pole (Reference Hill and Pole1992) as the new fossil-genera Smithtonia R.S.Hill & M.Pole and Willungia. Until that is done, assignment of ‘Decussocarpus araucoensis’ to Retrophyllum should not be made, even though its current name is nomenclaturally incorrect because of the illegitimacy of Decussocarpus and should not be used. ‘Decussocarpus araucoensis’ was first described as a species of Araucaria Juss. by Berry (Reference Berry1922) but was transferred to Podocarpus by Florin (Reference Florin1940), who commented that it was near to P. rospigliosii (i.e. Retrophyllum rospigliosii). Hill & Pole (Reference Hill and Pole1992), based only on an examination of albeit excellent quality photographs in Florin's Reference Florin1940 paper, considered that “probably some, but not all, of the specimens illustrated by Florin belong to this genus” [Retrophyllum]. More recently, Hill & Brodribb (Reference Hill and Brodribb1999: 667) repeated this assertion but were more definite in a footnote to a table in the same paper (Hill & Brodribb, Reference Hill and Brodribb1999: 662), in which they wrote, “Hill & Pole (Reference Hill and Pole1992) considered this species to belong to Retrophyllum, but they did not formally transfer it to that genus”.
In recent molecular phylogenetic studies, Retrophyllum in its traditional sense appears close to Afrocarpus and Nageia, the three genera forming a monophyletic subclade that is sister to Podocarpus (Conran et al., Reference Conran, Wood, Martin, Dowd, Quinn, Gadek and Price2000; Sinclair et al., Reference Sinclair, Mill, Gardner, Woltz, Jaffré, Preston, Hollingsworth, Ponge and Möller2002; Biffin et al., Reference Biffin, Conran, Lowe, Turner and Cernusak2011, Reference Biffin, Brodribb, Hill, Thomas and Lowe2012; Knopf et al., Reference Knopf, Schulz, Little, Stützel and Stevenson2012). Only the studies by Sinclair et al. (Reference Sinclair, Mill, Gardner, Woltz, Jaffré, Preston, Hollingsworth, Ponge and Möller2002), Herbert et al. (Reference Herbert, Hollingsworth, Gardner, Mill, Thomas and Jaffré2002) and Biffin et al. (Reference Biffin, Conran, Lowe, Turner and Cernusak2011, Reference Biffin, Brodribb, Hill, Thomas and Lowe2012) used species from all three informal groups A (South Pacific), B (New Caledonia) and C (South America) here recognised within Retrophyllum. Those by Sinclair et al. (Reference Sinclair, Mill, Gardner, Woltz, Jaffré, Preston, Hollingsworth, Ponge and Möller2002) and Herbert et al. (Reference Herbert, Hollingsworth, Gardner, Mill, Thomas and Jaffré2002) remain the most comprehensive in their sampling; Conran et al. (Reference Conran, Wood, Martin, Dowd, Quinn, Gadek and Price2000) sampled only the two species of group B, while Knopf et al. (Reference Knopf, Schulz, Little, Stützel and Stevenson2012) sampled only species from New Caledonia and South America (i.e. groups B and C) and Biffin et al. (Reference Biffin, Conran, Lowe, Turner and Cernusak2011, Reference Biffin, Brodribb, Hill, Thomas and Lowe2012) sampled only three of the six species treated here (one from each group). Herbert et al. (Reference Herbert, Hollingsworth, Gardner, Mill, Thomas and Jaffré2002) sampled the same four species as Sinclair et al. (Reference Sinclair, Mill, Gardner, Woltz, Jaffré, Preston, Hollingsworth, Ponge and Möller2002), i.e. excluding Retrophyllum piresii, for which DNA could not be obtained, and R. filicifolium (N.E.Gray) R.R.Mill, which at that time was not recognised as distinct from R. vitiense. Their results strongly supported the monophyly of Retrophyllum, despite the large disparities in morphology between the three morphological groups. For that reason, despite the large morphological differences, some of which appear to be quite fundamental, the genus is retained in its traditional circumscription in this paper. Herbert et al. (Reference Herbert, Hollingsworth, Gardner, Mill, Thomas and Jaffré2002) additionally used random amplified polymorphic DNA (RAPD) and restriction fragment length polymorphism to assist in separation of the two New Caledonian species Retrophyllum comptonii and R. minus.
Retrophyllum has been placed controversially by Bobrov & Kostrikin (Reference Bobrov and Kostrikin2000) and Melikian & Bobrov (Reference Melikian and Bobrov2000) in Nageiaceae, in the order Cephalotaxales. Although a relationship with Nageia is suggested by other evidence (including molecular data: Conran et al., Reference Conran, Wood, Martin, Dowd, Quinn, Gadek and Price2000; Sinclair et al., Reference Sinclair, Mill, Gardner, Woltz, Jaffré, Preston, Hollingsworth, Ponge and Möller2002; Biffin et al., Reference Biffin, Conran, Lowe, Turner and Cernusak2011, Reference Biffin, Brodribb, Hill, Thomas and Lowe2012; Knopf et al., Reference Knopf, Schulz, Little, Stützel and Stevenson2012), a removal from Podocarpaceae (even in a broad sense) is not, nor is its removal to a different order. Salter (Reference Salter2004) has pointed out that Bobrov & Melikian's removal of Retrophyllum to Cephalotaxales was based on a misinterpretation of the tissue layers of the mature seeds; they interpreted the seeds of Retrophyllum and Nageia as possessing two integuments, rather than a single integument and an epimatium.
Taxonomic Problems
Although a small group, the genus has been the subject of some debate. Four principal problems are addressed in this revision:
• the status of the genus and whether its widely disjunct species form a monophyletic group.
• species limits within Retrophyllum vitiense.
• identification of the two New Caledonian species Retrophyllum minus and R. comptonii, which can be very difficult to separate in the herbarium.
• whether Retrophyllum piresii is taxonomically distinguishable from R. rospigliosii, and if so at what rank.
Limits of the genus
Retrophyllum in the sense as originally defined by Page (Reference Page1989, Reference Page, Kubitzki, Kramer and Green1990) appears to be a morphologically unnatural genus that has been held together by the single character of heterofacially flattened shoots, which has been regarded as unique within Podocarpaceae. Although this is true for the extant genera of the family, two extinct genera with heterofacially flattened shoots (Smithtonia and Willungia) have since been described (Hill & Pole, Reference Hill and Pole1992), demonstrating that the character has evolved more than once in the family in the past. In fact, the six living species recognised here fall into three natural groups, here denoted Groups A, B and C, that are geographically separated today, although in each of them the mechanism whereby the ‘retrophyllous’ leaf arrangement is achieved is similar. The Southwest Pacific species divide into those with distichous leaves in both adult and juvenile phases (Group A: Fiji, Papuasia) and those with 4-ranked leaves in the adult phase but distichous ones in the juvenile state (Group B: New Caledonia). The South American species-pair (Group C) also has distichous leaves in both adult non-reproductive and juvenile phases, but on reproductive shoots the leaves are not ‘retrophyllous’. In all three groups, some degree of internode torsion appears to be involved in bringing the leaves into one plane, just as it is in Amentotaxus Pilg. of Taxaceae (Tomlinson & Zacharias, Reference Tomlinson and Zacharias2001); this results in a characteristic zigzag pattern of decurrent leaf bases along the twigs that is particularly noticeable in twigs of the penultimate order and is especially pronounced in Group C. Each of the three Groups A, B and C possesses unique combinations of characters of male and female reproductive organs, the degree of importance of which is such that, on the basis of morphology alone, each group could probably be segregated at generic rank, as has been done for other small genera of Podocarpaceae such as Lepidothamnus Phil., Lagarostrobos Quinn and Manoao Molloy. Furthermore, in the two groups for which seedlings are known (Groups B and C), these are totally different in their morphology; seedlings of Group B have epigeal germination, about 10 ‘cotyledons’ arranged decussately on a naked axis with very short internodes between them, and decussate phyllotaxis of the first few foliage shoots, while Retrophyllum rospigliosii of Group C has apparently hypogeal germination with no visible cotyledons, and spiral phyllotaxis of the initial foliage shoots, which are separated by relatively long internodes along an axis clad with leaves. Nevertheless, the three groups always form a monophyletic clade in molecular phylogenetic studies (e.g. Conran et al., Reference Conran, Wood, Martin, Dowd, Quinn, Gadek and Price2000; Sinclair et al., Reference Sinclair, Mill, Gardner, Woltz, Jaffré, Preston, Hollingsworth, Ponge and Möller2002). For that reason, the decision has been made to retain the present generic limits but to recognise three informal geographically defined Groups A, B and C within the genus, as outlined above and defined in detail below.
Group A species
Group A comprises the species occurring in the Southwest Pacific exclusive of New Caledonia. Most authorities have recognised only one species of Retrophyllum in this area, R. vitiense, with a range extending from Fiji through the Solomon Islands to New Guinea and the Moluccas (e.g. Eckenwalder, Reference Eckenwalder2009; Farjon, Reference Farjon2010). Seemann (Reference Seemann1862, Reference Seemann1863) provided the first descriptions of Retrophyllum vitiense (as Podocarpus vitiensis), based on material from Fiji collected by himself. Pilger (Reference Pilger and Engler1903) also knew the species only from Fiji, citing Seemann's collection and one other. Wasscher (Reference Wasscher1941) was the first author to realise that a species of Podocarpus sect. Polypodiopsis [i.e. Retrophyllum] also grew in New Guinea and adjacent islands. Under what he regarded as Podocarpus vitiensis, he cited two specimens from the south-eastern part of New Guinea and one from New Ireland in the Bismarck Archipelago and gave a very full description (as is typical of his work) that was based on those three specimens and one female specimen from Fiji. Therefore, although he was the first to describe male material of what is here treated as Retrophyllum filicifolium, he did not see female material of the New Guinea plant. Since then, much more material of Retrophyllum has been collected from New Guinea, and it is now known from both the eastern half of the island (Papua New Guinea) and the western half (West Papua and Papua). Netta Gray, in her revision of the Pacific members of Podocarpus sect. Polypodiopsis [i.e. Retrophyllum], also regarded Podocarpus vitiensis (= Retrophyllum vitiense) as occurring in Fiji, the Solomon Islands and New Guinea, but she also described a new species, Podocarpus filicifolius N.E.Gray, from Morotai in the Moluccas, just west of New Guinea (Gray, Reference Gray1962). This she separated from Podocarpus vitiensis on the basis of “the spreading scale leaves, thinner foliage leaves, the distinct receptacle 7 mm long supporting the seeds, and the spherical seeds” and used the last of those supposed differences (spherical seeds) to distinguish Podocarpus filicifolius from P. vitiensis. She also rather perceptively commented that the New Guinea specimens (all of them were by her included under Podocarpus vitiensis) “may still prove to represent a separate species”, but the immature ovules that she had seen were pear-shaped, as in Fijian Podocarpus vitiensis, and so she did not segregate them. De Laubenfels (Reference de Laubenfels1968) discovered that Gray's Podocarpus filicifolius was in fact based on a loose female cone of Nageia wallichiana (C.Presl) Kuntze and juvenile foliage of what he regarded in his paper as Podocarpus vitiensis. Consequently, most of the alleged differences that Gray had proposed between her new species and the Fijian Podocarpus vitiensis become irrelevant, and de Laubenfels (Reference de Laubenfels1968) therefore synonymised Gray's species with the latter. That remained his stance when in the following year he segregated the genus Decussocarpus from Podocarpus and regarded Podocarpus filicifolius as a synonym of D. vitiensis (de Laubenfels, Reference de Laubenfels1969). Until now, no one has revisited Gray's comment that New Guinea material might be taxonomically separable from Fijian Retrophyllum vitiense, possibly because Gray's taxon from Morotai was founded on mixed material. Nevertheless, examination of ample material demonstrates that there are numerous other differences, not observed by either Gray or de Laubenfels, between the New Guinea plants that have been hitherto included in Retrophyllum vitiense and the Fijian ones, and that the material from mainland New Guinea agrees with that from Morotai (excluding the foreign cone material). Accordingly, Gray's name is here reinstated and applied in an emended sense to encompass all plants of Retrophyllum seen from New Guinea and the Moluccas. Because Gray's type material is deemed insufficient, consisting as it does of juvenile leaves belonging to Retrophyllum plus a cone that does not belong to the genus, an epitype is designated in this paper to fix the application of the name with regard to the characters of adult foliage and female cones. Gray's protologue of Podocarpus filicifolius is here emended to exclude all mention of female cone characteristics, specifically all the text in the Latin diagnosis after “strobilis masculis ignotis”, everything after “foliage leaves” in the paragraph beginning “This tree differs from Podocarpus vitiensis in. . .”, the last sentence of the paragraph on p. 71 beginning “The female strobili”, and the supposed difference in seed shape in couplet C of the key to species (p. 71). The description given here provides full details of the male and genuine female cones of Retrophyllum filicifolium – the female being here described for the first time although, as mentioned above, Wasscher (Reference Wasscher1941) had already described the male cones.
Group B species
Three taxa belonging to Retrophyllum have been described from New Caledonia, but until 1949 only one of them was known: Podocarpus minor Carrière, now known as Retrophyllum minus. Carrière (Reference Carrière1867: 641) in his protologue said that this grew “au sommet de très-hautes montagnes, où il fut découvert par un jardinier anglais nommé Richard”, but the only actual specimen cited, Vieillard 1275, was from a low-lying locality (Lac Arnaud in the Plaine des Lacs) in the south of the island. From the very start, therefore, Retrophyllum minus seems to have been used to apply to all Retrophyllum of the island. The discoverer of the high-altitude plant mentioned in Carrière's protologue was most likely to have been N. Richards, who along with W. H. Duncan was a collector in New Caledonia for Ferdinand von Mueller (Morat, Reference Morat2010). Richards collected Araucaria biramulata J.Buchholz “on open stony land of a very high mountain” in or before 1862 (Richards 3, P, barcoded P00190084, originally named Araucaria cookii R.Br.). However, no specimen of Retrophyllum collected by him in New Caledonia has been traced at P by me, and none was cited by Guillaumin (Reference Guillaumin1911), who did, however, include three specimens of Araucaria collected by him. Nageia minor is therefore based purely on the single specimen cited, Vieillard's collection from low land in the south, as discussed in Appendix I of this paper. However, much of what is now recognised as the distinct species Retrophyllum comptonii was originally named Podocarpus minor in herbaria. Indeed, when Brongniart & Gris (Reference Brongniart and Gris1869) published the first description of the female cones of what they called Podocarpus minor, that description was based on two then newly collected specimens: Balansa 186 from Prony, which is Retrophyllum minus, and Balansa 1381 from woods near Téné and Bourail, which belongs to what we now know as R. comptonii. Brongniart & Gris's description of the albumen and embryo of Podocarpus minor could only have been based on the seed of Balansa 1381, and so this part of their description of P. minor applies only to Retrophyllum comptonii in today's taxonomy of the group. Seed colour, which varies from red to whitish-grey and has been used to separate taxa within the group (notably Podocarpus palustris), was not mentioned by Brongniart & Gris. However, an early collection of Retrophyllum minus by Pancher (s.n., labelled Vieillard 1275; see discussion, Appendix I) from Prony described the fruit as red, although only very young seed cones, not long past receptivity, are present on the sheet.
Buchholz (Reference Buchholz1949) described three new species of Podocarpus sensu lato from New Caledonia, the first two of which, P. comptonii J.Buchholz and P. palustris J.Buchholz, belong to Retrophyllum (the third, Podocarpus sylvestris J.Buchholz, does belong to Podocarpus as currently circumscribed: de Laubenfels, Reference de Laubenfels, Aubreville and Leroy1972). Both Podocarpus comptonii and P. palustris were distinguished by him from P. minor [Retrophyllum minus], for which, however, he provided no comparable description. Podocarpus comptonii was separated by its habit (a tall tree), its thinner adult leaves with midrib less than one quarter the width of the lamina, and by differences in female seed shape and wood colour. Podocarpus palustris was described as being amphibious and was distinguished from P. minor by: (1) the large caudices (trunks enlarged below: an adaptation to the amphibious habit); (2) the whitish wood of lower density (0.3); (3) the thicker, shorter, strongly reflexed seeds; (4) the smaller bracts on the female peduncle; (5) the smaller receptacle only 2 mm long; (6) the beak of what he called the ‘nut’ [i.e. the seed proper, only visible by dissection of a cone] shorter, more broadly conical and recurved; (7) the leaves broader than usual for P. minor relative to the length, with the margins less thick in the dried state, and deciduous on the branches of the current year, the individual leaves (called by Buchholz ‘leaflets’) 2–6 × 1–3 mm. The seeds of Podocarpus comptonii were stated to be red, while those of P. palustris were described as having a grey epimatium when ripe, thereby distinguishing it from the red epimatium of P. minor (and P. comptonii as described in its protologue). Buchholz (Reference Buchholz1949) noted that both species sometimes grew at the same locality (e.g. 22 km Station) while maintaining their character differences.
The description of these two new species by Buchholz (Reference Buchholz1949) brought a deal of confusion into the taxonomy of New Caledonian Retrophyllum that is still not satisfactorily resolved. There are two separate problems: the distinction between Retrophyllum minus and R. comptonii, and whether or not Podocarpus palustris is a distinct taxon.
Over most of the island, there is no problem over species separation of Retrophyllum comptonii and R. minus, because R. comptonii is the only one present, but in the south both species can occur sympatrically, along with the taxon Podocarpus palustris if that is regarded as distinct from R. minus. Of the various differential characters cited by Buchholz (Reference Buchholz1949) to distinguish Retrophyllum comptonii and R. minus, the width of the midrib (or ‘central band’ of de Laubenfels, Reference de Laubenfels1969) is the one that has more often been used (e.g. de Laubenfels, Reference de Laubenfels1969, Reference de Laubenfels, Aubreville and Leroy1972). Herbert et al. (Reference Herbert, Hollingsworth, Gardner, Mill, Thomas and Jaffré2002: 176), however, commented that this character was “exceedingly difficult to interpret” and that it was therefore “a poor arbiter where there is uncertainty over identifications” but provided no alternative morphological means of separating the two species. They did, however, find that the use of RAPD provided reliable molecular markers that could be employed to separate them. Gray (Reference Gray1962) had separated them on the basis of habit (large trees in Retrophyllum comptonii, small trees or shrubs in R. minus), foliage mostly flattened (R. comptonii) versus rarely flattened (R. minus) and twigs pinnately leaved (R. comptonii) versus foliage mostly decussate (R. minus). Farjon (Reference Farjon2010) attempted to get round the problem by keying out Retrophyllum minus first on the basis of specimens of it being ‘shrubs or small, dwarfed trees’ as compared with the other four species, all of which he considered to be ‘potentially tall trees’, by the foliage branches of R. minus being ‘ascending or spreading’ versus ‘spreading or more or less pendulous’ in R. comptonii and the other species, and by the leaf apex ‘always obtuse’ versus ‘acute, apiculate or some obtuse’. The habit/stature characters used by both these authors are difficult or impossible to interpret correctly from herbarium specimens unless these are accompanied by detailed notes on habit, which is rarely the case; additionally, although Retrophyllum minus is normally short in stature (0.9–3.5 m), it can occasionally reach a height of 8 m, thereby causing confusion with R. comptonii (2.5–30 m).
No one since Gray (Reference Gray1962) has tackled the issue of Podocarpus palustris, which later workers such as de Laubenfels (Reference de Laubenfels1969, Reference de Laubenfels, Aubreville and Leroy1972), Gaussen (Reference Gaussen1976) and Farjon (Reference Farjon2010) have all regarded simply as a synonym of Retrophyllum minus. Before Buchholz's work, Bernier (in sched., P) had differentiated two informal taxa within Podocarpus minor in southern New Caledonia: ‘type sylvestre’ and ‘type lacustre’, corresponding to P. minor sensu stricto (? and also southern, lowland plants of what Buchholz recognised as P. comptonii) and what Buchholz subsequently described as P. palustris. Most of Bernier's collections are labelled ‘type lacustre’ (Bernier 245, 246, 247, 248, 249, 250, 251; s.n. 7 iii 1948); of those seen, only Bernier 269 and 270 from Forêt Walker and Bernier 271 from Montagne des Sources are labelled ‘type sylvestre’. All of these have been determined for this revision as Retrophyllum comptonii. Bernier 271 was collected at an altitude of 900–1000 m at Montagne des Sources and is obviously that species. Bernier 269 and 270, however, were collected at only 200 m at Forêt Walker. Buchholz & Gray determined three sheets of Podocarpus comptonii at P as being possibly a new form or variety of Podocarpus comptonii; these were Bernier 203 (Forêt du Mois de mai), Buchholz 1350 (Walker's place) and Buchholz 1697 (Walker's place).
It is clear from determinavit slips (e.g. on Virot 658 at A, in 1953) that Gray originally intended to retain Podocarpus palustris as distinct from P. minor. However, that was not the case in her published revision (Gray, Reference Gray1962). In that work, Gray justified her synonymisation of Podocarpus palustris with P. minor by saying that “Dr Buchholz (ms. data) believed there to be a related species Podocarpus palustris which he described, but the differences he listed do not fall outside the normal range of variation in P. minor, and the difference in wood density is no more than expected from the slightly different ecological habitats”. Other apparent differences between Podocarpus minor and P. palustris can be found when herbarium material is examined. However, insufficient is known about the variation or constancy of these differences, and in this revision Podocarpus palustris is therefore regarded as a synonym of Retrophyllum minus.
Group C species
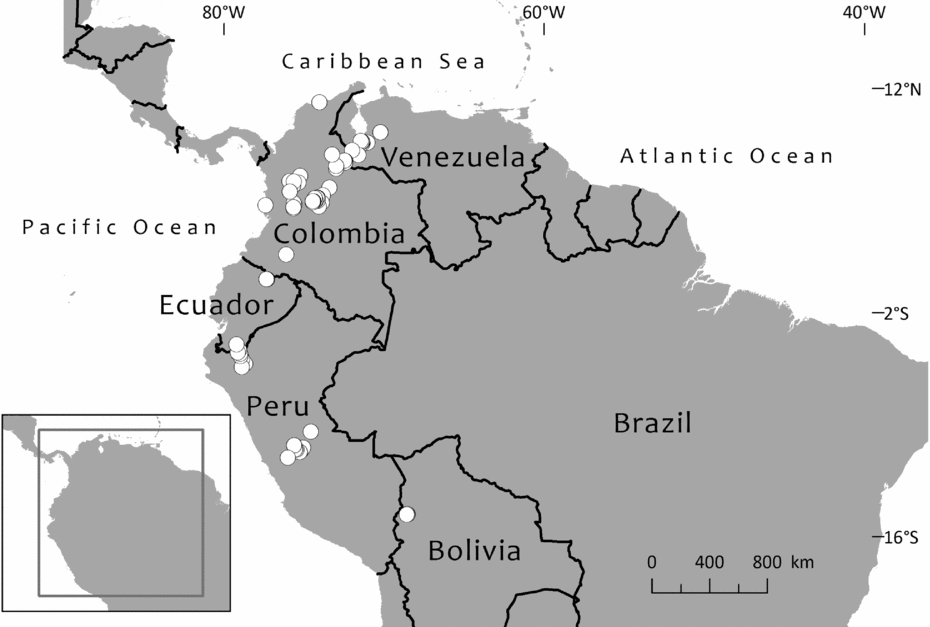
Until 1983, only one species of Retrophyllum was considered to grow in South America. This was Retrophyllum rospigliosii, first described as Podocarpus rospigliosii by Pilger (Reference Pilger1923) based on a collection from Peru bearing very young male cones. Female cones were apparently unknown until they were described, from Venezuelan material, by Schnee (Reference Schnee1944). The species is now known to be widespread along the northern Andes chain, occurring in Bolivia, Peru, Colombia, Ecuador and Venezuela, where it reaches its northernmost limit in the state of Trujillo, whence it has been recently collected (Dorr et al., Reference Dorr, Stergios, Smith and Cuello2000; Hokche et al., Reference Hokche, Berry and Huber2008). Paradoxically, it is now the case that female cones have been much more often collected than mature male ones, and there is some variation in female cone shape, which might have led Page (Reference Page1989, Reference Page, Kubitzki, Kramer and Green1990) to suggest that there could be more than two species of Retrophyllum in South America. However, Retrophyllum rospigliosii is not divided here. Regional treatments of the species have been provided by Schnee (Reference Schnee1944), Buchholz & Gray (Reference Buchholz, Gray and Steyermark1957) and de Laubenfels (Reference de Laubenfels, Luces de Febres and Steyermark1982) for Venezuela, Torres-Romero (Reference Torres-Romero, Pinto and Lozano1988) for Colombia and de Laubenfels (Reference de Laubenfels1994) for Peru.
The second South American species of Retrophyllum is R. piresii, first described as Decussocarpus piresii by Silba (Reference Silba1983). This is apparently a narrow endemic to the Serra Pacaás Novos in Rondónia, Brazil, and remains very poorly known from the type (a female specimen with ripe cones, represented in several herbaria) and two other wild-collected specimens as well as one of cultivated material. No male material appears to have been collected so far. As noted in the species account, there have been records of Retrophyllum piresii from similar habitats in Bolivia and Peru, but these cannot be confirmed at present. There is little published taxonomic information about the species apart from the protologue, a short treatment by Secco et al. (Reference Secco, Silva do Rosário, Rodrigues, Giulietti, Rapini, Gomes de Andrade, de Queiroz and Cardano da Silva2009) and a more detailed one by Farjon (Reference Farjon2010), who said that it should be provisionally kept distinct from Retrophyllum rospigliosii, a decision accepted here for reasons given later.
Graham (Reference Graham2008) documented the discovery of an unidentified Retrophyllum species from humid lowland swamp forest at Madre Selva Biological Station near Iquitos, Peru. Two male trees (nicknamed ‘Lonely George’ and ‘Lonely John’: Graham, Reference Graham2012) were found but at that time no females, and so the field botanists could not determine whether it was a new species, although it appeared to be different from Retrophyllum rospigliosii. The photograph published in Graham (Reference Graham2008) shows a tree with leaves similar to those of Retrophyllum piresii in their elliptic outline. The pollen cones are borne very profusely in the leaf axils; they are brown and about twice the length of the leaves. One female tree has since been found, as well as at least 20 seedlings and other mature trees, all over 100 ft [c.30 m] tall (Graham, Reference Graham2012). However, no herbarium material of this enigmatic taxon has been seen, and it is not known whether any exists. Cones from the single known female tree need to be collected to determine whether this taxon is conspecific with Brazilian Retrophyllum piresii or represents a third South American species of the genus.
In the Paris herbarium, there is also an undetermined specimen (10 xii 1875, Mosén 4393) from Sao Paulo, Brazil, labelled simply ‘Podocarpus’. Superficially it looks rather like a juvenile Retrophyllum, but closer examination reveals that it has net-veined, angiospermous leaves that have apiculate tips. Lindman (Reference Lindman1898: 25) identified the Stockholm duplicate of this number as sterile material of Holocalyx balansae Micheli (Fabaceae); for a recent revision of that genus see Mansano & Vianno Filho (Reference Mansano and Vianna Filho2010), although it does not cite the Mosén material.
Materials and Methods
This revision is based on examination of selected specimens from the herbaria (using standard codes according to Thiers, continuously updated) A, B (types), BM, E, GH, K and NY, as well as specimen images in the following online repositories and databases: COL (Instituto de Ciencias Naturales, Universidad Nacional de Colombia, 2004 and continuously updated, accessed 8 February 2013; Herbario Nacional Colombiano, no date, accessed 5 February 2015 etc.), JSTOR Global Plants (no date, accessed 3 September 2014 etc.: some types), L, MG (one isotype), MO (types), P, PNGplants (Conn et al., Reference Conn, Lee and Kiapranis2004 onwards, accessed 18 December 2014), R (one isotype), RB (JBRJ, no date, accessed 15 April 2014: one isotype), S (Krypto-S, no date, accessed July 2014 etc.: some types) and UDBC. Additional material at COL and UDBC was photographed for this research. The databases of COL, K (Kew, no date, accessed 3 September 2014 etc.), MEDEL, MO (TROPICOS), NOU (Herbier du centre IRD de Nouméa, no date, accessed 21 and 22 July 2014), S (Krypto-S, no date), WU (Vienna Herbaria) and Z (Zürich Herbaria, no date, accessed July 2014) were also consulted (that of MEDEL can no longer be found on the Internet so is not cited). Database records (indicated in specimen citations by the suffix ‘–database’) have been included only where there is no doubt as to correctness of identification, particularly if no duplicates of the specimen concerned have been examined either physically or as images. In specimen citations, the suffix ‘–photo’ denotes a photograph of a specimen, either mounted on a herbarium sheet (as in the case of much of Buchholz's material) or taken by a colleague, while the suffix ‘–image’ indicates a digital image in an online repository.
Herbarium specimens were examined using a stereo dissecting microscope and Leitz ×6 or ×8 loupes and photographed using a Panasonic Lumix TZ30 digital camera. Measurements were all made on dried material; when fresh, some organs can be larger, especially female cones, but in Retrophyllum there is not the same degree of lengthening of pollen cones with age as there is in genera such as Podocarpus. Some measurements were made on calibrated images using ImageJ version 1.49d (Rasband, Reference Rasband2014); calibration of Paris database images lacking scales was performed using the barcode label, measuring 50 × 20 mm, as a datum basis. Line drawings are provided for taxa that have not been previously illustrated or for which the known published illustrations are in some way unsatisfactory. Microsporophylls and microsporangia were, when possible, rehydrated for drawing by the artist. Where a species has been previously satisfactorily illustrated, an iconography is given listing known published illustrations of good quality and correctly identified.
References to Articles of the International Code of Nomenclature refer, unless stated otherwise, to the current Melbourne version (McNeill et al., Reference McNeill, Barrie, Buck, Demoulin, Greuter, Hawksworth, Herendeen, Knapp, Marhold, Prado, Prud'homme van Reine, Smith, Wiersema and Turland2012).
For each species, as part of the distribution summary, the Level 3 and 4 country names and codes of the Taxonomic Databases Working Group (TDWG) are listed according to Brummitt (Reference Brummitt2001). The names of World Wide Fund for Nature Bioregions and Ecoregions follow the schemes published for Latin America and the Caribbean by Dinerstein et al. (Reference Dinerstein, Olson, Graham, Webster, Primm, Bookbinder and Ledec1995) and for the Indo-Pacific by Wikramanayake et al. (Reference Wikramanayake, Dinerstein, Loucks, Olson, Morriaon, Lamoreux, McKnight and Hedao2002). Assignments to ecoregions were done using the Worldwide Fund for Nature's ecoregion map available at Wildfinder (no date).
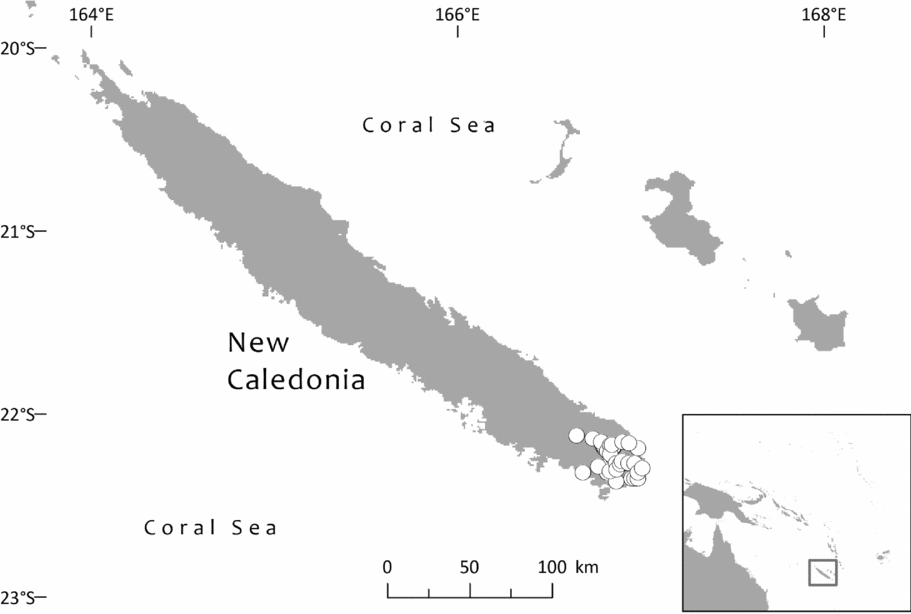
In specimen citations, country names are in Small Capitals, first-order subdivisions of countries (divisions in Fiji; provinces in Solomon Islands, Indonesia, Papua New Guinea, New Caledonia and Ecuador; states in Brazil and Venezuela; departments in Bolivia and Colombia; and regions [formerly provinces, until 2002] in Peru) in Bold and second-order subdivisions of countries (provinces in Fiji, Papua New Guinea, Bolivia and Peru; regencies in Indonesia; districts in Papua New Guinea; communes in New Caledonia; municipalities in Brazil, Colombia and Venezuela; and cantons in Ecuador) in Bold Italic. Solomon Islands has no second-order administrative divisions. Some countries also have third-order divisions (e.g. districts in Peru and municipalities in Bolivia); where known, these are indicated in italic. Assignment to communes in New Caledonia follows the database of NOU (Herbier du Centre IRD de Nouméa, no date). Some peaks in New Caledonia are at the boundary between two or more communes (e.g. Mt. Dzumac: Dumbéa and Païta), so some records are assigned to one commune and some to another. For Fiji, it has seemed helpful also to indicate the main islands in Bold Small Capitals, because the first-order administrative divisions sometimes divide islands or are divided between islands.
The paper includes a list of accepted names and synonyms (Appendix II) and a list of exsiccatae (Appendix III). Collectors’ initials are given in Appendix III but not in the specimen citations unless omitting them would create ambiguity (e.g. A.C. Smith, D. Smith and D.N. Smith). The New Caledonian collector H.S. MacKee spelled his surname in earlier collections as McKee; all specimen listings have been standardised as the more frequent MacKee, following Morat (Reference Morat2010), although his wife Margaret styled herself McKee (cf. McKee, Reference McKee1972, cited elsewhere in this paper). In Appendix II, specimens collected by one person but numbered in another person's series are listed twice for cross-referencing, under both the name of the actual collector and that of the person whose number sequence was used. This situation is most common with MacKee, in whose collection number series are specimens collected by numerous other individuals, but is also found in other numbering series, such as that of Vieillard, which includes material collected by Pancher and probably also Deplanche.
Maps are provided for all species. These were generated in ArcGis version 9.3 (ESRI, 2008) after geospatial coordinates had been added for specimens lacking such information. The main sources of geographical coordinates were GeoNames (no date) and Fuzzy Gazetteer (no date), as well as, for New Caledonia, the gazetteer coordinates in many specimen records in the database VIROT (Herbier du Centre IRD de Nouméa, no date; cited in square brackets after the locality). Phytogeography is indicated according to the scheme of Takhtajan (Reference Takhtajan1986).
Version 3.1 (second edition) of the categories and criteria of the International Union for the Conservation of Nature (IUCN, 2012) and version 10.1 of the guidelines for applying these (IUCN Standards and Petitions Subcommittee, 2013) were used to make the provisional conservation assessments for certain taxa treated in this paper.
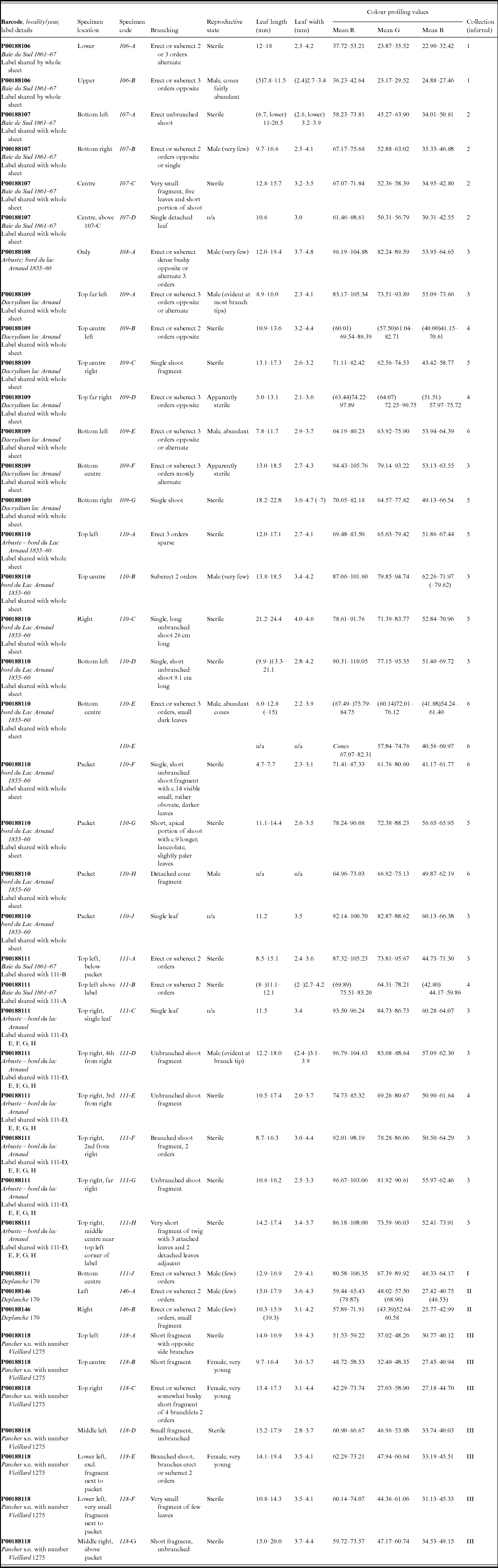
As part of the process for the lectotypification of the name Nageia minor Carrière, basionym of Retrophyllum minus (Appendix I), colour profiling was performed on the digital images of certain collections of R. minus at P using the image-processing and -analysis program ImageJ (Rasband, Reference Rasband2014). These included all those numbered Vieillard 1275 (the ‘type material’), as well as collections by Pancher and Deplanche (Appendix table 1). The original images were assumed to have been made using identical exposures, and no alterations were made to the original files. The colour profiles of portions of leaf measuring approximately 20 × 20 pixels (px, equivalent to c.1.7 × 1.7 mm on all the Paris specimens analysed, after calibration at 11.7 px/mm), as well as of a transverse line across the middle of that leaf, were generated using the ImageJ tool Analyze – Tools – Colour Profiler, which determines the proportions of red (R), green (G) and blue (B) in the image and provides a plot of intensity for each of the channels as well as tabulating the results (Ross, Reference Ross2007). Profiles were generated for several leaves on each specimen on each sheet; usually one profile was generated per leaf, but on very small fragments with few leaves, or single leaves, separate profiles were generated for areas c.20 × 20 px at the base and apex of the leaf. Areas of obvious discoloration or damage of the leaf surface were avoided whenever possible. The profile values given in Appendix table 1 relate to 20 × 20 px areas; those from the transverse lines showed slightly more variation, related to the often greater changes in topography of the dried leaf across the line. The profile values and corresponding plots allowed the accurate assignment of specimen fragments to the different elements that make up the mixed gatherings numbered Vieillard 1275, as well as ones by Deplanche and Pancher.
Taxonomy
Retrophyllum C.N. Page, Notes Roy. Bot. Gard. Edinburgh 45(2): 379 (22 Feb. 1989 [‘1988’]). – Podocarpus L'Hér. ex Pers. sect. Polypodiopsis C.E.Bertr., Ann. Sci. Nat., Bot. Sér. 5, 20: 65 (1874; see comments). – Nageia Gaertn. sect. Polypodiopsis (C.E.Bertr.) de Laub., Blumea 32: 210 (3 Feb. 1987). – [Decussocarpus de Laub., J. Arnold Arbor. 50: 340 (1969) sect. Decussocarpus (op. cit. p. 341), now a nom. illegit. (Art. 52) although legitimate when published.] – Type: Retrophyllum vitiense (Seem.) C.N. Page, based on Podocarpus vitiensis Seem.; the latter name is also the type of Decussocarpus de Laub. and of Podocarpus sect. Polypodiopsis C.E.Bertr. (non Polypodiopsis Carrière).
?[Polypodiopsis Carrière, Traité Gén. Conif. ed. 2: 710 (1867), nom. utique rej. (see comments)]. – Type: Polypodiopsis muelleri Carrière, loc. cit. (type specimen not indicated).
Nomenclatural note
Polypodiopsis Carrière, typified by P. muelleri Carrière from New Caledonia, may be synonymous with Retrophyllum, or it could be a member of the Proteaceae, such as Beauprea balansae Brongn. & Gris, as was suggested by Hutchinson (Reference Hutchinson1920). Polypodiopsis Carrière, an earlier homonym of the fern genus Polypodiopsis E.B.Copel., is now a nomen rejiciendum and cannot be used at genus rank (Mill & Weston, Reference Mill and Weston2004; Brummitt, Reference Brummitt2006 [‘2005’]; Barrie, Reference Barrie2006). However, when Bertrand (Reference Bertrand1874) apparently reduced Carrière's name to Podocarpus sect. Polypodiopsis, he designated a different name, Podocarpus vitiensis (now Retrophyllum vitiense), as type of the section, the name of which should therefore be attributed to Bertrand, not Carrière.
Etymology
Retrophyllum is derived from Latin, retro (‘backwards’), and Greek, phyllon, Latinised as phyllum (‘leaf’), on account of the orientation of the leaves, which are twisted so that along one side of the stem axis all the abaxial surfaces face uppermost, while along the other side of the axis all the adaxial surfaces are turned uppermost. The gender is neuter.
Trees or shrubs; dioecious. Trunk buttressed or not. Bark exfoliating in short vertical strips or small pieces (Pacific species: Groups A and B), or as large plates (South America: Group C). Vegetative shoots dimorphic with either both foliage and scale leaves or only scale leaves (Groups A and B) or monomorphic with foliage leaves only (Group C), heterofacially flattened at least in juvenile phase, in adult phase sometimes not fully so (New Caledonian species: Group B). Terminal buds developed on shoots, these either protected in some way (Groups A and B) or naked (Group C); bud scales present (Fiji and Malesia: Group A) or absent (Groups B and C). Leaves of juvenile phase always ± distichous, heterofacially turned at base so that all leaves along one side of the axis present their abaxial surface to the light and all those along the other side present the adaxial surface; those of adult phase either distichous and flattened in 2 ranks (Groups A and C) or wholly or partly in 4 ranks that show only minimal heterofacial turning (Group B). Adult leaves subsessile with bases decurrent and ± crossing over to a departure point on the opposite side and so creating a ± zigzag pattern along the branch (all groups, but particularly prominent in Group C); blades bifacially flattened, coriaceous, relatively thin (Groups A and C) or thick to very thick (Group B), with or without a visible midrib; resin canals 1 median below the vascular bundle (Groups A and B) or (1)3(5) below the vascular bundle plus 2–6 lateral bundles around the leaf margin (Group C); transfusion tissue present; accessory transfusion tissue absent. Pollen cones usually lateral but sometimes terminal in Retrophyllum vitiense, 1–8 together, potentially arranged in racemose inflorescences (Groups A and C) or groups (Group B) borne on a common peduncle; individual pollen cones ± pedicellate (Groups A and C) or sessile (Group B), cylindrical, ellipsoid or ovoid; microsporophylls imbricate, decussate (Group B: appearing spirally arranged) or spirally arranged (Groups A and C), triangular or ovate (Groups A and B) or lanceolate (Group C). Pollen 2-saccate. Female cones borne on current growth, as part of specialised reproductive shoots (Groups A and C) or terminating foliage shoots (Group B); cones sylleptic (Groups A and C) or proleptic (Group B), pedunculate; peduncle broadened distally (Group C) or not (Groups A and B), in the vegetative portion bearing adpressed scales (Group A) or spreading bracts (Groups B and C); carpophore absent; cone axis with either 1 or 2 (Group B) or 6–8 (Groups A and C) sterile bracts below the fertile bract; fertile bract free from the epimatium (Groups A and B) or with its basal half firmly adnate to it (Group C), distally navicular (Group C) or flat (Groups A and B). Prophylls absent. Receptacle vestigial, formed from the fertile bract and sometimes the last sterile bract (Groups A and C) or from 1 or 2 peduncle bracts plus the last sterile bract and fertile bract (Group B), cylindrical (Groups A and C) or obovoid (Group B). Ovule inverted. Epimatium present. Seed distally crested or not, with a beak at the micropylar end (Groups A and B) or lacking a beak (Group C). Germination epigeal (Group B) or hypogeal (Group C). Seedling phyllotaxis decussate (Group B), spiral (Group C) or currently unknown (Group A).
Present diversity and distribution
Six living species in three very distinct morphological lineages occurring respectively in: (A) Fiji, Solomons and eastern Malesia west to the island of Morotai in the Moluccas; (B) New Caledonia; and (C) tropical South America (eastern slopes of the Andes and extending into the Amazon Basin in Brazil).
Past diversity and distribution
Two (or more?) fossil species have also been included in the genus as traditionally circumscribed: †Australasia (New Zealand, Western Australia and Tasmania [undescribed material]) and southern South America (Argentina and ?Chile). There is apparently no record of the genus having occurred in Antarctica, which might be expected given the disjunct distribution of the living and known fossil species between the Australasia/Southwest Pacific area and South America.
Chromosome number and genomics
Diploid chromosome number: 2n = 20 (3 species cytologically known, from Groups A and B only). The karyotype of all known species comprises 10 pairs of metacentric chromosomes. The genome size is known only for Retrophyllum rospigliosii (of which the chromosome number is not yet known); at 12 pg, it is small for conifers but average within the Podocarpaceae, most species of which have small genome sizes of 8–18 pg, with only Falcatifolium (22 pg in F. taxoides) and Manoao colensoi (28 pg) being larger within the family (Zonneveld, Reference Zonneveld2012). Vieira et al. (Reference Vieira, Rogalski, Faoro, Fraga, dos Anjos, Picchi, Nodari, Pedrosa, de Souza and Guerra2016) have very recently published the complete plastome sequence of Retrophyllum piresii, based on material cultivated at the Museu Paraense Emílio Goeldi in Belém; I have seen herbarium material of this plant as well as photographs kindly sent by Chad Husby, and have verified the identification.
Key to species
1a. Vegetative shoots monomorphic, scale leaves absent; buds naked; female reproductive shoots subtended by a bract or by a modified (reduced) foliage leaf; female cones with the base of the fertile scale firmly adnate to the epimatium and with a navicular tip; bark exfoliating in large plates (Group C: South America) 2
1b. Vegetative shoots dimorphic, scale leaves present; buds protected, either by true bud scales or by modified leaves; female reproductive shoots subtended by a normal foliage leaf; female cones with the fertile scale free from the epimatium and not navicular; bark exfoliating in short vertical strips or small pieces (Malesia, Papuasia and New Caledonia) 3
2a. Peduncle of female cone bearing reduced leaves; foliage leaves lanceolate, acute to acuminate and frequently abruptly narrowed just below apex; adult shoots ± alternate (widespread from Venezuela to Peru) 5. R. rospigliosii
2b. Peduncle of female cone bearing small bracts; foliage leaves elliptic, obtuse, never abruptly narrowed below the tip; adult shoots opposite (one definite locality in Brazil) 6. R. piresii
3a. Female cones borne on specialised reproductive shoots that are either lateral or terminal; ultimate adult foliage shoots pendulous with leaves in 2 ranks; adult leaves with an evident midvein; buds protected by true bud scales (Group A: Malesia, Fiji, Solomons) 4
3b. Female cones terminal at ends of ordinary foliage shoots; ultimate adult foliage shoots erect or suberect with at least some leaves in 4 ranks; adult leaves with no visible midvein; buds protected by very reduced leaves, lacking true bud scales (Group B: New Caledonia) 5
4a. Leaves broadest near base; microsporophylls 44–80 in 11–20 whorls; female cone axis with 6–8 sterile scales that persist at least until the resting phase (Fiji and Solomons) 1. R. vitiense
4b. Leaves broadest near middle; microsporophylls 20–32 in 5–8 whorls; female cone axis with 4 sterile scales that are very quickly caducous, only present during the youngest stages (New Guinea, Moluccas) 2. R. filicifolium
5a. Adult leaves with the raised central area narrower than the marginal area; some adult leaves on ultimate shoots in 2 ranks, those towards shoot tips normally in 4 ranks; habit variable from shrub to tall tree; not aquatic 3. R. comptonii
5b. Adult leaves with the raised central area broader than or at least equalling the marginal area; all adult leaves on ultimate shoots in 4 ranks; aquatic shrub or ‘treelet’ with trunk rapidly tapering from a wide base 4. R. minus
Species descriptions
Group A. Bark exfoliating in short strips or small pieces. Vegetative adult shoots dimorphic. Terminal buds protected by bud scales. Leaves heterofacially flattened in both juvenile and adult phases. Adult leaves distichous in 2 ranks, relatively thin. Resin canal 1, positioned below vascular bundle. Pollen cones in racemose inflorescences subtended by scale-like bracts (or sometimes terminal and apparently solitary, as in the type of Retrophyllum vitiense), the raceme branches if present alternate or spiral, individual cones pedicellate, subtended by smaller scale-like bracts. Female cones on specialised lateral reproductive shoots, sylleptic; peduncle not broadened distally, in its vegetative portion bearing subadpressed scales. Cone axis with 4–8 sterile bracts. Fertile bract free from epimatium, flat. Receptacle vestigial, formed from the fertile bract and distal part of peduncle which may be slightly swollen, cylindrical or obovoid. Seed beaked at micropylar end. Details of germination and seedlings not known.
1. Retrophyllum vitiense (Seem.) C.N. Page, Notes Roy. Bot. Gard. Edinburgh 45(2): 380 publ. (22 Feb. 1989 [‘1988’]). – Podocarpus vitiensis Seem., Bonplandia 10: 366 (25 Dec. 1862) & J. Bot. 1: 33, t. 2 (1863). – Nageia vitiensis (Seem.) Kuntze, Revis. Gen. Pl. 2: 800 (1891). – Decussocarpus vitiensis (Seem.) de Laub., J. Arnold Arbor. 50: 342 (1969). – Lectotype (Farjon, Reference Farjon2010: 942 & 943): Fiji, Seemann 576, sheet 2/2 (lecto K, isolectos BM [2 sheets], E, GH–image, S–images). Fig. 1D.

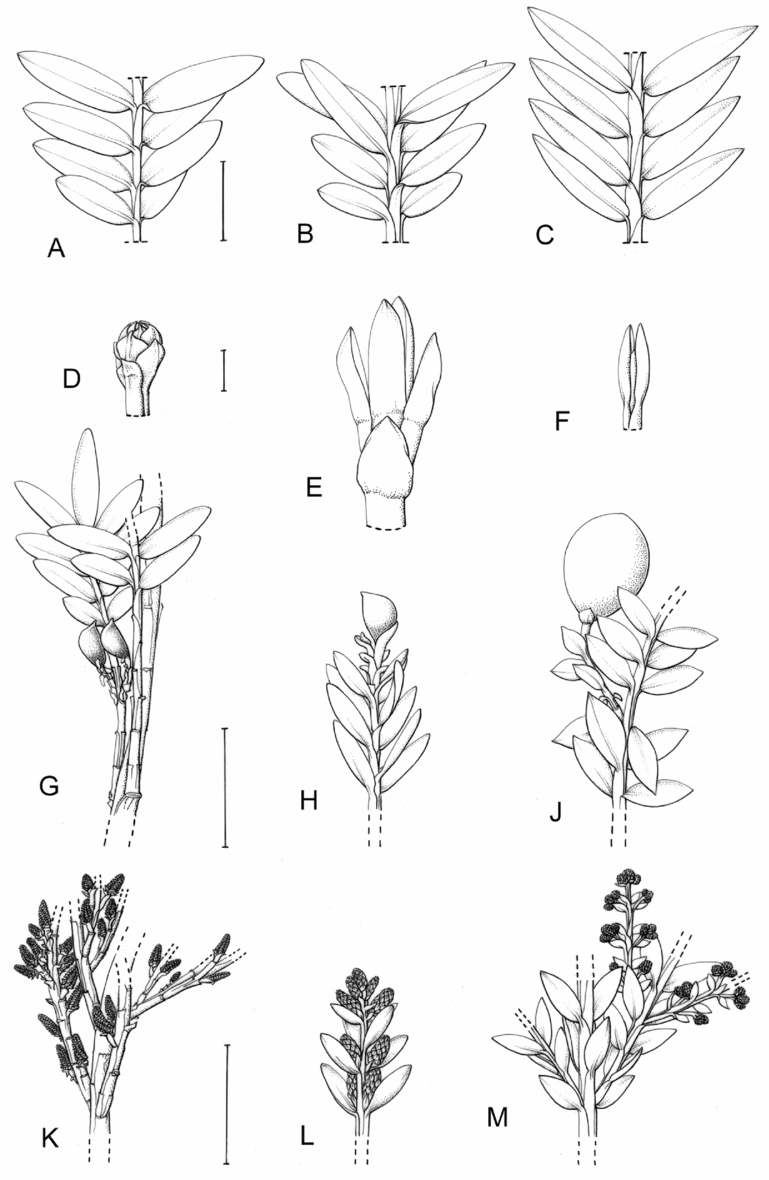
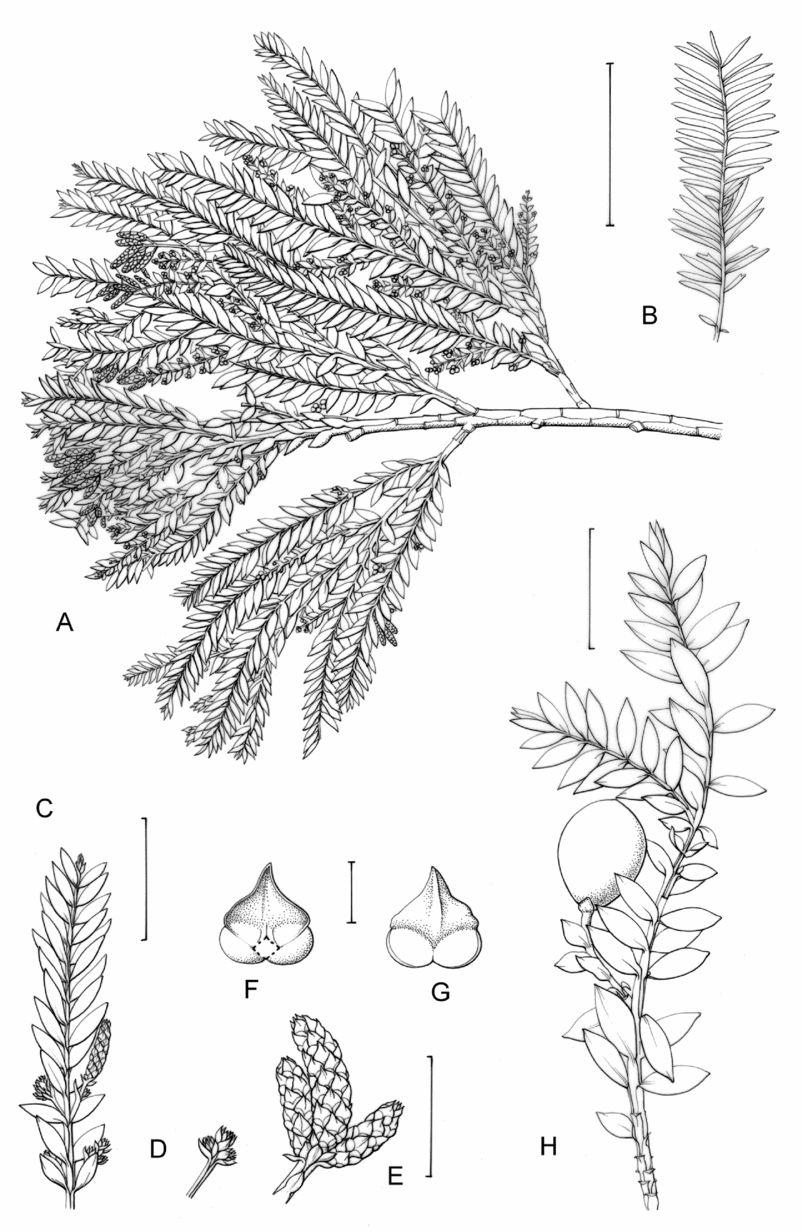
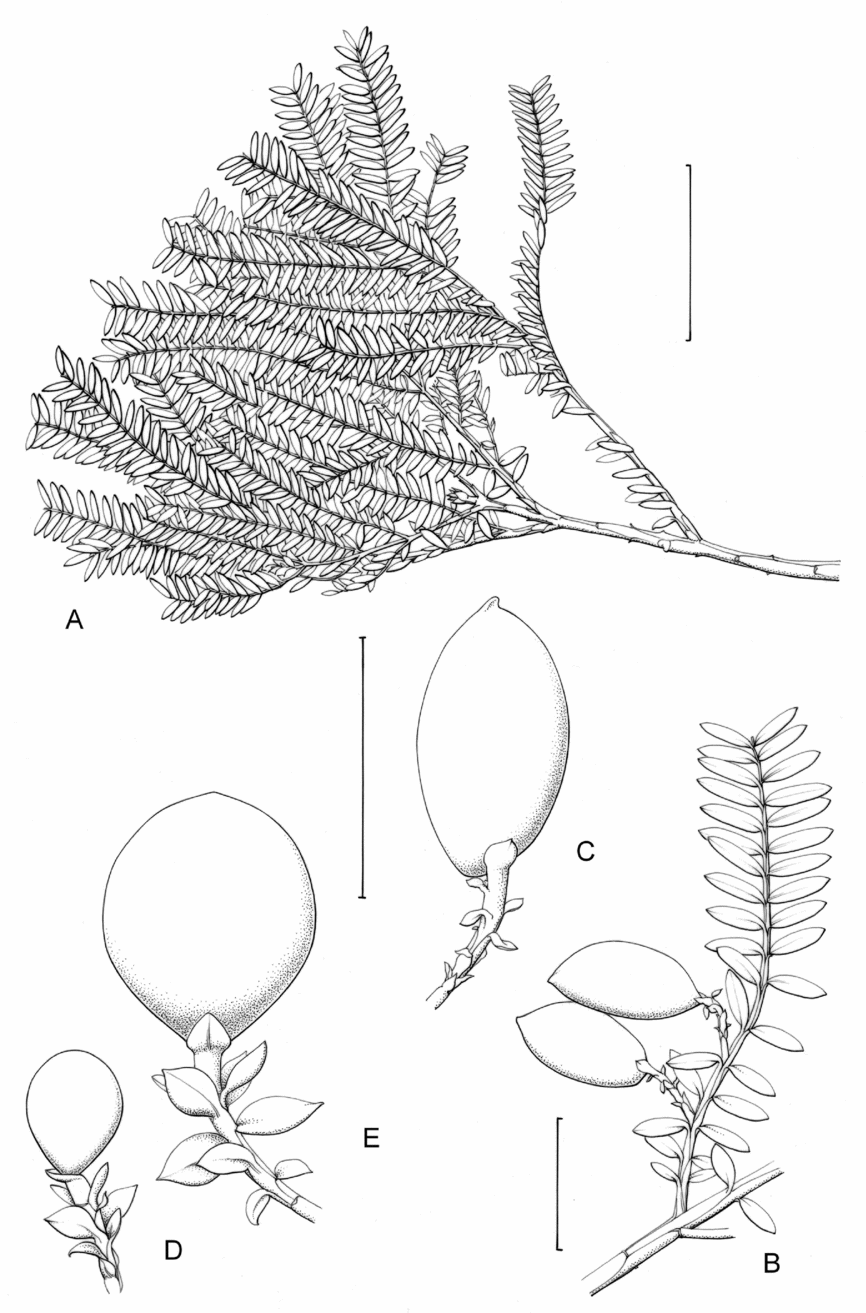
Fig. 1. Retrophyllum species, comparative drawings. A–C, Leaf arrangements × 2. A, Retrophyllum filicifolium (Group A) (Woods 345). B, R. comptonii (Group B) (Gardner et al. TNCA 5045). C, R. rospigliosii (Group C) (Wright 247). D–F, Terminal bud or equivalent, × 15. D, Retrophyllum vitiense (Group A) (Smith 7076). E, R. comptonii (Group B) (Gardner et al. TNCA 2036). F, R. rospigliosii (Group C) (García-Barriga 27584). G–J, Female cones, × 1.5. G, Retrophyllum filicifolium (Group A) (Woods 345). H, R. comptonii (Group B) (Gardner et al. 256). J, R. rospigliosii (Group C) (Bunting 4939). K–M, Male cones × 1.5. K, Retrophyllum filicifolium (Group A) (Carr 14160). L, R. minus (Group B) (McMillan 5139). M, R. rospigliosii (Group C) (R.T. Pennington 1433). Scale bars: A–C, 1 cm; D–F, 1 mm; G, H, J–M, 2 cm. Drawn by Claire Banks.
?[Torreya bogotensis Linden ex K.Koch, Wochenschr. Vereines Beförd. Gartenbaues Königl. Preuss. Staaten 14(25): 199 (1871), nom. utique rejic.: Mill, Reference Mill2010; Brummitt, Reference Brummitt2011].
Note on typification
Gray (Reference Gray1962: 73) indicated that the Kew example of Seemann 576 was the ‘holotype’ of Podocarpus vitiensis (basionym of Retrophyllum vitiense), with an isotype (not so indicated) at GH. This was incorrect because, as was noted by Smith (Reference Smith1979), Seemann (Reference Seemann1862: 366; Reference Seemann1863: 33) cited two syntypes in both the original protologue and in another publication the following year; the first of these was collected by W.G. Milne on the voyage of HMS Herald, and the second was Seemann 576. (Smith, Reference Smith1979: 101 in fact cites two Milne collections of this species, both unlocalised, one without number and the other Milne 33). However, de Laubenfels (Reference de Laubenfels1969: 344) repeated the designation of Seemann 576 at K as ‘holotype’ of Podocarpus vitiensis, with specimens at A and BM indicated as ‘isotypes’. These specimens should be regarded as lectotype (K) and isolectotypes (A, BM, GH) respectively, following Smith (Reference Smith1979: 100) and the more precise lectotypification by Farjon (Reference Farjon2010). The choice of a Kew specimen as lectotype by both the above authors is difficult to understand, because it is one of the two BM sheets, not either of the K sheets, that bears Seemann's drawings that were used in his two publications (1863, 1868). However, there is no controversy over the identity of any of these sheets, and Farjon's recent designation, which supersedes the earlier ones by de Laubenfels (Reference de Laubenfels1969: 342) and Smith (Reference Smith1979: 100), must therefore stand. Both latter authors independently chose Seemann 576 at K but without realising that there are two sheets of that number at K, one of them (annotated ‘1/2’, i.e. sheet 1 of 2) being a seedling and the other (‘2/2’) a branch from a young tree, and they could not have come from the same plant. Sheet ‘1/2’ at Kew is therefore not an isolectotype.
Iconography
Seemann, J. Bot. 1: t. 2 (Reference Seemann1863).
Etymology
Derived from Viti, alternative name for Fiji.
Vernacular names
ailumu (Solomon Is., Kwara'ae language: Mauriasi et al. BSIP 17025 in sched., K, L); dakua salusalu (Fiji: Parham, Reference Parham1964, Reference Parham1972; Damanu R10 in sched., K), ndakua salusalu or salu-salu (Fiji: Mead, Reference Mead1928; Smith, Reference Smith1979); ngapiru [Vanikoro (Solomon Is.: Nambalua – Piaito BSIP 7061 in sched., L); ?kau solo (Fiji: Seemann, Reference Seemann1862, Reference Seemann1863, Reference Seemann1868); tau solo (Fiji: Seemann, Reference Seemann1868, possibly a misprint for kau solo) Seemann's use (Reference Seemann1862 etc.) of the name kau solo in fact refers to Dacrycarpus imbricatus var. patulus, according to Smith (Reference Smith1979), who also said that the most frequently used name for Retrophyllum vitiense is ndakuua salusalu.
Distinguishing features
Retrophyllum vitiense is most closely allied to R. filicifolium (N.E.Gray) R.R.Mill (see below) from New Guinea and the Moluccas. Both have ± flat, distichous adult leaves, female cones borne laterally near the base of an ultimate foliage shoot, and dimorphic shoots bearing either foliage leaves or small decussate scale leaves. The dimorphic shoots separate both these species from the two South American species of Retrophyllum, which have monomorphic shoots. The flat, distichous, non-imbricate adult leaves and lateral female cones separate the two Papuasian species from the New Caledonian species Retrophyllum comptonii and R. minus, both of which have terminal female cones and adult leaves more or less in 4 ranks and ± imbricate (although juvenile and transitional shoots are distichous). Vegetative material of Retrophyllum vitiense is best separated from its nearest ally R. filicifolium by its tapered leaves widest close to the base, not near the middle. For differences in reproductive characters between these two species, see under Retrophyllum filicifolium.
Tree, 15–47 m, dbh 25–450 cm. Trunk sometimes spur-buttressed at base on large trees, bole straight and when adult unbranched except near top. Bark smooth, finally with faint vertical fissures, flaking into small longitudinal crumbly pieces, fissured, greyish-brown or (Solomons) dark brown, whiter in mature trees; inner bark yellowish-brown or (Solomons) reddish-brown; wood cream-coloured. Crown compact but much branched, pyramidal and finally rounded, with pendulous branchlets and twigs. Buds ovoid or obovoid; bud scales present, in 2 series, decussate, all equal, ovate and keeled, acute, brownish with narrow paler entire margin, initially persistent but later slowly caducous, not leaving scars. Ultimate juvenile shoots alternate, subopposite or opposite, 130–280 mm, with 23–72 (usually 35–60) pairs of foliage leaves, occasionally biramulate. Juvenile foliage leaves 4–11 mm apart, opposite-distichous, heterofacially turned at base, diverging at 60–80°, shortly petiolate, the petiole 0.2–0.5(–1.5) mm, lamina lanceolate or narrowly lanceolate, those near middle of branchlet 18–35 × (3.8–)4–6(–7) mm. Adult trees with dimorphic shoots, most shoots bearing foliage leaves but leader shoots bearing scale leaves; shoot flattening heterofacial with the decurrent leaf bases running straight down the internodes. Primary adult branches opposite-decussate or alternate. Penultimate adult branches erecto-patent with respect to subtending branch (although topographically deflexed-patent), straight or slightly curved with flexuous internodes, alternately bearing axillary decussate lateral foliage shoots and decussate ovate to orbicular bracts c.2 mm. Ultimate adult foliage shoots (70–)100–230 mm with (7–)11–60 pairs of leaves, straight or curved with slightly flexuous internodes. Leaf scars absent. Adult foliage leaves 4–8 mm apart, opposite-distichous, not imbricate, heterofacially twisted, shortly petiolate; petiole decurrent straight along length of internode, free part 0.2–1.7 mm; lamina of most leaves spreading but not forming a shallow ‘V’ along branch, widely erecto-patent, diverging at 45–70(–80)°, deep green, narrowly lanceolate to lanceolate (widest just above the base), those near middle of branchlets (12–)16–27(–32) × 3.2–6(–7) mm but lowest ones shorter and more ovate, all straight, coriaceous but relatively thin and flexible; margin not thickened, narrowly hyaline; surfaces equally amphistomatic, lacking a raised central area but with longitudinal vein-like striae between the rows of stomata; midrib evident, raised beneath, obscure throughout or at least distally above; apex subacute or obtuse, never mucronate; base rounded, obtuse or cuneate.
Pollen cones terminal or lateral, subtended by a bract, foliage leaf or (when terminal) pair of foliage leaves, solitary or 1–3 together, occasionally in a compound ‘inflorescence’ of 2 pairs of cones and a terminal cone, the individual cones when more than one present each subtended by a bract and shortly pedicellate; cones linear-cylindrical to narrowly cylindrical when terminal, cylindrical to ovoid when lateral; microsporophylls 44–80 per cone, in 11–15(–20) whorls; lamina triangular to ovate or somewhat wedge-shaped with buff-coloured scarious margin; microsporangia reniform, equalling the microsporophyll; pollen with hyaline body and whitish to hyaline sacci.
Female cones lateral, arising from near the base of an ultimate foliage shoot, 1 or 2 per shoot, pedunculate. Peduncle unbranched (bearing 1 cone axis) or forked at apex (bearing 2 cone axes), 2–12 mm, equalling or shorter than cone, longer than vestigial receptacle, erect or erecto-patent when young, with 3–5 pairs of bracts below the cone axis; distal part of peduncle not shed with cone. Peduncle bracts adpressed or subadpressed, ovate, c.1 × 0.5 mm, scarious-margined. Cone axis formed of 6–8 sterile bracts and 1 or 2 fertile bracts. Sterile bracts persisting till resting phase and then slowly caducous, narrowly ovate or ovate-elliptic, subacute, larger than peduncle bracts, at first horizontally spreading, later deflexed and finally caducous. Receptacle cylindrical. Fertile bract initially erect and longer than the epimatium, free from the epimatium and last sterile bract, with a narrowly wedge-shaped, prominently keeled proximal portion and free, ovate-elliptic, acute, non-keeled distal portion. Cones obovoid or pyriform, 15–23 × 10–12 mm; epimatium initially blackish-violet, later turning bright magenta and finally becoming bluish-red when ripe, not crested, with a conical, slightly hooked beak at micropylar end; micropylar beak with two short prongs that point ± vertically downwards towards the last sterile bract.
Taxonomic notes
Seemann was convinced that he was dealing with a new genus allied to Podocarpus (see Seemann, Reference Seemann1863: 33 as well as his annotation on the type, and Wasscher, Reference Wasscher1941: 424), although he nevertheless provisionally placed the species in Podocarpus. His perceptive taxonomic insight has since proved to be correct, because there is no doubt that Retrophyllum, in whatever sense it is defined, is distinct from Podocarpus.
Podocarpus filicifolius N.E.Gray, based on material from Morotai in the Moluccas, has usually been regarded as a synonym of this species, but examination of ample material both from Fiji and from New Guinea and Morotai has demonstrated that the plants from the latter areas are taxonomically distinct from Fijian material. They are treated below as Retrophyllum filicifolium.
Seeds of a fossil species allegedly closely allied to Retrophyllum vitiense were found in Pliocene beds in New Zealand by Evans (Reference Evans1937: 192, note; quoted by Florin, Reference Florin1940: 47). The fossil was not named, and the only other New Zealand fossil that has been definitely assigned to Retrophyllum is the Miocene species R. vulcanense M.Pole (Pole, Reference Pole1992). The possibility that Evans's seeds may have belonged to an extinct allied genus, such as Smithtonia or Willungia, should not be discounted.
Nomenclatural notes
Page (Reference Page1989) used Seemann (Reference Seemann1863) as the basionym reference, being presumably unaware of the earlier Bonplandia one (Seemann, Reference Seemann1862).
Linden [1869, nomen nudum (see Mill, Reference Mill2010); in Thurber (Reference Thurber1870, nomen nudum); Linden ex K. Koch (Reference Koch1871)], described Torreya bogotensis from Bogotá, Colombia; this was considered to be synonymous with Podocarpus vitiensis (Retrophyllum vitiense) by Bertrand (Reference Bertrand1874), but this is surely incorrect. If it really is a Retrophyllum at all (and there is some doubt), it is far more likely to be R. rospigliosii, which grows in Colombia. Linden's name was proposed for rejection under Art. 56 of the ICBN (McNeill et al., Reference McNeill, Barrie, Burdet, Demoulin, Hawksworth, Marhold, Nicolson, Prado, Silva, Skog, Wiersema and Turland2006) by Mill (Reference Mill2010), and this was unanimously recommended by the Nomenclature Committee for Vascular Plants (Brummitt, Reference Brummitt2011). Reveal (Reference Reveal2012) considered that the 1869 French description by Linden validated the name but Mill (Reference Mill2010) did not, and Brummitt (Reference Brummitt2011) reported that even the more detailed descriptive matter given by Koch (Reference Koch1871) was “just sufficient to validate the name”.
Phenology
Fl. Mar–Dec; fr. Jun–Feb (Smith, Reference Smith1979: 99).
Distribution
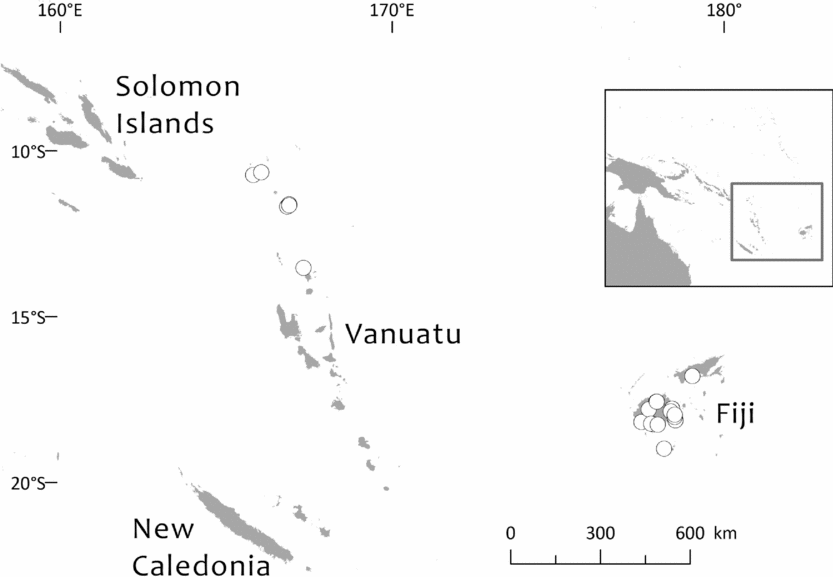
Southwest Pacific: Solomon Is. (Santa Cruz group), Vanuatu (only Banks Islands, Prov. Torba: absent from the main archipelago), Fiji. The Santa Cruz group (Solomons) and Banks Islands (Vanuatu) are adjacent. TDWG: 60 FIJ SCZ VAN. Map: Fig. 2.

Fig. 2. Global distribution of Retrophyllum vitiense.
Specimens seen and other records. Fiji. Unloc., vii 1860, Seemann 576 (K, 2 sheets annotated ‘1/2’ and ‘2/2’, sheet ‘2/2’ being lecto); isolecto BM–2 sheets, E, S–database; 2nd Seemann collection at S (S–database) without collector's number may be another isolectotype, as may a third “fragment ex Berlin” that lacks any information regarding collector or locality). Unloc., no date, Graeffe s.n. (BM, K). ‘Albizzi Levu’: otherwise unloc., Graeffe (K: Graeffe's locality has not been traced). Unloc., anon. (Department of Agriculture, Fiji) 5536 (L–image). Unloc., 300 m, 10 v 1962, Damanu L12 (K–database). Viti Levu: Western: Nausori Highland intermediate montane zone, 1900 ft [579 m], 8 iii 1962, Damanu NH15 (K). Ba: Nandarivatu, seedlings, 2800 ft [853 m], 21 ii 1927, Mead 1964 (K). Nandarivatu, 2800 ft [853 m], 26 ii 1927, Mead 1974 (K); ibid., 840 m, 9 iii 1927, Mead 1982 (K–database). Nandarivatu, valley of the Sigatoka river, Colo-north (= Tholo North), 800 m, 17 xi 1927, Gillespie 3865 (K); ibid., 900 m, 17 xi 1927, Gillespie 3865 (NY; note altitude discrepancy cf. K sheet). Nadarivatu, 2700 ft [823 m], v 1947, d'Espeissis 1460 (E, 2 sheets). Mt. Lomalagi (Nadarivatu), 3000 ft [914 m], 6 viii 1946, Vaughan 3254 (BM). Nadarivatu, 2700 ft [823 m], ix 1907, Gibbs 674 (BM). Nandarivatu, Easter [2–5 iv] 1926, Teulon s.n., comm. (J.D. & B.H.) Tothill 844 (K). Tholo North, vicinity of Nandarivatu, 750–900 m, Nauwanga, 4 ii–26 iii 1941, Degener 14483 (A, L–image, NY, S–database), 11484 (S–database). Tholo North, vicinity of Nandarivatu, 4 ii–26 iii 1941, Degener 14496 (NY). Mba, Tavua, 2.5 miles (by road) W of Nandarivatu, 2800 ft [853 m], 15 vii 1968, Webster & Hildreth 14270 with I. Kuruvoli (GH). Mba, Mandrongo, Nausori Highlands, W slopes of Mt. Mandrongo [Mangondro], 2000 ft [610 m; but the summit is only c.547 m fide GeoNames], 16 vii 1968, Webster & Hildreth 14277 with I. Kuruvoli (GH). Central: Serua: Naboutini, 4 vii 1962, Damanu R10 (K); ibid., 240 m, 5 xii 1962, Damanu R15 (K–database); ibid., 150 m, 28 iii 1963, Damanu R32 (K–database). Galoa, c.500 ft [152 m], 27 vii 1962, Damanu G7 (K). Near Korovisilou, Serua, 300 ft [91 m], 2 vii 1973, de Laubenfels P 508 (A, L–image); Korovisilou, 900 m, 12 xii 1968, Redrodro K110 (K–database). Road to Nambukilevu from Namboutini, c.1000 ft [305 m], 1 xi 1964, de Laubenfels P 309 (A). Tailevu: hills east of Wainimbuka River, in vicinity of Ndakuivuna, 100–200 m, 15–27 iv 1953, A.C. Smith 7076 (GH, L–image, NY, S–database, K–database). Tailevu/Naitasiri: Upper Rewa River valley, 3 vi 1992, Cox 1360 (NY, BRY–database). Naitasiri: Navolau, 17°52′S 178°24′E, 50 m, vi 1936, Peni 636 (K–database). Kadavu: Eastern:Kadavu: Kadavu Naikorokoro, 18°59′S 178°12′E, 240 m, 12 ix 1962, Damanu KU22 (K–database). Navutulevu, 18°14′S 177°48′E, 90 m, 4 vi 1962, Damanu NL10 (K–database). Vanua Levu: Northern: Cakaudrove: Thakaundrove, Yanawai River region, Mount Kasi, 300–430 m, 10–11 v 1934, A.C. Smith 1796 (GH, NY, K–database, S–database). Macuata: Labasa Sarava, 18°02′S 178°31′E, 300 m, 10 v 1962, Damanu L10 (K–database). Vanuatu. Torba: Banks Islands: Ureparapara, near Lehali village, 13°32′S 167°20′E, 300 m, 6 ix 1982, Hammer s.n. (K–database). Solomon Islands: Temotu: Santa Cruz I.: N.E. Santa Cruz, Carlisle Bay area, 900 ft [274 m], 22 x 1969, Mauriasi et al. BSIP 17025 (K, L–image). Ndeni I.: Graciosa Bay, 130 m, 11 xi 1969, Mauriasi et al. BSIP 17794 (K–database, L–image). Vanikoro I.: Lawrence River, 22 iii 1963, Whitmore BSIP 1580 (K–database, L–image); Vanikolo, 11°37′S 166°54′E, iii 1965, Piaito BSIP 7061 (K–database, L–image); nr Lemon River [11°39′S 166°54′E], 27 xi 1945, Walker BSIP 212 (A, K–database). Fiji (cultivated). Agr. exp. Garden, Naduruloulou, 215 i 1949, Lam 6932 (L–image). Australia (cultivated). New South Wales: Botanic Gardens, Sydney, viii 1908, Boorman s.n. (A). Java (cultivated). Jardin Botanique de Tjiboudas, viii 1959, Schnell 10647 (2 sheets, P–images).
Bioregions: Oceania; New Guinea & Melanesia (only the Santa Cruz and Vanuatu populations)
Ecoregions: OC0105 Fiji tropical moist forests, AA0126 Vanuatu rain forests.
Ecology
Dense lowland wet zone rain forest, montane rain forest or ridge forest, where it typically grows as scattered trees on slopes and in gullies in areas with rainfall of approximately 5000 mm per annum; 50–915 m. According to Mead (Reference Mead1928), this rain forest develops on the southeastern or ‘weather’ sides of the mountains. Associates include species of Agathis Salisb., Calophyllum vitiense Turrill, Gironniera celtidifolia Gaudich. (in the understorey), Parinari insularum A.Gray, Semecarpus vitiensis (A.Gray) Engl. and Pagiantha thurstonii (Horne ex Baker) A.C.Sm. (Keppel, Reference Keppel2005; Mead, Reference Mead1928, gives additional rain forest components). It can form a unique community in which it and Calophyllum vitiense are codominant (Keppel et al., Reference Keppel, Tuiwawa, Naikatini and Rounds2011).
Chromosome number: 2n = 20 (Hair & Beuzenberg, Reference Hair and Beuzenberg1958, as Podocarpus vitiensis)
Phytochemistry
The wood of Retrophyllum vitiense contains podocarpic acid, totarol, 19-hydroxytotarol and 4β-carboxy-19-nortotarol (Cambie et al., Reference Cambie, Cox, Croft and Sidwell1983), a unique combination of phenolics among the 10 species of Podocarpaceae studied.
Plant–animal interactions
The beetle Xyleborus rameus Schedl is known to bore into the seeds of Retrophyllum vitiense in Fiji (Greenwood, Reference Greenwood1977). The drywood termite Cryptotermes domesticus (Haviland) is known to attack timber of Retrophyllum vitiense in Fiji (Gray, Reference Gray1974).
IUCN conservation assessment (global: IUCN 3.1)
The most recent assessments are as LC (Farjon, Reference Farjon2010: 943; Thomas, Reference Thomas2013), but these both referred to the species in a wide sense, including what is here recognised as the separate species Retrophyllum filicifolium. Doyle (Reference Doyle, Farjon and Page1999), using an earlier IUCN grading system, evaluated the species as circumscribed here as LRlc on both Fiji and the Solomon Islands, also equivalent to IUCN 3.1: LC. Therefore it is considered that an assessment of LC is appropriate for the species as more narrowly circumscribed here, although it should be noted that Farjon commented that, in the wider geographical area of his circumscription, the species was subject to logging throughout its range.
Uses
Retrophyllum vitiense is one of the most highly prized timber trees of Fiji, being used for high-quality furniture, etc. and for flooring, exterior work and construction (Damanu 15 in sched., K). Mead (Reference Mead1928) commented that in his time it was one of the commonest and most important timber trees of Fiji. He also mentioned its use in boat-building. However, use of the species today should be avoided, because most imports are from non-sustainable forests (Rainforest Information Centre, no date, accessed 16 September 2015). Moreover, the wood is not very decay-resistant and is therefore unsuitable for use in some situations, such as ground contact (Osborne, Reference Osborne1967). The resin is inflammable and is used by Fijians to start fires (Degener 14483 in sched., A, NY and Degener & Degener, Reference Degener and Degener1953).
2. Retrophyllum filicifolium (N.E.Gray) R.R.Mill, comb. nov. – Podocarpus filicifolius N.E.Gray, J. Arnold Arbor. 43: 74 (15 Jan. 1962) quoad descr. foliorum tantum. – Lectotype designated here: Moluccas, Morotai, “tree, 15 m”, 1949, A. Kostermans s.n. (lecto L, foliage shoots only – image, originally determined by van Steenis as Podocarpus vitiensis Seem.; isolecto A, K). The lectotype specimen at L is a mixture of juvenile foliage of Retrophyllum filicifolium and detached fruits of Nageia wallichiana; the latter are here excluded as a result of lectotypification by the foliage element. The isolectotypes cited comprise foliage only. Epitype designated here: New Guinea, Northern District, ridge above Doma, 1500 m, 14 xi 1962, P.J.B. Woods 345 (epi E, barcode E00094518: comprising adult foliage, several attached young female cones, one attached older female cone and another in packet; isoepi K–database). Figs 1A, G, K, 3A–J.
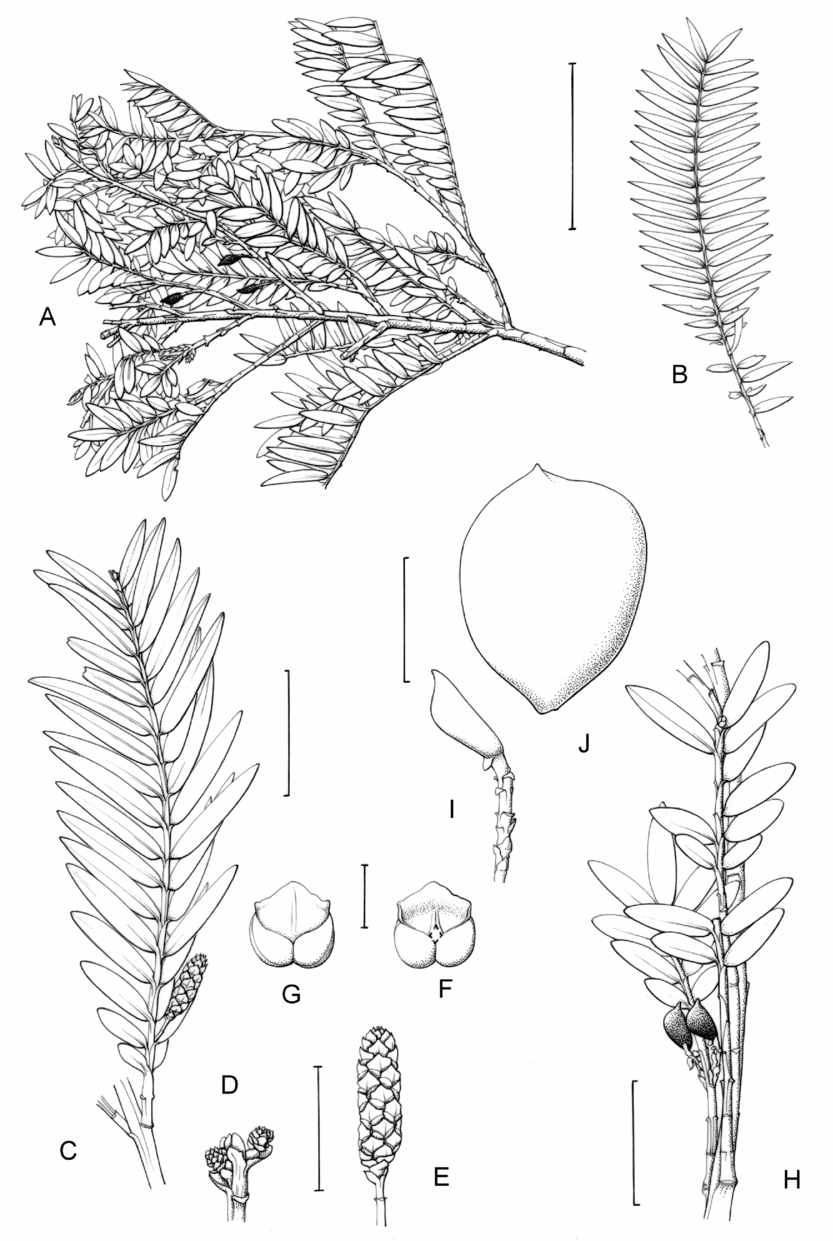
Fig. 3. Retrophyllum filicifolium. A, Branching arrangement (Woods 345). B, Juvenile foliage shoot (Woods 241). C, Adult male shoot (Carr 15666). D, Young male cones (Carr 15666). E, Mature male cone (Carr 15666). F, Microsporophyll and microsporangia, adaxial view (Carr 15666). G, Microsporophyll and microsporangia, abaxial view (Carr 15666). H, Adult female branch (Woods 345). I, Young female cone (Woods 345). J. Mature female cone (Woods 345). Magnifications: A, B, × 0.67; C, H, × 1.5; D, E, I, J, × 3; F, G × 15. Scale bars: A, B, 6 cm; C, H, 2 cm; D, E, I, J, 1 cm; F, G, 1 mm. Drawn by Claire Banks.
Podocarpus vitiensis sensu Wasscher (Reference Wasscher1941: 425–427), Gray (Reference Gray1962: 72–74), p.p. quoad pl. novoguineensis, non Seemann (Reference Seemann1862).
Decussocarpus vitiensis sensu de Laubenfels (Reference de Laubenfels1969: 342) p.p. excl. typ., quoad syn. Podocarpus filicifolius N.E.Gray (p.p.).
Nageia vitiensis (Seem.) Kuntze sensu de Laubenfels (Reference de Laubenfels, van Steenis and De Wilde1988: 394–395) p.p. excl. typ., quoad syn. Podocarpus filicifolius N.E.Gray (p.p.).
Nageia vitiensis sensu Takeuchi (Reference Takeuchi2010: 20) non (Seem.) Kuntze (1891).
Retrophyllum vitiense sensu Li (Reference Li and Marshall2007: 346), Abdillahi et al. (Reference Abdillahi, Stafford, Finnie and Van Staden2010: 7) p.p. quoad pl. novoguineensis.
Etymology: filicifolium, Latin from filix, ‘a fern’, and folium, ‘a leaf’
“The name refers to the fern-like appearance of the pinnately leaved twigs, reminiscent of the royal fern, Osmunda regalis” (Gray, Reference Gray1962: 74).
Vernacular names: lehil (New Britain: Frodin NGF 26292), moegò (New Guinea – Wissel Lakes, Kapaukoe language, Vink & Schram BW8730 as Retrophyllum vitiense)
Distinguishing features. Retrophyllum filicifolium has for long been confused with and included within R. vitiense, as explained in the Taxonomic Problems section of this paper
Male material of Retrophyllum filicifolium can be distinguished from R. vitiense by its pollen cones with fewer microsporophylls (typically 20–32 in 5–8 whorls as opposed to 44–80 in 11–20 whorls). Terminal pollen cones, which occur in some examples of Retrophyllum vitiense, including its type specimen, have not so far been observed in R. filicifolium. Material with young female cones can be separated from Retrophyllum vitiense by the fewer sterile bracts on the female cone axis (about 4, not 6–8) that are ephemeral and quickly caducous, not persisting till the cones reach resting phase, by the fertile bract with the proximal half lacking a keel, and by the obovoid, not cylindrical, receptacle. The ripe cones also appear to be slightly larger on the basis of the few that have been seen, being c.26 × 17 mm rather than 15–23 × 10–12 mm, although this may not hold when additional material is examined. Vegetative differences include the more quickly caducous bud scales that leave pale scars on the branchlet, the typically shorter juvenile and adult ultimate foliage shoots with fewer pairs of leaves (although there is overlap) and especially the broadest point of both adult and juvenile leaves being near the middle of the lamina rather than towards the base as in Retrophyllum vitiense. There is no geographical overlap between the two species, so if the provenance is known, identification is automatic.
Tree, 27–60 m, dbh 50–130 cm. Trunk sometimes spur-buttressed at base, when adult unbranched except near top. Bark smooth, flaking, fissured, dark brown or blackish on mature trees, light or medium brown on juveniles; inner bark reddish-brown, salmon or pink; wood pale orange, straw-coloured, yellow or white. Crown not widely spreading. Terminal buds on primary branches globose or ovoid; bud scales present, in 4 series, decussate, the lowest pair longer and slightly wider than the other three pairs, all ovate or ovate-rhombic and slightly keeled, acute, brownish with very narrow paler entire margin, caducous, leaving cream, transversely narrowly rhombic scars much paler than the branchlet; those on foliage shoots usually remaining dormant, ovoid. Ultimate juvenile shoots alternate, subopposite or opposite, 65–210 mm, with 7–35 (usually 11–20) pairs of foliage leaves, occasionally biramulate or sending off a lateral scale-bearing shoot. Juvenile foliage leaves 4–7 mm apart, opposite-distichous, heterofacially turned at base, diverging at 60–85°, shortly petiolate, the petiole 0.2–1 mm, lamina lanceolate or narrowly lanceolate with the lowest ones elliptic, oblong-elliptic, ovate or narrowly ovate and considerably smaller, those near middle of branchlet 18–40 × 4.2–6 mm, the apex often indistinctly mucronate. Adult (and juvenile) trees with dimorphic shoots, most shoots bearing foliage shoots but leaders bearing scale leaves; shoot flattening heterofacial with the decurrent bases running straight down the internodes. Primary adult branches not observed. Penultimate adult branches erecto-patent to widely patent with respect to subtending branch, straight or slightly curved, bearing opposite-decussate axillary lateral foliage shoots interspersed with remote pairs of decussate subadpressed to spreading ovate scales. Ultimate adult foliage shoots 20–90 (–120) mm with 4–18(–25) pairs of leaves, straight or curved, internodes slightly flexuous. Leaf scars absent. Adult foliage leaves 2–7 mm apart, opposite-distichous, pectinate, not imbricate, heterofacially twisted, subsessile to shortly petiolate; petiole decurrent straight along whole length of internode, free part 0.2–1.5(–2) mm); laminas spreading and at least sometimes forming a shallow ‘V’ along the branchlet (cf. label of epitype), widely erecto-patent, diverging at (45–)50–80(–90)°, mid to deep green, narrowly elliptic to elliptic or oblong (widest just below the middle), those near middle of branchlets 7–25(–30) × 3–4.5(–5) mm but lowest ones shorter, all straight, coriaceous and moderately thick; margin not thickened, very narrowly hyaline; abaxial surface flat to ± convex, adaxial surface ± flat, both equally amphistomatic, lacking a raised central area but with vein-like striae alternating with the stomatal rows; midrib evident, raised beneath but obscure throughout or at least distally above; apex subacute, obtuse or sometimes broadly rounded, usually not mucronate, the base rounded, obtuse or cuneate.
Pollen cones terminal on lateral shoots arising just above bud scales of previous season, subtended by a bract, 1–several together in groups that sometimes form a raceme-like inflorescence, ellipsoid or ovoid; microsporophylls 20–32(–40) per cone, in 5–8(–10) whorls; lamina triangular to ovate with buff-coloured scarious margin; microsporangia reniform; pollen hyaline.
Female cones borne laterally from near the base of an ultimate foliage shoot of the previous year's growth, the reproductive shoot subtended by a bract. Peduncle 5–10 mm, equalling or shorter than unripe cone and always shorter than ripe cone, longer than vestigial receptacle, erect or erecto-patent when young, becoming pendulous, with 3–5 pairs of bracts below the cone axis. Peduncle bracts adpressed or subadpressed, ovate to ovate-rhombic, c.1.5 × 0.3–0.5 mm, very narrowly scarious-margined. Cone axis formed of c.4 sterile bracts and 1 or 2 fertile bracts. Sterile bracts ephemeral, present at receptivity but then quickly caducous, elliptic, obtuse to rounded, larger than peduncle bracts, at first spreading but soon becoming deflexed and falling. Receptacle obovoid, purplish-brown, glaucous. Fertile bract initially erect, its proximal half fused to the epimatium and lacking a keel. Cones obovoid or pyriform, c.26 × 17 mm; epimatium green when young becoming red when ripe, glaucous when unripe, not crested, with conical, slightly hooked beak at micropylar end.
Distribution
Malesia: Moluccas (Morotai: type locality of Podocarpus filicifolius), New Guinea, Bismarck Archipelago (New Ireland, New Britain). Morotai marks not only the westernmost limit of this species, but also of the genus Retrophyllum in Asia. TDWG: 42 MOL 43 BIS NWG-IJ NWG-PN. Map: Fig. 4.

Fig. 4. Global distribution of Retrophyllum filicifolium.
Specimens seen and other records. Indonesia. North Maluku: Morotai Island Regency: Morotai, without detailed locality, 1949, tree, 15 m, Kostermans s.n. (lecto L–image, excl. fruits of Nageia wallichiana in packet; isolecto A, K). Papua: Jayapura: Dist. Hollandia, Cycloop Mountains, 1220 m, 28 vi 1961, van Royen & Sleumer 6073 (A, K, L–image, P–image). Res. Hollandia, Cycloop Mts (Ifar–Ormu), 1100 m, 2 xi 1954, Versteegh B.W. 913 (A, CANB–n.v., K, L–2 sheets, images). Paniai: Wissel Lake Region, slope and summit of Mt. Barara, 4 ix 1939, Eyma 5155 (L–image). Motito, Wissel Lakes, 1800 m, 18 v 1960, Vink & Schram BW 8730 (CANB–n.v., L–image, collection date wrongly entered in database as ‘18 vi’). Wamena: 6 km SW of Bernhard Camp, Idenburg River, 1200 m, ii 1939, Brass 12787 (A, BM, K–database, L–image); ibid., juvenile foliage of no. 12787, from a plant 4 m high, 1200 m, ii 1939, Brass 12787a (A, L–image). 6 km SW of Bernhard Camp, Idenburg [Taritatu] River, 1200 m, 17 ii 1939, Brass & Versteegh 12534 (A, L–image). ibid., “further material of no. 12787,” 1200 m, ii 1939, Brass 12912 (A, L–image). Papua New Guinea. Northern [Oro] (border with Central): ridge above Doma, 1500 m, 14 xi 1962, Woods 345 (epitype E, isoepitype K–database); near Doma village, 900–1500 m, 2 xi 1962, Woods 241 (E). Northern [Oro]: Sohe: Lala River, c.5000 ft [1524 m], 20 ii 1936, Carr 15666 (BM–2 sheets, CANB–n.v., L–image, NY, S–database). Alola, c.6000 ft [1829 m], 3 i 1936, Carr 14160 (A, BM–2 sheets, L–image, NY). Eastern Highlands: Lufa: Herowana, Crater Mt. WMA, N of airstrip, 6°39′3′′S 145°11′30′′E, 21 xi 2005, Kuria LAE 87216 & Oliver (LAE–n.v., NSW–n.v.). Hela: Koroba-Kopiago: Southern Highlands, c. 6 miles [9.5 km] E of Koroba station, 5°42′S 142°48′E, c.80 ft. [24.5 m] tall, 26 viii 1961, Pullen 2840 (LAE 96292–n.v.: de Laubenfels, Reference de Laubenfels1969: 343). West New Britain: Talasea: NE slope of Mt. Tangis, SW of Airagilpua, Talasea, 5°40′S 148°25′E, 3500 ft [1067 m], 17 xi 1965, Frodin NGF 26292 (A, CANB–n.v., L–image, NY). Mt. Tangis, NW slope, Talasea, 5°39′S 148°18′E, 2400 ft [732 m], 30 v 1966, Frodin NGF 26917 (A, CANB–n.v., L–image, NY). Kandrian: Umai River, Fullerborn Harbour, 6°06′S 150°40′E, 100 ft. [30.5 m], 5 iii 1965, Hammermaster & Sayers NGF 21842 (L–2 sheets, images); 7 miles SE of Benim village, Wariai subdist., Kandrian dist., 1000 ft [305 m], 5°47′S 148°57′E, 23 iii 1966, Henty & Frodin NGF 27359 (A, CANB–n.v., K, L–image, NY). New Ireland: Namatanai: Namatanai subprovince, Hans Meyer Range, 4°24′S 152°58′E, c.8 km (map distance) WNW of Taron on E coast, 1350 m, 29 x 1975, Sands 2381 et al. (A, CANB–n.v., K–database, L–image). Northern Hans Meyer Range, 70 km SE of Namatanai, 850 m, 3°03′S 152°40′E, 1 xi 1984, Gideon & Obedi LAE 77181 (BISH–n.v., CANB–n.v., E, K–database, L–image).
Bioregions: Wallacea; New Guinea & Melanesia
Ecoregions: AA106 Halmahera Rain Forests, AA0112 New Britain–New Ireland Montane Rain Forests, AA0116 Northern New Guinea Montane Rain Forests.
Ecology
According to field notes on specimen labels, Retrophyllum filicifolium is a typical species of tall primary montane rain forest or ridge forest, associated with Dacrycarpus (Endl.) de Laub., emergent palms such as Gulubia Becc. and other trees including Agathis, Podocarpus L'Hér. ex Pers., Serianthes Benth., Syzygium R.Br. ex Gaertn., Casuarina rumphiana Miq., and sometimes in shade among bamboos. It occurs at altitudes of 300–1800 m, mostly between 900 and 1500 m. It would seem to occur chiefly at higher altitudes than Retrophyllum vitiense which, in Fiji and the Solomon Islands, grows mainly between 100 and 900 m.
Provisional IUCN conservation assessment: LC
This species is here reinstated for the first time after a lapse of more than 40 years (when it was synonymised with Podocarpus vitiensis by de Laubenfels, Reference de Laubenfels1968), and there has therefore been no previous conservation assessment for it as a separate species. The species appears to be widespread on New Guinea, and it is therefore regarded as Least Concern, although it is, or has been, subjected to logging.
Uses
None have been recorded. However, they are likely to be similar to those of Retrophyllum vitiense.
Group B. Bark exfoliating in short strips or pieces. Vegetative shoots dimorphic. Terminal buds protected by reduced foliage leaves; true bud scales absent. Leaves heterofacially flattened in both juvenile and adult phases. Adult leaves in 4 ranks, thick to very thick; juvenile ones distichous. Resin canal 1, situated below vascular bundle. Pollen cones in groups at branch tips, on a common peduncle (sometimes ± sessile), individual cones sessile, subtended by scale-like bracts and the lower ones in the group axillary to leaves; microsporophylls decussate (appearing spirally arranged), triangular or ovate. Female cones terminating ordinary foliage shoots, proleptic; peduncle not broadened distally, in its vegetative portion bearing spreading bracts; cone axis with 1 or 2 sterile bracts below fertile bract. Fertile bract free from epimatium, flat. Receptacle formed from 1 or 2 peduncle bracts plus the last sterile bract and the fertile bract, obovoid. Seed beaked at micropylar end. Germination epigeal. Seedling phyllotaxis decussate. – New Caledonia.
3. Retrophyllum comptonii (J.Buchholz) C.N. Page, Notes Roy. Bot. Gard. Edinburgh 45(2): 380 (22 Feb. 1989 [‘1988’]). – Podocarpus comptonii J.Buchholz, Bull. Mus. Hist. Nat. Paris sér. 2, 21: 284 (30 Jul. 1949). – Decussocarpus comptonii (J.Buchholz) de Laub., J. Arnold Arbor. 50: 344 (15 Jul. 1969). – Nageia comptonii (J. Buchholz) de Laub., Blumea 32(1): 211 (3 Feb. 1987). – Type: New Caledonia, Mt. Mou, J. Buchholz 1684 (holo ILL–image, photos A, P; iso ILL–image, K–image, MO, NY, S–image [S-C-1294]). Paratypes Mt. Mou, Buchholz 1085 (ILL–image; isoparatypes elsewhere as cited below); Mt. Mou, Buchholz 1449 (ILL–image; isoparatypes elsewhere as cited below); Mt. Humboldt, Buchholz 1697 (ILL–image), 1697a and 1697s. Fig. 1B, E, H.
Iconography
De Laubenfels, Fl. Nouv. Caléd. 4: 51, pl. 11 f. 1–8 (1972), as Decussocarpus comptonii.
Etymology
This species is named after Robert Harold Compton (1886–1979), Cambridge botanist who, after the First World War, became director of Kirstenbosch Botanical Garden (South Africa) in 1919. Compton collected Retrophyllum comptonii several times during a field expedition to New Caledonia in 1914, although he was not the first to do so; a much earlier collection by Vieillard has been seen.
Vernacular names: palissandre (Chevalier, Reference Chevalier1957: 114; Suprin, Reference Suprin2011b)
Distinguishing features
Retrophyllum comptonii in most of its wide range has the capacity to become a tall tree up to at least 30 m, although in the far south of the island it tends to be much shorter. It is rarely if ever an aquatic. The adult foliage is mostly opposite decussate, with only the leaves in the upper part of the tree in 4 ranks. The adult leaves have a raised central area that is always narrower than the leaf margins and when dried is marked either by two (not three) parallel ridges or longitudinal wrinkles. The surface of the seed is scalloped and ridged, these low and not prominent.
Tree or shrub, 2.5–30 m tall, 12–80 cm dbh. Trunk apparently not buttressed, the bole straight and lacking branches for 10 m or more in large trees. Bark smooth when young, ± rough on older trees, peeling in short vertical strips or ragged pieces, fissured, dark grey, greyish-brown, tan or reddish-brown; inner bark brownish; wood cream or whitish. Crown pyramidal, finally rounded. Buds ovoid, protected by very reduced foliage leaves; true bud scales absent. Juvenile shoots with 1 or 2 very small leaves at base and 22–35 pairs of foliage leaves in up to 3 growth increments. Juvenile leaves 5–7 mm apart, opposite, distichous and pectinate, heterofacially turned at base, diverging at 55–65°, sessile or subsessile, lamina narrowly lanceolate or linear-lanceolate, 20–35 × c.2.5 mm on seedlings, somewhat smaller on older saplings, gradually tapered to a subacute, narrow and frequently slightly mucronate tip. Adult trees with dimorphic shoots, most shoots bearing foliage leaves but a few leader shoots bearing scale leaves and these either emitting lateral foliage shoots or terminating in foliage shoots; shoot flattening fully heterofacial on juvenile and transitional shoots but only partly so on adult branches. Primary adult branches irregularly arranged, divaricate or widely spreading, opposite, alternate or in whorls of 3. Penultimate adult branchlets erecto-patent to divaricate, ± curved, initially clad with distant, alternate, small, elliptic-ovate subadpressed scales with decurrent bases, their free laminas finally deciduous. Ultimate adult foliage shoots ± clustered at tips of penultimate branches, erecto-patent to suberect, flexuous, 40–70 mm with 8–15 pairs of leaves, subtended by 1 or 2 pairs of very reduced scale-like foliage leaves. Leaf scars absent. Lowest true adult foliage leaves smaller and relatively broader than the others; adult leaves in 4 ranks or some (on transitional shoots) opposite-decussate and distichous, crowded, 3–4 mm apart, sessile with partly twisted bases, most showing heterofacial twisting, scarcely or not imbricate, erecto-patent to suberect, mid to dark green; lamina of leaves in middle of shoot elliptic, oblong-elliptic, narrowly lanceolate or lanceolate, 6–15 × 2–5 mm, straight, thick and coriaceous; margin not thickened, narrowly hyaline; surfaces unequally amphistomatic, with more stomata on adaxial surface; midrib not visible on either surface but both surfaces with a raised, narrowly elliptic central area narrower than the margins and either longitudinally wrinkled or marked by two parallel ridges when dried; apex obtuse or broadly rounded; base obtuse or cuneate.
Pollen cones lateral (axillary) or terminal on lateral shoots of current growth, subtended by a leaf or scale-leaf, 1–5 together, pedunculate (individual cones sessile when > 1 together); peduncle shorter than cone(s), erecto-patent, bearing 1 pair of ± adpressed scales; peduncle scales greenish, keeled, ovate with decurrent base, acute, with very narrow, buff scarious margin; cones ellipsoid or ovoid, 4–6(–12) × 2.5–3 mm, straight, reddish, or greenish with a pink tinge; microsporophylls decussate but appearing spirally arranged, 16–20(–28) per cone in 4 or 5 short/3 or 4 long ‘spirals’; lamina greenish sometimes tinged pink or red, broadly triangular or deltate and abruptly narrowed to an acute, apiculate apex, with very narrow whitish hyaline margin, longer than the microsporangia; microsporangia pinkish, elliptic; pollen white or cream.
Female cones terminal on lateral foliage shoots of current growth, shortly pedunculate. Peduncle in line with shoot or erecto-patent, shorter than remainder of cone but longer than receptacle, bearing 1 (occasionally 2) cone(s), bearing 8–10 bracts in 4 or 5 decussate pairs; distal part of peduncle shed with cone. Peduncle bracts light green turning brownish green, not keeled but convex abaxially, elliptic or ovate-elliptic, 1.5–4 × c.1–1.5 mm, subacute to obtuse. Proximal sterile bracts (cone axis) absent other than the one subtending the fertile bract. Cone subtended by a non-fleshy receptacle formed from 1 or 2 peduncle bracts plus either one sterile and one fertile bracts or two fertile (or part-fertile) bracts. Receptacle narrowly obovoid or narrowly pyriform, light green at first becoming orange. Sterile bract normally 1, light green becoming pale orange, stiffly spreading to erecto-patent, elliptic to ovate-elliptic, obtuse, with scarious margin. Fertile bract light green becoming pale orange, initially erect, with median longitudinal groove, wholly free from epimatium, with reflexed, ovate free tip with acute apex. Cones pyriform, 20–25 × 13–20 mm; epimatium green becoming scarlet or deep red when ripe (occasionally blue-black, fide Munzinger et al. 1680 in sched., P), with short conical apical crest; seed beaked at micropylar end, the beak usually straight, occasionally curved. Seed surface with two ridges leading to the micropylar beak, not porous and not adapted to water dispersal.
Germination epigeal. Seedlings with two brown, erect, soon deciduous cataphylls that envelop the base of the stem and have greenish linear lanceolate tips that protect the first leaves; initial leaves of main axis cotyledon-like, c.6 pairs, separated by short, naked internodes, widely spreading and the lowest pair becoming recurved, their blades linear, 21–28 × 1.5–2 mm, longer than the leaves on the initial branchlets, basally not twisted, opposite-decussate, amphistomatic.
Distribution
New Caledonia (throughout the island except the northern half of the western coast). TDWG: 60 NWC. Map: Fig. 5.

Fig. 5. Global distribution of Retrophyllum comptonii.
Specimens seen and other records. New Caledonia. Unloc., Baudouin 542 (P–image); unloc., no date, Bernier s.n. (P–image, barcode P00188269); unloc., Pancher s.n. (P–image, barcode P00188209); unloc., Sarlin 228 (P–image) and 238 (P–image); collection details obscured, Bernier 268 (K–database, P–image, possibly a composite sheet of up to three separate gatherings?; Bernier 269 is from Plaine des Lacs); Province Nord: Hienghène: Contrefort est du Mt. Panié, 1000–1400 m, 10 ix 1966, MacKee 15594 (P–image); ibid., 800 m, 10 ix 1966, MacKee 15639 (P–image, seedlings); Mont Panié [20°35′S 164°46′E], 700–900 m, 19 ix 1966, Schmid 1422 (NOU–database). Mont Panié, 800 m, 3 viii 1978, MacKee 35577 (P–image); Mt. Panié, c.20 air-km NW of Hienghène, 950 m, 6 xii 1979, McPherson 2191 (MO–database, P–image, NOU–database); Mont Panié [20°35′S 164°46′E], 950 m, 3 iii 1983, Lauri 45 (NOU–database). Mt. Panié, above Haut Coulna, on SW slopes, 20°36′82[sic]′′S 164°44′40′′E, 1060–1250 m, 30 x 1999, McPherson & van der Werff 17834 (P–image, NOU–database); Mt. Panié, NE slopes on summit route via eastern trail, 20°35′00′′S 164°46′14′′E, 1158 m, 7 xii 2002, Gardner et al., Third New Caledonia Araucaria Exped. (TNCA) 5045 (E). Kaala-Gomen: Mont Taom (Crête Ouest), 900 m, 24 iv 1982, MacKee 40376 (MO–database, P–image, NOU–database), 40377 (P–image). Kouaoua: décharge Montmartre, 750 m, 14 ii 1978, Veillon 3514 (P–image, NOU–database, Z–database). Poindimié: Wagap, Vieillard 3264 [p.p.] (P–image, original label bearing manuscript name “Podocarpus Guillainii”). Sommet du Mt. Grandie (Haute Amoa), 900–960 m, 14 v 1968, MacKee 18807 (NOU–database, P–images, 2 sheets), 18808 (NOU–database, P–image); massif de Grandie, pente SE, c.700 m, 31 iii 1988, Veillon 6783 (P–image, NOU–database). Dans une cuvette au massif du Tchingou, c.1250 m, 18 iv 1951, Hürlimann 1220 (P–image, Z–database); Massif de Tchingou, 750 m, 13 xii 1983, Morat 7621 (P–image); Massif de Tchingou, 20°53′36′′S 165°00′42′′E, 1160–1175 m, 1 iv 2001, McPherson & Munzinger 18100 (P–image, MO–database, NOU–database); ibid., 20°54′15′′S 165°01′19′′E, 760–850 m, 5 iv 2001, McPherson & Munzinger 18149 (MO–database). Pouébo: Bois du montagne à Balade, 1855–1860, Vieillard 1265 (P–image). Mont Colnett [20°29′S 164°41′E], c.1000 m, 1 x 1997, Suprin 2628 (NOU–database); sur la crête SW du Mt. Colnett, c.1200 m, 12 ix 1951, Hürlimann 1964 (P–image, S–database), 1966 (P–image, Z–database); Mont Colnett (pente est), 1100 m, xi 1981, Nasi s.n. (MacKee 39997) (P–image); ibid., 1100 m, xi 1981, Nasi (MacKee 40011) (P–image); Mt. Colnett, E slopes, 20°30′00′′S 164°42′52′′E, 1000 m, 29 x 2003, McPherson 19070 et al. (P–image, MO–database); ibid., 1000 m, 29 x 2003, McPherson 19073 et al. (P–image, MO–database, S–database). ‘Ignambi, 18 iii 1914’, Compton 608 (S–database under Retrophyllum minus, 3 accessions in spirit coll., nos. S-A-3669, S-A-3681, S-A-3684; the locality, date [only given for S-A-3681] or collection number of all must be wrong because Compton collected at Mt. Mou in the S of the island on that date, see Compton 607 below); Ignambi, > 3500 ft [1067 m], 30 vii 1914, Compton 1524 (A–photo, BM, K); Ignambi, Compton 1527 (S–database under R. minus); Ignambi, 3000 ft [914 m], 1 viii 1914, Compton 1587 (BM, A–photo); am Gipfel des Ignambi, 4 vi 1925, Däniker 2901a (Z–database); en montant vers le sommet de l'Ignambi, c.1250 m, 17 viii 1951, Hürlimann 1842 (P–image, Z–database); sur la crête du massif Ignambi le long de la route de Gomen, c.1170 m, 25 viii 1951, Hürlimann 1832 (P–image, Z–database); summit of Ignambi, on trail from Oubatche to Gomen, c.1000 m, 12 vi 1956, Foster 160 (P–image); near top of Mt. Ignambi, c.10 km S of Panébo, c.1300 m, 7 xii 1963, Green 1783 (A); Mont Ignambi, 1100–1200 m, 19 viii 1965, Bernardi 10347 (L–image, P–image, Z–database); E slopes of Mont Ignambi, 20°26′39′′S 164°34′58′′E, 1200 m, 6 iii 1999, Cretinon & Gardner, ICCP New Caledonia Exped. (1999): 73 (E); Ignambi [20°27′S 164°35′E], 1250–1300 m, 2 v 2002, Lowry 5747 (MO–database, NOU–database). Pouembout: Kopéto [21°10′S 165°00′E], 940 m, 8 viii 2006, Rigault & Barrière s.n. (Barrabé 365) (NOU–database). Ad montem Paéoua metalliferum, 900 m, 13 viii 1965, Bernardi 10131 (K, L–image, P–image, Z–database); ibid., 900–950 m, 13 viii 1965, Bernardi 10149 (K–database, L–image, P–image, S–database, Z–database); Mt. Paéoua, 900–1100 m, 4 vii 1967, MacKee 17032 (P–image), 17056 (K, NOU–image, P–image, Z–database); Mt. Paéoua, c.1100 m, 16 x 1986, Veillon 6062 (P–image, NOU–database); Paéoua, 21°10′36.0′′S 165°05′14.2′′E, 1077 m, 5 xii 2005, Gardner et al. 256 (E). Poya (N): Mt. Boulinda, c.1102 m, 26 iv 1965, Veillon 120 (P–image, NOU–database; P sheet has ‘Blanchon’ blacked out and ‘Veillon’ substituted below, with Blanchon's initials J.P. remaining; Veillon's are J.M.). Boulinda [21°14′S 165°09′E], 1102 m, 26 iv 1965, Veillon 122 (NOU–database). Mont Boulinda, c.1200 m, 28 iv 1965, Schmid 137 (P–image); Mt. Boulinda, pente nord, 1150–1300 m, 28 viii 1967, MacKee 17354 (P–image), 17357 (NOU–database, P–image, Z–database), 17358 (P–image). Monte Boulinda, 450–900 m, 12–15 iv 1968, Bernardi 12754 (K, P–image, Z–database); Massif de Boulinda, N of Poya, c.850 m, 28 viii 1981, McPherson 4121 (GH, P–image, MO–database, NOU–database, Z–database). Aoupinié [21°10′S 165°18′E], 1000 m, 16 ix 1981, Suprin 1407 (NOU–database). Province Sud: Bouloupari: Dent du St Vincent, vii 1909, Le Rat 71 (P–image, seedlings, also duplicate without locality details, P–image). Mont Do, 1000 m, 28 xi 1966, Veillon 949 (P–image, NOU–database); c.15 km towards NW, Mont Do, upper region towards top, 700–1014 m, 28 xi 1966, Ehrendorfer 6600-138-38 (WU–image). Mont Do [21°45′S 165°59′E], 1000 m, 24 v 1977, Musselman 5415 (NOU–database). Bourail: au-dessus de Téné, près de Bourail, 18 iii 1869, Balansa 1381 (P–image; duplicate without locality information, P–image). Pente boisée d'un contrefort du massif Mé Maoya (Haute Houaïlou), 800–900 m, 28 xii 1962, MacKee 9886 (CANB–database, L–image, P–image, K–database). Poya, Mé Maoya Massif, southern edge of Këiyoümê in disused mine, 21°26′37.8′′S 165°21′43.5′′E, 1012 m, 2 xi 2008, Hollingsworth et al. 194 (E, P–image). Mé Aoui, 850 m, 18 vi 1981, Cherrier (MacKee 39235) (NOU–database, P–image). Canala: Crête Mt. Nakada, 21°37′S 166°03′E, 1050 m, 6 x 1977, MacKee 33961 (MO–database, NOU–database). Near Prokoméo radio tower, NW of Canala, 21°30′S 165°51′E, 700 m, 3 ii 1983, McPherson 5477 (MO–database). Mt. Nakada, 21°37′35′′S 166°03′22′′E, 800–900 m, 17 iv 2001, McPherson & Munzinger 18254 (P–image, MO–database); ibid., 21°37′82 [sic]′′S 166°03′35′′E, 780 m, 18 iv 2001, Munzinger 764 & McPherson (P–image). Dumbéa: Mt. Dzumac, 1000 m, 28 iv 1951, Guillaumin & Baumann-Bodenheim 12717 (Z–database), 12725 (P–image, Z–database), 12727 (P–image, Z–database); N face of Mt. Dzumac, c.1000 m, 27 ix 1963, Green 1216 (A, K–database, NOU–image). Pic de Kouwehé [Couvélé], 700–1000 m, 4 ix 1882, Brousmiche 697 (P–image). Au fond de la vallée derrière la mine Sunshine, c.730 m, 15 iii 1951, Hürlimann 1062 (P–image, K–database, Z–database). Near Pic du Rocher, on mountain crest along trail to Pic du Rocher, Plateau Montagne des Sources, nr Pic du Rocher, 950 m, 15 x 1947, Buchholz 1222 (NY, P–image, K–database, S–database; photo of trees in situ, A). Un vallon au dessous de la route vers la Montagne des Sources au Pic Buse, c.530 m, 11 vii 1951, Hürlimann 1573 (P–image, Z–database). Mont Dzumac, 900 m, 25 vii 1981, McPherson 3958 (NOU–database). Mont Dzumac [22°02′S 166°28′E], 880 m, 5 i 1982, Suprin 1612 (NOU–database, 2 sheets); ibid., 1050 m, 4 v 1982, Suprin 1830 (K–database, NOU–database). Mont Dzumac, ‘0 m’ [sic: from map, must be c.600 m], 11 ix 1984, Jaffré 2585 (NOU–database, 2 sheets). Le Mont-Dore: Thy [22°10′S 166°31′E], 980 m, 19 x 1982, Brinon 1359 (NOU–database). Mont Koghis, [22°10′S 166°31′E], 1000 m, 26 x 1982, Zlarnik 30 (NOU–database). Inland from Baie des Pirogues, 16 x 1923, White 2120 (A, 2 sheets; S–database). Moindou: Mé Ori, 900–1000 m, 3 xii 1969, MacKee 21219 (P–image, K–database, MO–database, NOU–database, Z–database). Along old lumber road to top of Mt. Mé Ori, above Katrikoin, 21°31′S 165°39′E, 8 ix 1980, McPherson 3066 (MO–database, 2 sheets). Bourail, E slopes and summit area of Mé Ori (ascent starting from Katrikoin), 21°31′31.7′′S 165°39′56.4′′E, 31 x 2008, Hollingsworth et al. 153 (E, P–image). Me Adeo pente O, c.750 m, 24 iii 1988, Veillon 6747 (P–image, NOU–database). Païta: Mont Humboldt, 1400 m, 16 ix 1902, Schlechter 15331 (A–photo of BM sheet, BM, E, K–2 sheets, L–image [currently as R. minus, May 2015], P–image, S–database, Z–database); ibid., 1400 m, Schlechter 15332 (P–image); Mt. Humboldt, 1200–1300 m, Buchholz 1578 (A–photo, seed; NY, S–database); Mt. Humboldt, 1400 m, 20 ix 1951, Baumann-Bodenheim 15393 (P–image, Z–database); ibid., 1400 m, 20 ix 1951, Baumann-Bodenheim 15411 (P–image, Z–database); Mt. Humboldt, c.1100 m, xi 1973, Schmid 4838 (P–image, NOU–database); Mont Humboldt, Réserve Spéciale Botanique, 29 xi 2005, Gardner et al. 108 (E); Mont Humboldt [21°53′S 166°24′E], 1380 m, 2 xii 2009, Grignon et al. 559 (NOU–database). Mt. Dzumac, 800 m, 1 xii 1957, de Laubenfels P153 (S–database); Mont Dzumac, [22°02′S 166°27′E], 950 m, 6 xii 1964, Blanchon 1246 (NOU–database); Mt. Dzumac, 760 m, 29 xi 1964, de Laubenfels P 415 (K); Mt. Dzumac, 8 vii 1965, Aubréville & Heine 229 (P–image); Mont Dzumac, 22°06′–22°08′S 166°27′–166°32′E, 800–900 m, 8 vii 1965, Bernardi 9520 (P–image). Crête entre Mt. Dzumac et Mt. Ouin, c.1000 m, 22 xii 1962, Barets 8 (P–image); ibid., 900 m, 11 x 1967, MacKee 17670 (P–image); ibid., 900 m, 17 iv 1968, MacKee 18694 (P–image, seedlings); ibid., 900 m, 7 vii 1969, MacKee 20244 (P–image); ibid., 6 i 1982, MacKee 40185 (P–image, K–database, NOU–database, Z–database). Haute Ouinné, côte orientale, 750 m, 18 i 1948, Bernier 267 (P–image); Ouinné sup., 700 m, 29 iv 1951, Guillaumin & Baumann-Bodenheim 12815 (P–image, Z–database), 12843 (P–image, Z–database), 12861 (P–image, Z–database); ibid., 900 m, 30 iv 1951, Guillaumin & Baumann-Bodenheim 12910 (Z–database), 12960 (P–image). Monts Dzumac and down to the Rivière Ouinné, 800 m, 22°02′54′′S 166°28′47′′E, 9 iii 1999, Cretinon & Gardner, ICCP New Caledonia Exped. 1999: 90 (E); Monts Dzumac road down to the Rivière Ouinné, 800 m, 22°02′54′′S 166°28′47′′E, 3 vi 2001, Gardner et al., New Caledonia Araucaria Exped. 2001: 1026 (E, 2 sheets). Mont Mou, 1861–1867, Vieillard 3064 (GH, P–image, original labels of both sheets bearing manuscript name “Podocarpus Guillainii”); sommet du Mont Mou, 1866, Vieillard 3264 [p.p.] (P–image, with manuscript name “Podocarpus Guillainii”). Mt. Mou, 1910–11, Godefroy s.n. (P–image); Mont Mou, 3500 ft [1067 m], 18 iii 1914, Compton 607 (BM, A–photo; Compton 608, at S and said to be collected from Ignambi, could not have been collected on 18 iii 1914 as the S database record says); summit of Mt. Mou, 3500 ft [1067 m], x 1923, White 2033 (E, P–image, K–database); Gipfelcrête des Mt. Mou, 21 ii 1926, Däniker 2902 (P–image, Z–database); crête sommitale du Mont Mou, c.1150 m, 19 vi 1938, Virot s.n. (A, P–image); ibid., c.1100 m, 4 ix 1938, Virot 38 (A, P–image); crête sommitale du Mont Mou, 1150 m, 19 ii 1939, Résineux no. 8, coll. Virot (A, P–image); Mont Mou, 1100 m, 28 ix 1947, Buchholz 1085 (paratypes ILL–2 sheets, images; isoparatypes A–photo, GOET–image, NY, P–image, K–database, S–database); Mt. Mou, 1100 m, 4 xii 1947, Buchholz 1447 (A–photo), 1449 (paratype ILL–image, isoparatypes A–photo [seed kernel]), K, NY, P–image, S–database: paratype and isoparatypes of Podocarpus comptonii); Mont Mou, 1100 m, 4 xii 1947, Buchholz 1452 (K–database, NY, S–database); Mt. Mou, 1100 m, 9 ii 1948, Buchholz 1684 (holo ILL–image, A and P–photos of holotype, iso ILL–image, K–image, MO, NY, S–image); Mt. Mou, 1100 m, 9 ii 1948, Buchholz 1684s (small seedlings, ILL–image, P–image); Mt. Mou, 1100 m, 5 vi 1948, Buchholz 1791 (NY, S–database); Mont Mou, Pic des Mousses, 1000–1100 m, 23 i 1949, Skottsberg 202 (P–image); Mont Mou, Pic des Mousses, 23 i 1949, Selling 202 (S–database); Mont Mou, 1000 m, 22 xi 1949, MacDaniels 2323 (P-1041) (NY–database). Mt. Mou, 1200 m, 23 viii 1950, Baumann-Bodenheim 5654B (Z–database); Mt. Mou, 1200 m, 13 iii 1951, Guillaumin & Baumann-Bodenheim 11257 (P–image, Z–database); ibid., 1200 m, 13 iii 1951, Guillaumin & Baumann-Bodenheim 11261 (P–image, Z–database), 11282 (P–image, Z–database), 11299 (P–image, Z–database), 11301 (P–image, Z–database); Mount Mou, c.20 miles NW of Nouméa and c.5 miles N. of Païta, 26 vi 1952, McMillan 5015 (P–image); Mt. Mou, below N. summit, 1100 m, 25 xi 1955, MacKee 3516 (A, E, P–image); sommet du Mont Mou, Blanchon 341 (P–image); Mont Mou, 1200 m, 9 x 1957, de Laubenfels P129 (S–database, spirit coll.; SBT–n.v., cited by de Laubenfels, Reference de Laubenfels1969: 345); ibid., 9 ix 1960, Erdtman s.n. (3 collections, S–database); Mt. Mou (c.25 km N of Nouméa), c.1200 m, 7 x 1963, Green 1268 (A, K–database, NOU–database); Mt. Mou, 1140 m, 18 xi 1964, de Laubenfels P 360 (A); ibid., juvenile, 1140 m, 18 xi 1964, de Laubenfels P 361 (A); Mont Mou, 28 v 1965, Boisseau (hb. MacKee 12725) (P–image); Mont Mou, 22°03′–22°04′S 166°19′–166°22′E, 30 vii 1965, Bernardi 9879 (L–image, P–image, Z–database); Mt. Mou, along trail starting near Sanatorium c.7 air-km NNW of Païta, 1100 m, 8 ix 1979, McPherson 1867 (P–image, MO–database, NOU–database); Mt. Mou, along trail from near Sanatorium to summit, 1150 m, 8 viii 1981, McPherson 4020 (P–image, K–database, MO–database, NOU–database); Mont Mou, close to summit on N ridge slope, 1000 m, 22°04′S 166°20′E, 27 v 2001, Gardner et al., New Caledonia Araucaria Exped. 2001: 1014 (E). Poya (S): Contrefort Ouest du Mé Maoya au dessus de la Mine Emma, 1350 m, 11 vii 1965, Corbasson (hb. MacKee 13037) (P–image, K–database); ibid., 1400–1450 m, 2 x 1965, MacKee 13492 (K, P–image); Thio: Mont Poueari (SE Thio) [21°40′S 166°14′E], 1140 m, 13 x 1994, Suprin (hb. MacKee 46368) (P–image, NOU–database). Forêt de Sailles, 21°39′58′′S 166°14′49′′E, 1100 m, 12 iv 2001, Munzinger 1200, Suprin & Carriconde (P–image, det. Suprin incorrectly as Prumnopitys ferruginoides); ibid., forêt au bas du col juste avant le mont Pwénari, 21°39′58′′S 166°14′49′′E, 1200 m, 8 xii 2001, Munzinger 1307, Suprin & Carriconde (MO–n.v., P–image, NOU–database). Forêt de Sailles vers Thio, parcelle 3 no. 25909, 21°39′56′′S 166°14′38′′E, 849 m, 18 xi 2009, Grignon et al. 584 (P–image, NOU–database). Mont Ninga [21°45′S 166°08′E], 19 vi 1975, Sévenet 958 (NOU–database). Pic de Na Kado, Brousmiche s.n. (P–image, named as Decussocarpus minor (Carr.) de Laub. by de Laubenfels). Mt. Nekandi, 1200–1300 m, 7 xi 1967, MacKee 17908 (P–image). Nékando [21°51′S 166°26′E], 1100 m, 28 xi 1983, Veillon 5659 (NOU–database). Yaté: Montagne des Sources, Pic des Conifères et Campement “Bernier”, 900–1000 m, x 1947, Bernier 271 (P–image and duplicate without locality information, P–image). Montagne des Sources, 850 m, 9 vii 1949, Selling 208 (S–database); au dessous du Camp Bernier à la Montagne des Sources, c.800 m, 21 ii 1951, Hürlimann 931 (NY, P–image, Z–database); Montagne des Sources, 22°05′–22°09′ S 166°32′–166°38′ E, 6 vii 1965, Bernardi 9445 (L–image, P–image, Z–database); ibid., 8 ix 1960, Erdtman & Chevalier s.n. (S–database); ibid., 8 ix 1960, Erdtman s.n. (3 sheets, S–database); Montagne des Sources [22°07′S 166°35′E], 600 m, 6 vii 1965, Veillon 281 (NOU–database). Crête sommet Montagne des Sources, 22°07′S 166°36′E, 900 m, 7 x 1969, MacKee 20928 (NOU–database). Montagne des Sources [22°07′S 166°35′E], 850 m, 24 i 1982, Suprin 1636 (NOU–database). Montagne des Sources, Parc Territorial de la Rivière Bleue, highest summit area to which trail leads, 22°06′52.06′′S 166°36′19.68′′E, 1011 m, 11 xii 2002, Gardner et al., Third New Caledonia Araucaria Exped. (TNCA) 2036 (E). Forêt du Mois de mai, 150 m, viii 1947, Bernier 203 (P–3 sheets, images, one juvenile and lacking locality or date); Forest du Mois de mai, Plaine des Lacs near Walker's place, 4 xi 1947, Buchholz 1350 (K–database, P–image, NY, S–database); Plaine des Lacs, Mois de mai, 4 xi 1947, Buchholz 1350a (NY, S–database); Forêt Walker, Haute Yaté, Plaine des Lacs, c.200 m, 6 xi 1947, Bernier 269 (P–image, seedlings); ibid., same details, Bernier 270 (P–image); Mois de mai forest, Plaine des Lacs, 160–200 m, 6 xi 1947, Buchholz 1359 (A, P–image, S–database), 1359a (P–image, MO–database, S–database); Forest du Mois de mai, 180–250 m, 13 ii 1948, Buchholz 1697 (A–photo, seed kernel; ILL–image, K, NY, S–database; paratype and isoparatypes), 1697a (S–database), 1697s (ILL–image, NY); forest du Mois de mai near Walker's place, Plaine des Lacs, 180–200 m, no date, Buchholz 1697 (P–image, isoparatype, label details differ slightly from others of this number); forêt “Mois de mai” (Riv. blanche), 13 vii 1951, Baumann-Bodenheim 14057 (P–image, Z–database); ibid., 14 viii 1951, Baumann-Bodenheim 15178 (NY, P–image, Z–2 sheets, database); ibid., 14 viii 1951, Baumann-Bodenheim 15197 (P–image, Z–database); Rivière Blanche, 200 m, 24 vii 1954, H.D. Ingle I. 66 (L–image, MEL–n.v.); Mois de mai, 300 m, 22°07′S 166°39′E, 15–29 iii 1987, Watt 519 (NY–image, juvenile, fragment). Parc Territorial de la Rivière Bleue, Mois de mai, 22°07′11.2′′S 166°39′36.8′′E, 230 m, 27 xi 2005, Gardner et al. 68 (E). [Forêt] des Electriques (Rivière blanche sup.), [c.350 m], 17 vii 1958, Hürlimann 3173 (Z–database, 2 sheets); Rivière Bleue, 5 viii 1951, Baumann-Bodenheim 15028 (NY, P–image, Z–2 sheets, database); Rivière Bleue [22°06′S 166°38′E, c.200 m], 4 viii 1963, Blanchon 399 (NOU–database, det. Blanchon, needs confirmation); forêt de la Rivière Bleue, 150 m, 1 vi 1994, Pintaud 51 (juv., P–image); Plaines des Lacs, sources de la Kuébini, 24 vi 1980, Cherrier (hb. MacKee 39255) (P–image); margin of Rivière Bleue at Pont Germain, 22°06′05.2′′S 166°39′28.9′′E, 111 m, 27 xi 2005, Gardner et al. 85 (E). Kouakoué, piste Haute Ouinni, 22 ix 1977, Veillon 3305 (P–image, NOU–database); Kouakoué [21°58′S 166°31′E], 1300 m, 22 ix 1977, Morat 5676 (NOU–database). Haute Yaté, Rivière Bleue, 200 m, 1 x 1981, MacKee 39724 (A, E, NOU–database, P–image); Mt. Kouakoué, 21°57′29′′S 166°32′20′′E, 26 xi 2002, Munzinger 1661, Tronchet et al. (P–image, NOU–database); Mt. Kouakoué, 21°57′31′′S 166°32′11′′E, 26 xi 2002, Munzinger 1680, Tronchet et al. (P–image, NOU–database); Kouakoué, along trail SW of camp, 21°57′49′′S 166°32′07′′E, 12 v 2006, Lowry II et al. 6821 (P–image, MO–database, NOU–database); Kouakoué [21°57′S 166°31′E], 950 m, 3 xi 2009, Grignon et al. 470 (NOU–database). Forested slopes above a tributary of the Rivière Ni, 22°00′17′′S 166°28′42′′E, 950–1000 m, 10 xi 2003, McPherson 19209 & Mouly (P–image, MO–database). Mamié, between Ointapoin and Tiova, 22°06′23.8′′S 166°53′03′′E, 552 m, 27 x 2008, Hollingsworth et al. 90 (E, P–image).
USA (Cultivated). Georgia: Atlanta Botanical Garden, Fuqua Conservatory, 16 xii 2009, Silba B-640 (accession 9040015/1996-0015, coll. A. Watt in New Caledonia) (NY–image).
A specimen collected by Bernard Suprin (in herb. MacKee, no. 46368, P, barcoded P001655140) very closely resembles Retrophyllum minus (treated below) in branching pattern but is from a high-altitude locality in the north of New Caledonia and belongs to R. comptonii, with which it agrees in leaf characters. Vieillard 1265 from Balade (extreme NE New Caledonia) also has to belong to Retrophyllum comptonii on geographical and habitat grounds, although it was determined as Podocarpus minor Parl. by Guillaumin in 1943 – this was 6 years before Podocarpus comptonii (now Retrophyllum comptonii) was segregated from P. minor (now R. minus). The later annotation slip “= Nageia minor Carrière cf. de Laubenfels, Blumea 32: 211, 1987” is misleading and represents an uncritical up-dating of the nomenclature on the 1943 determinavit slip rather than a new determination; this is also a feature of several other specimens in the P herbarium. The specimen not unexpectedly has the characteristics of Retrophyllum comptonii. On the other hand, a specimen from Port Boisé (Le Mont-Dore) that de Laubenfels in 1972 determined as Decussocarpus comptonii has in this revision been reassigned to Retrophyllum minus, along with others from the same locality that had previously been identified as Decussocarpus minor. Gray (Reference Gray1962: 75) cited an example of Vieillard 1275 from Mt. Mou at P under Podocarpus comptonii, but I have not seen such a specimen and it is not among those imaged in the P database: all examples of Vieillard 1275 that I have seen are from low-lying localities and are Retrophyllum minus. It is possible that the specimen Gray (Reference Gray1962) cited is the one now barcoded P00188118, which is an unnumbered Pancher collection but was given the number Vieillard 1275 and mentions Mt. Mou among several collecting localities, which may not all relate to that number.
Veillon 2962 from Ouégoa is currently determined at P as Decussocarpus comptonii, but the corresponding sheet at NOU is indicated as having been identified as Falcatifolium taxoides (Brongniart & Gris) de Laub. by Veillon on the date of collection. After examining images of both sheets, on account of its leaf and pollen cone morphology I concur with Veillon's determination.
Bioregion: New Guinea & Melanesia
Ecoregion: AA0113 New Caledonia Rain Forest.
Ecology
Dense humid rain forest, moss forest or dry montane forests on mountain slopes and by rocky riverbanks; chiefly on ultrabasic rocks but also occurring on gneiss (Mt. Ignambi, Mt. Colnett) and limestone (Mt. Panié); 150–1600 m. The lower altitudinal range was given as 600 m by Farjon (Reference Farjon2010), even though de Laubenfels (Reference de Laubenfels, Aubreville and Leroy1972) had cited numerous collections from locations such as Mois de mai that are at much lower altitudes; more recent collections from as low as 150 m in the Plaine des Lacs area have also been made, and the species descends to near sea level at Baie des Pirogues in the extreme south of Grande Terre. In these low-lying locations, the species grows together with Retrophyllum minus and some individuals are very difficult to separate from it. Indeed, it is possible that some plants of Retrophyllum minus, including the epitype collection, with female cones maroon-red or greenish red, are in fact fertile hybrids between R. comptonii and the lacustrine form of R. minus, equivalent to Buchholz's Podocarpus palustris, although there is as yet no evidence to support this hypothesis other than their intermediate morphology. Associates of Retrophyllum comptonii in its more typical montane forest habitats include other gymnosperm trees such as Acmopyle pancheri Pilg., Araucaria humboldtensis J.Buchholz, A. laubenfelsii Corbasson, A. montana Brongn. & Gris, A. muelleri (Carrière) Brongn. & Gris, Austrotaxus spicata Compton, Falcatifolium taxoides (Brongn. & Gris) de Laub., Podocarpus sylvestris J.Buchholz and various angiosperm trees such as species of Nothofagus Blume.
Little or nothing is known about the dispersal of this species. The attractively coloured red ripe female cones suggest an adaptation to dispersal by vertebrates such as birds, as postulated by Farjon (Reference Farjon2010), although the flesh has been described as tasteless (McPherson 2191 in sched.). Pigeons such as Ducula goliath (Gray), the New Caledonian Imperial Pigeon, might act as dispersers, although no fruits of Podocarpaceae were recorded in the diet of that species in one limited study in the months of March and April (Barre et al., Reference Barre, Wichatitsky, Lecoq and Maillard2003).
Chromosome number: 2n = 20 (Hair & Beuzenberg, Reference Hair and Beuzenberg1958, as Podocarpus comptonii)
Phytochemistry
Cambie et al. (Reference Cambie, Cox and Sidwell1984) recorded the presence of the phenolics totarol and 19-hydroxytotarol in the wood of Retrophyllum comptonii. These were the only two phenolic compounds isolated; podocarpic acid and 4β-carboxy-9-nortotarol were both absent, in contrast to their earlier report on Retrophyllum vitiense (Cambie et al., Reference Cambie, Cox, Croft and Sidwell1983).
Mycological associations
The ascomycete fungus Caliciopsis podocarpi Huguenin was first described from two specimens; the host of the holotype was Retrophyllum minus and that of the other collection was R. comptonii (Huguenin, Reference Huguenin1969: 300). This was the first instance of Caliciopsis Peck occurring on Retrophyllum or indeed any member of ‘Podocarpus sensu lato’ (in the sense of Buchholz & Gray, Reference Buchholz and Gray1948), although the genus was later recorded from Podocarpus nubigenus (Butin, Reference Butin1970).
IUCN conservation assessment: LC (Farjon, Reference Farjon2010: 938; Jaffré et al., Reference Jaffré, Munzinger and Lowry2010: 1489; Thomas, Reference Thomas2010a)
The species is one of the most widespread of all New Caledonia's conifers, occurring throughout the island.
Uses
According to Farjon (Reference Farjon2010), the species has been extensively logged, especially at lower altitudes, for its high-quality wood used locally for flooring, furniture and similar purposes.
4. Retrophyllum minus (Carrière) C.N. Page, Notes Roy. Bot. Gard. Edinburgh 45(2): 380 (22 Feb. 1989 [‘1988]’, ‘minor’). – Nageia minor Carrière, Traité Gén. Conif. ed. 2, 2: 641 (15 Jan. 1867). – Podocarpus minor (Carrière) Parl. in A. DC., Prodr. 16(2): 509 (1868). – Decussocarpus minor (Carrière) de Laub., J. Arnold Arbor. 50: 352 (15 Jul. 1969). – Type citation: [New Caledonia] “Habite dans la Nouvelle-Calédonie, au sommet de très-hautes montagnes, où il fut découvert par un jardinier anglais nommé Richard. – D'après M. Vieillard (herbier de la Nouvelle Calédonie no. 1275 – in Herb. Mus. Par.) on le trouve aussi au bord du lac Arnaud.” – Lectotype designated here: New Caledonia, Yaté, “bord du Lac Arnaud”, Vieillard 1275 (P: sheet barcoded P00118110, top left sterile specimen, here designated 110-A: see Appendix I). Isolectotypes (all Vieillard 1275, P): sheet barcoded P00188109, sterile specimens at top centre right here designated 109-C and at bottom right here designated 109-G; sheet barcoded P00188110, unbranched sterile specimen c.26 cm long at right here designated 110-C and shoot in packet with c.9 larger leaves here designated 110-G. Epitype designated here: New Caledonia, “from banks of River des Lacs [sic] at site of former village below mine excavations of Madeliene [sic] mine Plaine des Lacs, 140 m or probably less. Tree 2–3 m high; wood sample with wood yellowish density near 0.5. Seeds maroon red, elongated pear-shaped, larger than coll. made of var. at 22 km Sta.”, 22 ii 1948, J.T. Buchholz 1729 (epi NY, isoepi ILL–n.v. [photos A, P], K, MO–n.v. [fide TROPICOS database], S–database). – For full details of the very difficult and complex typification of this name, see Appendix I. Fig. 1L.
[Podocarpus minor (Carrière) Parl. var. palustris J.Buchholz, in sched. Buchholz 1421, nom. nud.]. – Podocarpus palustris J.Buchholz, Bull. Mus. Natl. Hist. Nat. (Paris) sér. 2, 21: 284 (1949). – Type: New Caledonia, “Small tree growing in water, with stems flaring at base, dark gray bark, brownish beneath. 2–3 m high, some stems 40–50 cm in diameter at water line. Wood white soft of density about 0.3. Trees subject to flooding at times the leaves becoming covered with ferrugenus [sic] silt. Seeds smaller than in species coll. on Rio des Lacs”, 27 xi 1947, J. Buchholz 1421 (holo ILL–image barcoded ILL00010021, iso ILL–2 sheets–images barcoded ILL00010019 and ILL00010020, A–photo, K, MO–image (stamped 1712087), NY, P–image barcoded P00188158, RSA–image, S–image, TEX–image barcoded 00370048, WIS–image barcoded 0057120).
Iconography
De Laubenfels, Fl. Nouv. Caléd. 4: 51, pl. 11 f. 9–15 (1972), as Decussocarpus minor.
Etymology
Retrophyllum minus was originally described by Carrière (Reference Carrière1867) in the genus Nageia as N. minor Carrière. The other six species that he enumerated in that genus all had leaves measuring 40–160 mm in length, whereas he gave the length of the leaves of Nageia minor as 12–17 mm. The epithet minor (Latin for ‘smaller’; minus when neuter) therefore most probably alludes to the much smaller leaves of Retrophyllum minus compared with the leaves of the other six Nageia species, although there are no clues in his protologue as to why the epithet was chosen. Although less likely, it might also refer to the smaller stature of Retrophyllum minus compared with those six Nageia species, for which Carrière only gave actual height details for two. The epithet has nothing whatever to do with Retrophyllum minus being the smallest species in the genus Retrophyllum, as stated by Farjon (Reference Farjon2010: 938) and implied also by Gray (Reference Gray1962: 76), because, with the exception of R. vitiense (1863), of which Carrière was apparently unaware as he gave no treatment of it under any genus, all other species of Retrophyllum were first described between 1923 and 1983, long after Carrière described Nageia minor. Buchholz's epithet palustris alludes to the fact that the species is very frequently aquatic.
Vernacular name: bois bouchon (Jaffré, Reference Jaffré1988; Suprin, Reference Suprin2011a) – French for ‘cork wood’, referring to the very light, corky texture of the wood (an adaptation to its aquatic habitat)
Distinguishing features
Retrophyllum minus is a low shrub or small tree that is typically aquatic. The adult foliage is all in 4 ranks. The very thick adult leaves have a raised central area that when dried is marked by longitudinal wrinkles or by three (not two) ridges, and is typically wider than the leaf margins, although this is variable and plants with a narrower central area can be confused with Retrophyllum comptonii unless other characters are also examined. The seed surface is rough, porous and very buoyant, an adaptation to the aquatic habitat.
Small tree or shrub, frequently aquatic, normally 0.9–3.5 m tall but rarely attaining 8 m; 20–50 cm diam. at water-line. Trunk rapidly tapering from a wide, buttressed base, sometimes split from near base. Bark rough when old, peeling in short vertical strips or ragged pieces, fissured, dark grey, greyish brown or tan (colour often masked by ferruginous silt); inner bark brownish or reddish-brown; wood cream or whitish, very light. Crown open, irregular. Buds shortly ovoid to shortly ellipsoid, protected by very reduced foliage leaves; true bud scales absent. Primary branches irregularly arranged, divaricate, widely spreading or ascending; foliage branches clustered at tips of branches, erecto-patent to erect, straight, curved or flexuous. Shoots dimorphic but appearing mostly monomorphic, with only occasional scale-bearing shoots that soon pass into foliage shoots. Shoot flattening heterofacial on juvenile shoots but adult shoots ± lacking flattening. Juvenile foliage shoots with (1–)2–4 small leaves at base and 17–35 pairs of foliage leaves in 2–3 growth increments. Juvenile leaves (3.5–)4.5–6(–7.5) mm apart, opposite, distichous and pectinate, heterofacially turned at base, diverging at (40–)50–60(–70)°, sessile or subsessile, lamina narrowly lanceolate, linear-lanceolate or lanceolate-elliptic, 25–33 × 2.8–4 mm, gradually tapered to a narrow, obtuse tip. Adult foliage shoots subtended by 1–3 pairs of very reduced scale-like foliage leaves, the lowest true foliage leaves smaller and relatively broader than the others; leaves opposite-decussate in 4 ranks, crowded, sessile with bases not twisted and all with abaxial surfaces outermost, the upper leaves of each shoot partially imbricate, erecto-patent to erect, either yellow- to mid-green or grey-green; lamina of leaves in middle of shoot narrowly elliptic, elliptic, narrowly lanceolate-elliptic, lanceolate-elliptic or narrowly lanceolate, straight, very thick and coriaceous; margin thickened, extremely narrowly hyaline; midrib not visible on either surface but both surfaces with a raised elliptic central area as wide as or wider than the margins and either longitudinally wrinkled or marked by three longitudinal lines when dried; apex obtuse, broadly rounded or occasionally subacute; base cuneate or shortly attenuate.
Pollen cones lateral (axillary) or terminal on lateral shoots of current growth, subtended by a leaf or scale-leaf, 1–7 together, pedunculate (individual cones sessile when > 1 together); peduncle shorter than cone(s), erecto-patent, bearing 1–3 decussate pairs of spreading scales; peduncle scales green or grey-green, not keeled, ovate or ovate-lanceolate with ± long decurrent base and ± patent free part, subacute, with or without a narrow scarious margin; cones ellipsoid, ovoid or subglobose, 4–8 × 2–3 mm, straight; microsporophylls decussate but appearing spirally arranged, 24–40 per cone, the lamina green, ± heavily tinged violet, broadly triangular, deltate or triangular-ovate, abruptly narrowed to an acute, apiculate apex, with extremely narrow whitish hyaline margin, shorter than the microsporangia; microsporangia free, pinkish, elliptic or semicircular, stomium longitudinal, abaxial; pollen white to hyaline.
Female cones terminal on lateral foliage shoots of current growth, shortly pedunculate. Peduncle in line with shoot or erecto-patent, shorter than remainder of cone but longer or shorter than receptacle, bearing either 8–10 or 4 bracts in 4–5 or 2 decussate pairs respectively; distal part of peduncle shed with cone. Peduncle bracts light green or grey-green, keeled, elliptic, ovate-elliptic or ovate-rhombic, subacute to obtuse. Cone subtended by a non-fleshy to slightly fleshy receptacle formed from 1 or 2 peduncle bracts plus the sterile and fertile bracts. Receptacle narrowly obovoid, narrowly pyriform, narrowly obconical or cylindrical, light green or greyish-violet. Sterile bract normally 1, stiffly spreading to erecto-patent, oblong-elliptic, ovate-elliptic or elliptic, obtuse or subacute, with very narrow scarious margin. Fertile bract light green or greyish-violet, initially erect, with median longitudinal groove, wholly free from epimatium, with spreading, ovate free tip with acute apex. Cone obovoid (sometimes shortly so), ellipsoid or pyriform; epimatium either green at first, then blushing red and finally maroon-red when ripe, or grey-green and not turning red, with a shortly conical (sometimes indistinct) apical crest; seed beaked at micropylar end, the beak straight or curved. Seed with rugulose, porous texture, adapted to floating in water. Germination epigeal. Seedlings: initial leaves of main axis c.5 pairs, linear, 15–18 × c.1.5 mm excluding decurrent base, basally not twisted, opposite-decussate in a spiral arrangement, amphistomatic, separated by short, naked internodes, the youngest ones narrowly linear, apiculate and 23–31 × 1.5–2.2 mm, older ones becoming opposite in one plane, linear-elliptic or narrowly elliptic, shorter (11–20 × 1.9–3.0 mm) and bluntly mucronate at tip.
Taxonomy
Retrophyllum minus is a variable species. Some states, including the type, have ascending branches, leaves yellow- to mid-green when fresh, and female cones initially green turning red or finally maroon, as in Retrophyllum comptonii. Other states, including virtually all the aquatic material assigned by Buchholz to Podocarpus palustris and collected since, have more widely divaricate or patent branches, leaves grey-green, and female cones that are grey-green or whitish and apparently not turning red when ripe. Such plants also appear to have more microsporophylls per pollen cone (30–40) compared with plants at the other end of the spectrum of variation, which have only about 25 microsporophylls per cone. The plants with almost white, extremely glaucous ripe female cones are very distinct and easily recognisable in the field as belonging to Retrophyllum minus. This is not the case with the state having pinkish-green to red ripe cones, which often shows Retrophyllum comptonii-like characteristics. The variation within the species clearly influenced Buchholz to describe one extreme, that with cones not turning red when ripe, as Podocarpus palustris. Plants assignable to this and to typical Retrophyllum minus often occur together at the same site, however. The two states appear to be very distinct, especially when reproductive organs are present, and the fact that many of the distinguishing features are reproductive rather than vegetative also makes the case for recognising varieties within the species stronger than it might otherwise be. Matters are complicated, however, by the occurrence of low-altitude populations of Retrophyllum comptonii sympatrically with R. minus in the Plaine des Lacs area, and it is therefore possible that hybridisation may occur. Indeed, as mentioned under Retrophyllum comptonii, the morphological characters of ‘typical’ Retrophyllum minus (i.e. including its type material) are intermediate between those of Podocarpus palustris and Retrophyllum comptonii, and a hybrid origin of those populations of R. minus agreeing with its type therefore needs testing. For this reason, varieties are not here recognised within the species.
Material from the Port Boisé area (hb. MacKee 19303, 19304, 19305) was determined at Paris as both Decussocarpus minor and D. comptonii by de Laubenfels in 1972. All three sheets seem identical in foliage characters and are more similar to Retrophyllum comptonii than they are to R. minus. However, plants from the same population were analysed using RAPD by Herbert et al. (Reference Herbert, Hollingsworth, Gardner, Mill, Thomas and Jaffré2002), who found that the Rivière Trou Bleu population, coded by them as RTB, grouped with Retrophyllum minus, not R. comptonii, using this technique. Hence, all the specimens from this locality are here assigned to Retrophyllum minus.
Distribution
New Caledonia (Grande Terre, Province Sud: Prony, Baie du Sud, Lac en Huit, Rivière des Lacs, Plaine des Lacs). Map: Fig. 6.

Fig. 6. Global distribution of Retrophyllum minus.
In the Rivière des Lacs area Retrophyllum minus coexists with plants that are assignable to Retrophyllum comptonii and as noted above some plants possess characters intermediate between the two species, suggestive of hybridisation. Most of the area of the two lakes forming Lac en Huit, one of the classic localities for Retrophyllum minus, is in Yaté commune, but the southern third of the eastern lake is in Le Mont-Dore, as well as the RM9 road (‘Route de l'Aérodrome’) along the south side of both lakes and all of the CR7 road (‘Route du Déversoir’ – the Nouméa–Yaté main road). Records from this area have therefore been assigned to one or other commune, or an unassignable boundary zone, depending on field notes and their accuracy. The whole of Grand Lac (Lac Arnaud) is in Yaté.
Specimens seen and other records. NEW CALEDONIA. Unloc., Le Rat 253A (S–database). Unloc., no date, Petit 138 (P–image); Bernier 245/1 (seedlings; P–image, barcode P00188175); Bernier 245/4 (P–image, barcode P00188172); Aubréville & Heine 241 (P–image); unloc., 1950–1951, Baumann-Bodenheim 6378 (P–image, juvenile and adult); unloc., 1861, Deplanche 170 (P–image); unloc., 1863, Deplanche 170 (P–image, central area of leaf rather narrow and transitional to R. comptonii but determined in 1950 as Podocarpus minor by Buchholz & Gray; A–photo). Without definite locality, “Hauteur un mètre sur les plateaux couverts du marais fort arides. 8 m sur les flancs escarpees des Montagnes du même sol, fruit rouge,” Pancher (Vieillard 1275) (BM–also seemingly (re?)numbered Hance 17247, K). Province Sud: Le Mont-Dore: Baie du Sud, 1861–1867, Vieillard 1275 (A, GH, K–2 sheets, P–2 sheets, images and also A–photos). Prony, without date, Franc 207 (BM [photo A], GH, K: note discrepancy in numbering and dating of other sheets, see below). Bords d'un torrent situé au fond de la Baie de Prony, ix 1868, Balansa 186 (2 sheets, P–images). Prony, Baie de Kué, 3 m, x 1903, Cribs 1493 (P–image, K–database); Prony, Le Rat 149 (P–image). Prony, x 1913, Franc 207 (P–image); ibid., x 1913, Franc 207 (Série A) (2 sheets, P–images); ibid., i 1914, Franc 207 (A); Prony, undated, Franc 207 (Série A) (A); Prony, 100 m, Pancher s.n. (P–image: label bears manuscript names ‘Podocarpus nana Parl.’, ‘Dacrydium austrocaledonicum Vieillard’ and ‘Podocarpus Guillainii Vieillard’, as well as the inscription “C'est la même plant que Vieillard 1275”, the first five words of which are in different handwriting to the rest of the label, which also gives locality information for specimens collected at Cougui 800 m and Mt. Mou 1200 m that are, from the altitudes, assignable to Retrophyllum comptonii). Prony, where road crosses the Rivière Bleue, very close to the river margin, 50 m, 22°18′35′′S 166°49′55′′E, 28 iii 1999, Cretinon & Gardner, ICCP New Caledonia Exped. 1999: 17 (E). Rivière des Pirogues, 31 x 1923, White 2261 (E, K, P–image). Le Carénage, just above sea, 8 iv 1955, MacKee 2373 (E, L–image, P–image); c.5 miles from Baie du Carénage, on road between Baie du Carénage and highway from Nouméa to Yaté, 600 ft [183 m], 22 vii 1952, McMillan 5139 (A, E, K, L–image, P–image). Port Boisé, embouchure du Port Boisé, vallée de Koué, 100 m, 28 ii 1976, Viratelle (hb. MacKee 32355) (P–image). Port Boisé, embouchure du ruisseau Trou Bleu, 20 viii 1968, Lavoix (hb. MacKee 19303 & 19304, both det. de Laubenfels as Decussocarpus minor) (P–images, 19303 also NOU–database, 19304 also K–database); ibid., Lavoix (hb. MacKee 19305, det. as D. comptonii or R. comptonii) (P–image, K–database, NOU–database). Port Boisé, Rivière Trou Bleue, Cretinon & Gardner, ICCP New Caledonia Exped. 1999: 50 (E; also specimen collected 25 iv 2001 from E cultivated accession 19990384C, E); ibid., 1 iii 1999, Cretinon & Gardner, ICCP New Caledonia Exped. 1999: 50A (E, seedling). Lac en Huit [22°16′S 166°52′E], 7 x 1957, de Laubenfels P115 (SBT–n.v., fide de Laubenfels, Reference de Laubenfels1969: 345; S–database); ibid., 19 v 1977, Musselman 5280 (NOU–database). Rivière Bleue, route de Prony [22°17′S 166°46′E], xi 2007, Munzinger 4830 (NOU–database). Le Mont-Dore / Yaté boundary: Plaine des Lacs, 22°14′–22°16′S 166°50′–166°57′E, ad ripam lacus “en 8” (Lac en Huit) dicti, 250 m, 3 vii 1965, Bernardi 9369 (L–image, P–image, Z–database); along banks of La Madeleine (Rivière des Lacs), S of Nouméa–Yaté road, edge of Lac en Huit, c.250 m, 30 viii 1980, McPherson 2996 (P–image); Lac en Huit, 150 m, 15 xi 1955, MacKee 3382 (A, L–image, P–image). Yaté: Bords du lac Arnaud, 1855–1860, Vieillard 1275 p.p. (lectotype and isolectotypes as specified above and in Appendix I, P–images); ibid., Vieillard 1275 p.p. (non-lectotype material as specified in Appendix I, P–image). Grand Lac (Plaine des Lacs), 250 m, 30 iii 1942, Virot 658 (A, P–2 sheets, images); western margin of Grand Lac, 22°15′52′′S 166°54′30′′E, 250 m, 1 iii 1999, Cretinon & Gardner, ICCP New Caledonia Exped. 1999: 44 (E). Plaine des Lacs, Lac en Huit, au niveau du déversoir (berge du lac), 250 m, 8 i 1987, Jérémie & Tirel 1572 (P–image). Lac en Huit, 22°16′16.0′′S 166°53′13.5′′E, 256 m, 24 xi 2005, Gardner et al. 02 (E). Lac en Huit, northern shore-line of second lake, 22°15′20.3′′S 166°53′51′′E, 241 m, 24 xi 2005, Gardner et al. 04 (E). Plaine des Lacs, prior to 19 xii 1889 (date of receipt at P), Raoul s.n. (P–image); Plaine des Lacs, Le Rat 607 (BM and photo of this sheet A; P–image), 751 (P–image), 1040 (P–image); Bord des rivières de la Plaine des Lacs, ix 1905, Le Rat 2587 (P–image). Plaine des Lacs, bords de la rivière Pouéné, ix 1905, Le Rat 2621 (2 sheets, P–images, one without locality information). Plaine des Lacs, 22 km [station], c.750 m, viii 1947, Bernier 204 (P–image); 22 km station, 31 x 1947, Bernier 245/3 (female, P–image, barcode P00188173); Plaine des Lacs, 22 km Station, 4 xi 1947, Buchholz 1347 (paratypes of Podocarpus palustris ILL, 2 sheets (ILL00010017 and ILL00010018)–images, isoparatypes of Podocarpus palustris K, NY, P–image, RSA–image, S–database; photo of trees in situ, A); ibid., same date, Buchholz 1348 (paratype of Podocarpus palustris ILL–image (ILL00010016), isoparatypes of Podocarpus palustris GOET–image, K, NY, P–image, RSA–image, S–database, WIS–image); ibid., 22 xi 1947, Buchholz 1421 (holotype of Podocarpus palustris ILL–image (ILL00010021), isotypes of Podocarpus palustris ILL–images (ILL00010019, ILL00010020), K, MO–image, NY, P–image, RSA–image, S–image, TEX–image; photo of seeds, A); Buchholz 1474 (paratypes of Podocarpus palustris ILL–2 sheets (ILL00010015 and ILL00010022)–images [photo of latter specimen at A], isoparatype of Podocarpus palustris S–database (photo)]; ibid., 14 ii 1948, Buchholz 1705 (K, seedling, P–image, 2 seedlings; S–database). Guépyville, radier du 22 km, 150 m, 27 v 1948, Bernier 251 (P–image, K–database). Route de Yaté, km 22, viii 1949, Sarlin 73 (P–image). Route Bon Secour, 22 km ck., R[ivière] Blanche, 200 m, 19 vii 1954, Ingle I.44 (L–image, MEL–n.v.). 22 km station, 10 vii 1957, de Laubenfels P112 (SBT–n.v., fide de Laubenfels, Reference de Laubenfels1969: 347; S–database). Plaine des Lacs, Mine Madeleine, 140 m or less, 22 ii 1948, Buchholz 1729 (epitype NY, designated here; isoepitype ILL–n.v. [photos A, P; A example with somewhat different text on label from others], K, MO–n.v. [fide TROPICOS], S–database). Plaine des Lacs, on road from Nouméa to Yaté, 10 vii 1956, “this specimen is from same tree as that from which Buchholz obtained his type”, Foster 200 (topotype of Podocarpus palustris, P–image). Plaine des Lacs, village Anna Madeleine, delta du Dacrydium n.sp., 230 m, 7 iii 1948, Bernier 246 (P–image); ibid., juvenile, 7 iii 1948, Bernier s.n. (P–image); ibid., 6 vi 1948, Bernier 249 (P–image, K–database, Z–database). Madeleine, Plaine des Lacs, 30 iii 1951, Guillaumin & Baumann-Bodenheim 11749 (P–image, Z–database); ibid., 30 iii 1951, Guillaumin & Baumann-Bodenheim 11811 (P–image, Z–database). Plaine des Lacs, Rivière des Lacs, la Chute, 239 m, 13 iii 1964, Staufer & Blanchon 5807 (L–image, P–image, K–database, NOU–database, Z–database); Plaine des Lacs – Bords du lac de Yaté (sous ligne haute tension après riv. Madeleine) – la Chute, 13 iii 1964, Blanchon 736 (P–image, NOU–database). Along banks of La Madeleine (Rivière des Lacs), S of Nouméa–Yaté road, near bridge, 23 xi 1979, McPherson 2132 (P–image, K–database, NOU–database); ibid., beside Chute de la Madeleine [22°13′S 166°50′E], 29 v 1980, McPherson 2748 (P–image, NOU–database); riverbanks below Chutes de la Madeleine, 10 viii 1981, Stone 14836 (P–image). Rivière des Lacs (rive droite) 5 km en Aval de la Chute, 200 m, 29 vi 1986, MacKee 43169 (CANB–database, P–image, K–database, NOU–database, Z–database). Forêt “Mois de mai”, Riv. blanche, 22 vii 1951, Baumann-Bodenheim 13923 (P–image, Z–database). Serpentine area, Rivière Blanche c.30 miles E of Nouméa on road to Yaté, Plaine des Lacs, 600 ft [183 m], 21 vii 1952, McMillan 5120 (A, E, L–image, P–image, K–database). Ex-radier Anna-Mad.[eleine] (locality given on composite label on sheet 245/4), Bernier 245/2 (male; P–image, P00188174). Plaine des Lacs, Anna Madeleine, 150 m, 22 i 1967, Aymard (hb. MacKee 16325) (P–image). Anna-Madeleine, rivière [22°09′S 166°54′E], 100 m, 23 xi 1979, Hartley 15064 (CANB–database, L–image, NOU–database). La Madeleine, 8 xii 1981, Dickison 223 (NOU–database). Rivière des Lacs (La Madeleine) en amont des chutes, 20 ix 2007, Dagostini et al. 1340 (P–image, NOU–database); Trois Bras [22°09′S 166°54′E, c.320 m], 18 ix 1980, Suprin 693 (NOU–database). Near bridge over ‘River des Lacs’ [sic] on road to Yaté, 21–22 ii 1948, Buchholz 1719 (P–image, packet only, its contents not visible; seeds similar to Buchholz 1729, fide label); Plaine des Lacs, Campement 22, 5 vii 1949, Selling 78 (S–database); Plaine des Lacs, i 1914, Franc 207 (Z–database: specimens seen of this number have all been from a different locality, Prony); Plaine des Lacs, Rivière des Lacs, 6 x 1924, Däniker 228 (Z–database); ibid., 11 x 1924, Däniker 228 (P–image, Z–database); Plaine des Lacs, 8 ii 1926, Däniker 228a (Z–database); Plaine des Lacs, Rivière des Lacs, Plaine des Lacs, rades de la Rivière des Lacs, c.100 m, 6 vi 1948, Bernier 250 (P–image); Plaine des Lacs, c.250 m, 14 xii 1949, MacDaniels 2544 (GH); Vallée des Lacs, 5 x 1950, Guillaumin & Baumann-Bodenheim 6511 (Z–database, 2 sheets); ibid., pont, 3 x 1950, Guillaumin & Baumann-Bodenheim 6580 (P–image, Z–database, 2 sheets); ibid., 5 x 1950, Guillaumin & Baumann-Bodenheim 6582 (P–image, juvenile; Z–database); ibid., 5 x 1950, Guillaumin & Baumann-Bodenheim 6594 (P–image; Z–database, 2 sheets); ibid., 7 x 1950, Guillaumin & Baumann-Bodenheim 6766 (P–image, Z–database). Rivière des lacs, route de Yaté, c.100 m, Bernier 245/2 (P–image). Plaine des Lacs, route de Yaté, near new bridge over Madeleine River, 5 xi 1959, Thorne 28565 (L–image, P–image). Bassin de la Rivière Yaté, Laverie Lafleur du 22ème km, 19 viii 1957, Service des Eaux et Forêts, Nouméa 225 (S–database); au bord de la Rivière des Lacs, au S du pont, 150 m, 9 vii 1958, Hürlimann 3113 (Z–database); Rivière des Lacs, 22°10′50′′S 166°50′56′′E, 200 m, 1 iii 1999, Cretinon & Gardner, ICCP New Caledonia Expedition 1999: 26 (E). Plaine des Lacs, bords de la Riv. à Chute, 25 vi 1963, Blanchon 208 (P–image, NOU–database); Plaine des Lacs, 29 vi 1965, Aubréville & Heine 130 (P–image); Tournée des Lacs, 3 vii 1965, Aubréville & Heine 170 (P–image). Süd-Bai, zwischen der Bai N'Go und Touaourou, viii–xii 1903, Rohrdorf 178 (Z–database); Touaourou, 350 m, x 1903, Cribs 1752 (P–image; highest altitude known for this species). Vallée de Toémo (entre Goro et Port-Boisé), 30 m, 4 v 1990, Cherrier (hb. MacKee 44895) (P–image, NOU–database as Retrophyllum comptonii). Bord de la Kuébini, 14 ii 1984, Lauri & Gay 165 (P–image, NOU–database). Rivière des Kaoris, Baie des Requins, 30 m, 7 iii 1948, Bernier 247 (P–image). Cascade de N'Goro, 50 m, 7 v 1948, Bernier 248 (2 sheets, P–images). Marais Kiki, 28 ix 1950, Baumann-Bodenheim 6370 (Z–database, 2 sheets); Marais Kiki, Plaine des Lacs, 150 m, 22 x 1954, MacKee 1118 (A, E); ibid., MacKee 1119 (E, P–image); bassin de la Rivière Yaté, Marais “Kiki”, 19 viii 1957, Service des Eaux & Forêts, Nouméa 226 (S–database, spirit coll.); au bord du Marais Kiki, 148 m, 17 vii 1958, Hürlimann 3157 (Z–database). Near forks of Yaté river, 3 xii 1957, de Laubenfels P160 (SBT–s.n., fide de Laubenfels, Reference de Laubenfels1969: 347; S–database). Along Riv. des Lacs near beginning of Route du Carénage, 22°10′S 166°50′E, 150 m, 11 xii 1973, Webster 19205 (GH, P–image, NOU–database). Carènage – Creek Pernod [22°12′S 166°49′E, c.190 m], 1 xii 1964, Blanchon 1160 (P–image, NOU–database). Plaine des Lacs, Crique Pernod, 16 xi 1950, Guillaumin 8339 (P–image, S–database, Z–database); ibid., 16 xi 1950, Guillaumin 8345 (P–image, Z–database). Plaine des Lacs–Carénage road at crossing of Creek Pernod, 200 m, 15 xi 1955, MacKee 3377 (A, L–image, P–image; E, dated 15 xi 1953). Rivière des Lacs, near the junction with the Pernod Rau., c.160–170 m, 24 ix 1963, Green 1187 (A, P–image, K–database); Creek Pernod [22°12′S 166°49′E], 15 i 1970, Jaffré 352 (P–image, NOU–database); along road to Plaine des Lacs, at crossing of Creek Pernod, 22°11′01′′S 166°50′39′′E, 5 xii 1996, Lowry II et al. 4683 (MO–database, P–image, mis-determined as Decussocarpus comptonii); Pernod Creek, 4 xi 1984, Page 22031 (E = no. 22032 at ORSTOM); ibid., Page 22034 (E = no. 22032 at ORSTOM: some larger leaves lanceolate); ibid., Page 22033 (E); ibid., Page 22030 (E). Rivière des Lacs, 100 m before the fork in the river which divides into Creek Pernod, 22°10′45′′S 166°50′50′′E, 130 m, 29 xi 2002, Gardner et al., Third New Caledonia Araucaria Exped. TNCA 5000 (E); ibid., Gardner et al., Third New Caledonia Araucaria Exped. TNCA 5001 (E). Lake Suprin, 22°17.247′S 166°59.504′E, 238 m (Hope, Reference Hope2015); Lake Boulet, 22°16.973′S 166°58.587′E, 222 m (Hope, Reference Hope2015).
USA (Cultivated). Florida: Miami: Montgomery Botanical Center (Coral Gables), cultivated in nursery, 20 xii 2009, J. Silba B-654 (NY–image).
Bioregion: New Guinea & Melanesia
Ecoregion: AA0113 New Caledonia Rain Forest.
Ecology
Retrophyllum minus as delimited here, and by de Laubenfels (Reference de Laubenfels, Aubreville and Leroy1972), is exclusively a low-altitude species. It grows along lake and shallow river margins in areas subject to seasonal flooding, from near sea level up to 350 m but typically below 170(–250) m, very often as a swamp tree. The observation in the protologue, “Habite dans la Nouvelle-Calédonie, au sommet de très-hautes montagnes. . .” presumably applies to material of Retrophyllum comptonii, which does inhabit mountain summit habitats as well as lowlands. Before the separation of Podocarpus comptonii from P. minor by Buchholz (Reference Buchholz1949), the name Podocarpus minor was applied indiscriminately to New Caledonian plants of Retrophyllum from both lowland swamps and high mountains. Retrophyllum minus grows in single-species stands or with other tall shrubs, such as Dacrydium araucarioides Brongn. & Gris. Retrophyllum comptonii also occurs at some localities, especially in the Plaine des Lacs. Plants from lowland swamp habitats (true Podocarpus minor, = Retrophyllum minus) were often given the informal designation “type lacustre”, especially on labels of specimens collected by Bernier, while plants later segregated as Podocarpus comptonii (= Retrophyllum comptonii) were labelled “type sylvestre”. This species has been regarded as a rheophyte by van Steenis (Reference van Steenis1981: 168), and its seeds float, aided by their porous epimatium (Carlquist, Reference Carlquist and Carlquist1974: 223). Jaffré (Reference Jaffré1988) described an association in which the two dominant species were Retrophyllum minus and Dacrydium guillauminii J.Buchholz, which are otherwise two of the rarest plant species of New Caledonia. Retrophyllum minus was, according to Jaffré (Reference Jaffré1988), found on unstable soil in small coves in the 1- to 5-m-wide alluvial zone along the riverbanks. Other associates include Cloezia aquarum (Guillaumin) J.W.Dawson, Homalium kanaliense Briq., Melaleuca brongniartii F.Muell., Neocallitropsis pancheri (Carrière) de Laub., Pancheria communis Baker f. and Xanthostemon aurantiacus Schltr. Fruiting periods of Retrophyllum minus tend to coincide with periods of high water levels (Cornu et al., Reference Cornu, Sarrailh and Marion2001).
Analysis of late Quaternary sediment cores from Lac Xere Wapo in the eastern Plaine des Lacs region has revealed the presence of Retrophyllum minus pollen and wood as far back as 120,000 years ago, but that there have been some marked fluctuations in abundance; the species was abundant in the earliest zone, XW-4 (120,000–90,000 years ago), but there was an abrupt rise and fall in abundance in zone XW-3 (90,000–80,000 years ago) and a dramatic decline in zone XW-2 (80,000–45,000 years ago) that is countered by a rise in Araucaria pollen. In the most recent zone (XW-1, 45,000 years ago to present), Retrophyllum again becomes one of the dominant species but Dacrydium is the most dominant (Stevenson & Hope, Reference Stevenson and Hope2005).
Chromosome number: 2n = 20 (Hair & Beuzenberg, Reference Hair and Beuzenberg1958, two separate counts as Podocarpus minor and P. palustris)
Mycological associations
See under Retrophyllum comptonii.
Conservation status: EN B1ab(iii,v)+2ab(iii,v); C2a(i) (Thomas, Reference Thomas2010b)
At the Madeleine site, which has been subjected to much trampling by the public, who have used the rare trees as diving platforms (Province Sud, 2012), there has been extensive planting since 1999 of Retrophyllum minus (1200 trees: Province Sud, 2012) and other rare gymnosperms, such as its associates Dacrydium guillauminii and Neocallitropsis pancheri. Germination success of Retrophyllum minus there has been very good (Cornu et al., Reference Cornu, Sarrailh and Marion2001; Province Sud, 2012).
Uses
No uses of Retrophyllum minus are reported in recent literature. Sebert (Reference Sebert1874), however, commented that, among the New Caledonian Podocarpaceae, Podocarpus minor was “l'espèce la plus interessante sous le rapport industriel”; this opinion of course also embraced Retrophyllum comptonii, which at that time was included in a broad concept of Podocarpus minor as previously stated.
Group C. Bark exfoliating as large plates. Vegetative shoots monomorphic. Terminal buds naked. Adult leaves distichous and heterofacially flattened in 2 ranks, relatively thin. Resin canals (1)3(5) median plus 2–6 lateral ones round leaf margin. Pollen cones in racemose inflorescences subtended by leaves, the raceme branches opposite, individual cones ± pedicellate, subtended by leaf-like bracts; microsporophylls spirally arranged, lanceolate. Female cones borne on specialised lateral reproductive shoots, sylleptic; peduncle broadened distally, in its vegetative portion bearing small spreading bracts that may or may not resemble reduced leaves; cone axis with 6–8 sterile bracts below fertile bract. Fertile bract with its basal half firmly adnate to epimatium, navicular. Receptacle formed from fertile bract and last sterile bract, cylindrical. Seed not beaked at micropylar end. Germination hypogeal. Seedling phyllotaxis spiral. – South America.
5. Retrophyllum rospigliosii (Pilg.) C.N. Page, Notes Roy. Bot. Gard. Edinburgh 45: 380 (22 Feb. 1989 [‘1988’]). – Podocarpus rospigliosii Pilg., Notizbl. Bot. Gart. Berlin 8: 273 (1 Feb. 1923). – Decussocarpus rospigliosii (Pilg.) de Laub., J. Arnold Arbor. 50: 347 (15 Jul. 1969). – Nageia rospigliosii (Pilg.) de Laub., Blumea 32(1): 211 (3 Feb. 1987). – Type: Peru, Oxapampa, N. Esposto 556 (holo B–extant, images seen; photos of holo at A, F [negative #11579, and a second duplicate photo of the B holotype mounted on a herbarium sheet], GH, NY); iso S (2 sheets: SBT W 430–image, S–Regnell 7799–image, both fragments ex Berlin) and S spirit collection–database). Farjon (Reference Farjon2010: 941) indicated that the holotype was at USM (Universidad Nacional Mayor de San Marcos, Peru) but, if there is a duplicate there, it is highly improbable that Pilger saw it and, if it exists, it will be another isotype. Figs 1C, F, J, M, 7A–H, 9D, E.

Fig. 7. Retrophyllum rospigliosii. A, Branching arrangement (Pennington 1433). B, Juvenile foliage shoot (Roldán et al. 3168). C, Adult male shoot (Pennington 1433). D, Young male cones (Pennington 1433). E, Mature male cone (Pennington 1433). F, Microsporophyll and microsporangia, adaxial view (Carr 15666). G, Microsporophyll and microsporangia, abaxial view (Pennington 1433). H, Adult female branch (Bunting 4939). Magnifications: A, B, × 0.67; C, H, × 1.5; D, E, × 3; F, G, × 15. Scale bars: A, B, 6 cm; C, H, 2 cm; D, E, 1 cm; F, G, 1 mm. Drawn by Claire Banks.
?Torreya bogotensis [Linden, Cat. n. 25 (1870) et ex Thurb. in Bull. Torrey Bot. Club 1: 7 (1870) nom. inval.]; Linden ex K.Koch, Wochenschr. Vereines Beförd. Gartenbaues Königl. Preuss. Staaten 14(25): 199 (1871), nom. utique rej. (Mill, Reference Mill2010; Brummitt, Reference Brummitt2011: 1207). – Type not indicated.
Podocarpus montanus sensu Knuth in Fedde, Repert. Spec. Nov. Regni Veg. Beih. 43: 95 (1926) non (Humb. & Bonpl. ex Willd.) Lodd. (1836).
Etymology
Podocarpus rospigliosii is named after Dr Carlos Rospigliosi Vigil, who led the first expedition sent out in May 1918 by the newly founded Museo de Historia Natural in Lima, of which Rospigliosi was the inaugural director, and in which both Nicolás Esposto (collector of the type of P. rospigliosii) and Rospigliosi participated. In 1922, Rospigliosi sent specimens of Podocarpus rospigliosii to Pilger in Berlin, who described it as new some months later.
Vernacular names: pino, pino hayuelo, pino de montaña, pino colombiano (Colombia: Villamizar & Suevara 63, UDBC), pino hembrito (Colombia, Santander: Cardenas & Oliveros 198, COL–database), chaquiro crespo and chaquiro colorado (Colombia: Vásquez Correa, Reference Vásquez Correa2010), pino negro (Colombia: Villamizar & Suevara 63, UDBC), diablo fuerte (Cajamarca, Peru: Vicuña-Miñano & León, Reference Vicuña-Miñano and León2003), pino romerón, pino silvestre (throughout Colombia); pino de Pacho (Cundinamarca, Colombia); romerillo macho (Becerra-Montalvo & Zevallos-Pollito, Reference Becerra-Montalvo and Zevallos-Pollito2014), romerillo blanco, diablo fuerte, alcumano, ulcumano, utcumanu (NW Peru: Vicuña-Miñano & León, Reference Vicuña-Miñano and León2003; Vicuña-Miñano, Reference Vicuña-Miñano2005), pino criollo (Mérida, Venezuela: Aranguren & Márquez, Reference Aranguren and Márquez2011)
Some of the above, such as diablo fuerte and ulcumanu, are shared with other species of Podocarpaceae.
Distinguishing features
Retrophyllum rospigliosii is distinguishable from the only other currently known South American species of its genus, R. piresii, by its more lanceolate leaves that are acute to shortly acuminate and frequently abruptly narrowed just below the tip, and by its more shortly ellipsoid to subglobose female cones that are narrowed at the base. The foliage shoots are at all stages arranged ± alternately, whereas in Retrophyllum piresii the adult shoots (the only state known) are opposite. The peduncle of the female cone bears reduced leaves, not scales as in Retrophyllum piresii. Several of these characters were not noted when the original description of Retrophyllum piresii was drawn up and serve as additional justification for the recognition of two separate species. Eckenwalder (Reference Eckenwalder2009), however, reduced Retrophyllum piresii to synonymy within R. rospigliosii on the grounds that, in his opinion, the specimen known to him fell within the range of variation of the latter species. Retrophyllum rospigliosii can be distinguished from the Fijian R. vitiense and the New Guinea R. filicifolium (the only other species in the genus that have all leaves not imbricate) by its bark exfoliating in large plates (not small pieces), the naked buds (in R. vitiense and R. filicifolium protected by bud scales), the foliage shoots lacking scale leaves, the blade of the microsporophyll longer than the microsporangia (not equalling or shorter than them), the more acute female cone peduncle bracts that lack even a narrow scarious margin, the much more pronounced zigzag arrangement of the decurrent leaf bases (which in fresh material are bright green and contrast strongly with the brownish colour of the rest of the branchlet) and the ellipsoid to subglobose (not obovoid) female cones. Prumnopitys montana (Humb. & Bonpl. ex Willd.) de Laub., which has in the past been confused at least once with Retrophyllum rospigliosii (Knuth, 1926: see synonymy), has spirally arranged leaves that appear alternate-distichous and the shoot flattening is homofacial, not heterofacial, so that the leaves along both sides of the shoots are turned with their abaxial surfaces uppermost; the laminas are also almost all obovate, rather than lanceolate as in R. rospigliosii.
Evergreen tree 20–45 m; dbh to 250 cm (Vicuña-Miñano & León, Reference Vicuña-Miñano and León2003). Trunk of mature trees buttressed at base, unbranched for up to 28 m although branching in young trees begins at c.3 m. Bark of trunk smooth, bluish-black, exfoliating in large plates; wood reddish-yellow. Crown ovoid or umbrella-shaped, much branched. Branchlets of last two orders erecto-patent, straight or slightly curved; ultimate branchlets usually alternate, adult ones 25–140(–210) mm with 5–32(–50) pairs of leaves, juvenile ones up to 270 mm (perhaps more) with up to at least 40 pairs of leaves. Leaf scars absent. Buds naked. Leaves on last two orders of branching all of one type (scale leaves absent). Leaves on penultimate axes soon caducous except when subtending branch bases or when branch does not develop further branching, arranged in 2 stepped opposite-distichous parallel ranks; decurrent bases crossing over to opposite side of branchlet and forming a green zigzag pattern that contrasts strongly with the dark brown bark when fresh; lamina larger than those of ultimate shoots, ± ovate-elliptic. Juvenile leaves of ultimate shoots unequally amphistomatic, 4–5 mm apart, opposite-distichous, pectinate, subsessile; lamina horizontally spreading, mid to deep green, narrowly lanceolate or lanceolate, 15–23 × 3–6 mm, straight, coriaceous, initially dorsally keeled when unfolding but both surfaces becoming flat, with vein-like striae between rows of stomata, midrib slightly raised on both surfaces, apex subacute or obtuse, base obtuse. Adult leaves equally or unequally amphistomatic, 3–5 mm apart throughout on most foliage shoots but up to 10 mm apart on leader shoots and sometimes in lower 1/3 of foliage shoots, opposite-distichous, pectinate, not imbricate, subsessile (free part of petiole 0.3–0.5 mm); lamina spreading and forming a shallow ‘V’ along branch, erecto-patent, diverging at 45–60(–70)°, mid to deep green, elliptic, lanceolate, ovate, ovate-elliptic, obovate-elliptic or obovate, 7–14 × 3–5 mm, straight, thinly coriaceous, the margin only slightly thickened, both surfaces flat with vein-like striae alternating with the stomatal rows, midrib only slightly raised but distinct on both surfaces, the blade frequently (but not always) abruptly narrowed distally below the acute or shortly blunt-acuminate apex that is never mucronate, the base obtuse or cuneate, the petiole decurrent along the whole length of the internode.
Pollen cones subtended by a foliage leaf, lateral in leaf axils of current growth, solitary or in groups or primary clusters of up to 3, often with an opposite pair of cones below; common peduncle present when cones in clusters but otherwise absent, individual cones shortly pedicellate and subtended by a pair of bracteoles; peduncle (when present) with 4–6 decussate, ovate, acute scales; cones greenish brown when young turning pinkish brown, ovoid when young becoming cylindrical, straight or curved from base, 8–12 mm when shedding pollen; microsporophylls 28–36, decussate in 7–9 whorls of 4, lamina longer than microsporangia, triangular-lanceolate, narrowly lanceolate or lanceolate, with entire or finely crenulate whitish hyaline scarious margin, the apex acute and incurved; microsporangia reniform; pollen snow-white or cream.
Female cones borne on current year's growth, terminal on specialised lateral reproductive shoots bearing reduced, ovate, acute leaves. Peduncle deflexed-patent to pendulous, its lower portion bearing 1 or 2 pairs of ovate-elliptic, leaf-like peduncle bracts and passing into the cone axis which bears 3 or 4 pairs of decussate, elliptic to obovate leaf-like sterile bracts (sometimes with interspersed small scales) with acute apex and glaucous on adaxial surfaces; apical part of cone axis (above last pair of sterile bracts) forming an indistinct cylindrical receptacle that becomes violet when ripe and is often shed with the cone together with the fertile bract. Fertile bract initially erect, with its basal part adnate to epimatium, distal part navicular and free. Female cone shortly ellipsoid to subglobose, 16–30 × 12–18 mm (the seed proper c.15 × 11 mm); epimatium violet and ± glaucous especially when not fully ripe, somewhat asymmetrical with the adaxial side rather more convex than the abaxial, with a narrowed base and very short conical distal crest that sometimes breaks off and with a straight, conical beak at micropylar end; micropylar beak with two short prongs that point slightly obliquely (towards the abaxial side) downwards towards the last sterile bract; cone resiniferous within; seed proper rounded at the distal end, narrowed to a conical spine at the micropylar end.
Germination apparently hypogeal (first foliage shoot emerging at soil level, cotyledons not visible). Seedlings with alternate-spiral phyllotaxis, the main axis with relatively long internodes and rather widely spaced, alternate-spiral leaves similar to those of the foliage shoots; foliage shoots with 12–25 pairs of leaves (the lower ones with fewer pairs than the upper), the leaves in 4 ranks (2 sets of 2) that are flattened distichously to form a shallow ‘V’ along the shoot with the largest leaves near the middle of the shoot.
Notes
Adult foliage shoot length (and consequently number of leaves) is very variable, being dependent at least partly on position on the tree and reproductive condition; individual ultimate foliage shoots in the upper or fertile regions of the tree are much shorter than those lower down (which also have longer leaves than those higher up). Neill & Salinas 7202 and Morales et al. 867, both from Ecuador, are atypical in having adult foliage leaves diverging from the axis mostly at angles greater than 65°, with many almost at a right angle especially in the former specimen. The adult foliage shoots of Neill & Salinas 7202 are also unusually long (to > 20 cm, with up to 50 pairs of leaves), whereas those of Morales et al. 867 are much shorter. The habitat and altitude (tropical forest at 600–675 m) of these two gatherings are also atypical for Retrophyllum rospigliosii. The habitat and foliage angle of both specimens are more characteristic of Retrophyllum piresii, which has not been recorded from Ecuador. However, Neill & Salinas 7202 has the alternate branching and lanceolate, acute leaves that are typical of Retrophyllum rospigliosii and consequently it is assumed to be a slightly divergent form of that species. In Morales et al. 867 most branches are opposite as in Retrophyllum piresii, but a few are alternate. Another specimen [Peru. Puno: Río Candamo, fila at mouth of Río Guacamayo, ridge top forest with cloud forest aspects, 13°30′S 69°50′W, 870 m, 28 v 1992, Gentry et al. 77310 (MO)] is also problematic. It is again from a rather low altitude for Retrophyllum rospigliosii, and the branches are opposite as in R. piresii. Unfortunately, it is sterile so it is not known whether it would have the distinctive long ellipsoid female cones that are so characteristic of Retrophyllum piresii. It was determined as the latter species [on the field label, as Nageia piresii (Silba) de Laub.] by de Laubenfels in 1994, which remains the current determination, but its true status remains uncertain.
Distribution
Northern and Western South America. W Venezuela (Táchira, Mérida, Trujillo), E Colombia (Antioquia, Cundinamarca, Magdalena, Norte de Santander, Santander del Sur), Ecuador (Napo, one collection; also see Jørgensen & Léon-Jánez, 1999), NW & C Peru (Gray & Buchholz, Reference Gray and Buchholz1948: 120; Torres-Romero, Reference Torres-Romero, Pinto and Lozano1988; Brako & Zarucchi, Reference Brako and Zarucchi1993: 2; Vicuña-Miñano, Reference Vicuña-Miñano2005: 286), NE Bolivia (Dpto. La Paz: Prov. Bautista Saavedra and Prov. Franz Tamayo, 3 collections: Zenteno-Ruiz, Reference Zenteno-Ruiz2007 and more recently collected specimens cited below). Andean Region (Northern Andean Province). TDWG: 82 VEN 83 BOL CLM ECU PER. Map: Fig. 8.

Fig. 8. Global distribution of Retrophyllum rospigliosii.
Specimens seen and other records. Venezuela. Mérida: otherwise unloc., 7000 ft [2135 m], 1846, Funck & Schlim 1208 (P–image); 1928, Pittier 12756 (S–database); [distributed] 1959, Bernardi s.n. (K). Campo Elias: Los Quebraditos, above Jají, 2590 m, 21 iv 1944, Steyermark 55999 (K, MO, NY, S–database). Bosque de San Eusebio, 2100–2400 m, 30 x 1962, Ruiz Terán 1148 (K). La Azulita: Environs de La Carbonera, 2000–2500 m, ix 1952, Humbert 25588 (NY–database, previously determined as Podocarpus harmsianus and Prumnopitys harmsiana). La Carbonera, 2250 m, 30 i 1953, Bernardi 335 (NY); La Cabonera [sic], 15 km NW of Ejido, 2200 m, 19 vi 1953, Little Jr. 15245 (NY–database). La Carbonera, ± 25 km W. of Mérida, 2400 m, 22 iv 1965, Breteler 4495 (COL–photo, MO, NY, RB–image, S–database). La Carbonera, 17 ii 1972, Dobson 1277 (CONN–database). La Carbonera, 2400 m, 6 i 1980, de Laubenfels 754 (A, male and bark; K–database, L–image, male and bark); ibid., 2400 m, 6 i 1980, de Laubenfels 755 (A, K–database, L–image); ibid., 2400 m, 6 i 1980, de Laubenfels 756 (A, L–image, female; K–database); La Azulita road, 50 km from Mérida, 1700 m, 11 iii 1980, Sobel & Strudwick 2130 (NY). Rio Capaz, Via Azulita, 20 vii 1951, Curran 2147 (NY). Libertador: Environs de Mérida, 1600–2200 m, 14 ix 1952, Humbert 26148 (P–image, seedlings). Santos Marquina: La Mucuy, 18 x 1955, García-Barriga 15490 (COL–image and photos). Táchira: Jáuregui: Savana Grande between La Grita and Yeguines, 1911, Jahn 99 (S–database). Junín: Municipia Delicias, Caserío Villa Páez y sus alrededores, 1900–2300 m, 6 iii 1962, Ruiz Terán 918 (K, MO). Entre Villa Páez y Betania, cerca de la frontera de Colombia, 2000–2400 m, 15 xi 1975, Bunting 4898 (NY); ibid., 2050–2350 m, 11 x 1976, Bunting 4939 (NY, K–database); faldas debajo del Páramo de Tamá, cerca de la frontera Colombo-Venezolana, arriba de Betania y Tamá, cerca de la Quebrada Buena Vista, 2300–2450 m, 22–24 v 1967, Steyermark et al. 98706 (S–database); Trujillo: Boconó: P. N. Guaramacal, alrededores de la Laguna de los Cedros, 1830 m, 9 i 2004, Stergios et al. 20755 (E, F–n.v., MER–n.v., MERF–n.v., MO–n.v., PORT–n.v., NY–n.v., US–database (barcode 00772960)–n.v., VEN–n.v.).
Colombia. Unloc., 1828–1890, Hb. Triana 665 (BM). Cundinamarca [Prov. Gualivá]: Albán: Albán, 1962, Goitia s.n. (UDBC 10797–image and photos). Vereda Santa Ana, finca San Pablo, 1800 m, 1 ix 1985, Schmidt-Mumm 324 (COL–photo). San Francisco: Cordillera Oriental, Finca « El Carmero » El Tablazo entre Subachoque y San Francisco, 1900–2100 m, 26 i 1944, García-Barriga 11038 (COL–photo, MA–n.v.); ibid., 26 i 1944, García-Barriga 11041 (COL–image). Vereda “San Miguel”, 2100 m, 18 iv 1983, Barrera 50 (COL–photo). Sasaima: Estación San Bernardo, entre Sasaima y Albán, 1700–1800 m, 2–5 viii 1945, Dugand & Jaramillo 3962 (COL–photo). Sasaima, vereda San Bernardo, carretera a Villeta, entre los kilometros 66–70, 1860 m, 30 vi 1945, Garcia-Barriga 11586 (COL–photo). Sasaima, vereda de Apocentos (antiqua hacienda de Apocentos), 10–12 vii 1960, García-Barriga 17259 (NY, COL–photo). Sasaima, San Bernardo, quebrada La María y Río Dulce, 1750–1950 m, 20–30 xi 1962, García-Barriga 17584 (NY). Sasaima, San Bernardo, quebrada La María y Río Dulce, 1750–1950 m, 20–30 xi 1962, García-Barriga 17585 (BM, COL–photo). Sasaima, Vereda San Bernardo, orillas del Río Dulce, 1780 m, 31 xii 1962, García-Barriga 18014 (COL–image and photos). Sasaima, Hacienda San Pablo, 1800–1900 m, 1949, Schneider 905 (S–database). Sasaima, Vereda las Mercedes, 1200 m, García-Barriga 20159 (COL–n.v.). Sasaima, Vereda de Guane, entre los ríos Gualibá y Guane, Finca La Concepción, 8–11 i 1974, García-Barriga 20468 (COL–image). Vergara: Mpio. de San Francisco [sic], Vergara, 2 ii 1981, Barrera 45 (COL–photo), 46 (COL–photo). [Prov. Guavio] Guasca: Guasca, i 1936, Pérez-Arbeláez 4798 (COL–photo). [Prov. Rionegro] Pacho: Mpio. Pacho, otherwise unloc., 2000 m, ix 1962, Goitia s.n. (UDBC 2060–images and photos); ibid., 1965, Mahecha s.n. (UDBC 5436–image and photos). Pacho, 18 x 1934, Pérez-Arbeláez 3150 (COL–2 sheets, photos). Pacho, Vereda Patasía, Hacienda Patasía, 1650–1820 m, 4 viii 1947, García-Barriga 12492 (COL–photo, K, NY). Pacho, Hacienda de Patasía, 1800 m, 21 vi 1948, Uribe Uribe 1734 (COL–photo). Pacho, 1859 m, 1 vi 1944, Bohorquez R. 475 (COL–photo). [Prov. Tequendama] Tena: Laguna de Pedro Palo, finca La Laguna, por carreteable desde Patio Bonito, 7 iii 1988, Franco et al. 2430 (COL–photo). [Prov. Ubaté] Ubaté: Mpio. Ubaté, without precise locality, Mahecha s.n. (UDBC 11190–image and photos). Antioquia: [Subreg. Norte] Angostura: Vereda El Guásimo, Finca El Guásimo, 2312 m, 10 viii 2007, A.M. Vásquez 3 (MEDEL–database). [Subreg. Oriente] Guatapé: Vereda Santa Rita, Finca Vertedero (Empresas Publicas de Medellín), 1967 m, 22 viii 2007, A.M. Vásquez 5 (MEDEL–database). [Subreg. Suroeste] Fredonia: Fredonia, 1850 m, 2 viii 1930, Archer 541 (US–n.v., photo NY; PH–database, S–database; seed separate, with Soukup 1801 from Peru, see below). Jardín: Vereda El Salado, Río Docató, Finca Las Manguitas, 5°33.1′N 75°53.0′W, 1500 m, 11 iv 1990, Betancur et al.1817 (COL–photo, MO, NY). [Subreg. Valle de Aburra] Envigado: Vereda Santa Catalina, 2050 m, 7 viii 2007, Vélez-Puerta 1999 (MEDEL–database). Medellin: El Poblado, Carrera 49, primera entrada a EAFIT, 1530 m, 14 xii 2005, Vélez 6946 (MEDEL–3 sheets, database). Huila: [Subreg. Subsur] Acevedo: Parque Nacional Natural Cueva de los Guácharos, centro de potrero de Leonidas, 2090 m, 11 ix 1979, Henao 237 (COL–photo). Magdalena: Santa Marta: Sierra Nevada de Santa Marta, Cerro San Lorenzo, 1850 m, 11 vii 1968, Villamizar & Guevara 63 (UDBC 2223–image and photos); ibid., 2100 m, 11 ix 1965, Espinal-T. & Delgado-F. 1790 (COL–photo). Quindio: Salento: Barsinal, 1970 m, i 1853, Triana 1800 (BM). Pereira: Acosta: Parque Regional Natural Ucumari, Sectores La Pastora y Los Chorros, 2200 m, 9 ii 2000, Roldán et al. 3168 (MO). Risaralda: Santa Rosa de Cabal: Vereda La Suiza, Sector Riobarbo, Finca Lisbrán, 1841 m, 10 xii 2007, Rodríguez-C. et al. 505 (COL–image). Norte de Santander: [Subreg. Occidente] Abrego: Linea divisoria entre los Deptos. Santander del Norte y Cesar, entre Abrego y Las Jurisdicciones (Cerro de Croque), 3440–3750 m, 22–23 v 1969, García-Barriga & Jaramillo-Mejía 19866 (MA–n.v., NY, COL–photo; assigned to Norte de Santander by Torres-Romero, Reference Torres-Romero, Pinto and Lozano1988, and Vargas Rincón, Reference Vargas Rincón2011). [Subreg. Sur-oriente] Toledo: Hoya de Samaria, 2000–2100 m, 29, 30 x 1941, Cuatrecasas et al. 12768 (COL–photo; cited by Vargas Rincón, Reference Vargas Rincón2011, as Decussocarpus rospigliosii). Santander del Sur: [Prov. Comunera] Gámbita: Vereda de Guausa, margen izquierdo carretera, 2070 m, 28 vi 1999, Cardenas & Oliveros 198 (COL–image). [Prov. Soto] Piedecuesta: Piedecuesta, Cristales en bosquete, 2100 m, 15 xi 1989, Roncancio 23 (UDBC 2915–image and photos); ibid., 12 xii 1989, Roncancio 024 (COL–photo). Tona: Tona, 2295 m, 20 xi 1989, Roncancio 055 (COL–photo); Tona, Vereda Guarumales, finca La Plazuela, 7°09′00′′N 72°59′29′′W, 2288 m, 13 xi 2003, Quirós Quirós 75 (COL–2 sheets, image and photos).
Ecuador. Napo: El Chaco: 4 km al E de Lumbaqui, 1 km al E del puente sobre Río Aguarico, arriba del Río Aguarico, 0°05′N 77°20′W, 600 m, 5 viii 1986, Neill & Salinas 7202 (NY, K–database, MO–database; atypical, see comments). Sucumbios: Gonzalo Pizarro: Lumbaqui, derecho de Vi OCP, 0°04′16′′N 77°18′00′′W, 675 m, 29 v 2002, Morales et al. 867 (MO, atypical, see above). Zamora-Chinchipe: Chinchipe: Parque Nacional Podocarpus, La Esmeralda (Coperativa San Francisco de Numbala Alto), 4°22′S 79°03′W, 2250 m, i 1995, Palacios & Tirado 13026 (MO). Región de la Cordillera del Cóndor, sector sur, Parroquia San Francisco de Vergel, Cuenca alta del Río Vergel, Pica Sangola, 4°44′09′′S 78°56′59′′W, 2200 m, 16 iii 2005, Quizhpe et al. 1092 (MO); ibid., cerca a La Canela, Sendero a Las Palmales, 4°35′44′′S 78°59′08′′W, 2190 m, 5 iii 2007, Quizhpe & Wisum 2461 (MO–database). Road south of Podocarpus National Park and Tapichalaca Reserve, c.4 km N of Valladolid, Río Valladolid, upper Río Chinchipe watershed, 4°30′43′′S 79°07′24′′W, 2150 m, 3 xii 2006, Neill & Jost 15337 (MO–database). Palanda: Camino paralelo a quebrada Flor Amarilla, cruce sobre la quebrada San Francisco, limite con el Parque Nacional Podocarpus, 4°00′00′′S 79°13′00′′W, 1900–2200 m, i 1995, Palacios & Tirado 13159 (MO–database).
Peru. Cajamarca: San Ignacio: Chirinos: La Palma, 10 km NW de Chirinos, 5°25′S 78°53′W, 1780 m, 5 ii 1988, Gentry et al. 61207 (MO). Huarango: El Triumfo-Convento, 5°13′S 78°40′W, 1000–1400 m, 30 vi 1996, Campos & Rodríguez 2825 (MO). San José de Lurdes: Buenos Aires, 4°55′S 78°51′W, 1870 m, 28 x 1995, R. Vásquez & Jaramillo 20459 (MO). Pasco: Oxapampa:Huancabamba: Parque Nacional Yanachaga-Chemillén, Parcela Permanente 1 ha Oso Playa, 10°17′58′′S 75°36′35′′W, 2200 m, 31 xi [sic] 2006, Monteagudo et al. 12942 (MO–image); ibid., 2200 m, same date [with same error!], Monteagudo et al. 12986 (MO–image); ibid., 3 xi 2006, Monteagudo et al. 13078 (MO–image); ibid., 6 xi 2006, Monteagudo et al. 13230 (MO–image); ibid., arbol no. 403, 11 xi 2006, Monteagudo et al. 13353 (MO–image, NY–database). Parque Nacional Yanachaga-Chemillén, remedición de parcela Oso-Playa 1.0 ha, 10°17′58′′S 75°36′56′′W, 2200 m, 20 vi 2008, Monteagudo et al. 16407 (MO–database). Oxapampa: Oxapampa, Esposto 556 (holo B–image, iso GH–image, NY–image, fragment of holo S). Oxapampa, otherwise unloc., viii 1942, Soukup 1801 (US–n.v.; image NY; sterile shoot; loose seed is annotated, “this seed belongs in packet with W.A. Archer 541, Fredonia, Colombia”; S–database). Oxapampa, R.T. Pennington 1422 (E); ibid., R.T. Pennington 1426 (E); ibid., R.T. Pennington 1427 (E); ibid., R.T. Pennington 1431 (E); ibid., R.T. Pennington 1433 (E). Chorobamba, 10°33′S 75°33′W, 1700 m, 5 vii 1985, D.N. Smith & Brack E. 7634 (NY, F–image, MO–n.v.; cultivated). Palcazú: Comunidad Nativa Santa Rosa de Palcazú, sector Santo Domingo, 10°26′50′′S 75°03′25′′W, 470 m, 13 x 2008, Huamán et al. 313 (MO–database, cultivated). Chontabamba: 2 de Mayo, Hacienda La Victoria, 10°35′S 75°28′W, 1890 m, 28 v 1982, D. Smith et al. 1752 (NY). Villa Rica, viii 1955, Soukup 4401 (GH). Villa Rica, 10°43′S 75°16′W, Chacras above town, 1500 m, 21 xi 1998, Daza & T.D. Pennington 16464 (E). Centro Bocaz, camino y trocha a Purus, 10°38′S 75°11′W, 1590 m, 19 ix 2003, Perea 381 et al. (NY–database). Base of road towards Pajonal/Villa Rica, near Oxapampa, 10°38′05′′S 75°20′16′′W, 2093 m, 17 ix 2001, Weigend 5795 et al. (NY–database). Junín: Junín:Junín: Caserio de Pampas Raimondi, 11°05′S 76°0′W, 2040 m, 23 xi 1973, Espejo 29ELE (K–database). Ucayali: Coronel Portillo:Iparia: falda dentro las cuencas del Rio Ariapo y Rio Iparia, afluentes del Rio Ucuyali, Reserva Comunal el Sira, 1550–1600 m, 9°27.85′S 74°33.95′W, 1 xi 2009, Graham 5179 (E, MOL–image).
Bolivia. La Paz: Franz Tamayo: Parque Nacional Madidi (ANMI), Sector Macheineo, Camino viejo de la carretera de Mohima a Pata, 14°39′18′′S 68°34′30′′W, 1873 m, 27 v 2011, Gardner et al. (2011): 30 (E). Parque Nacional Madidi (ANMI), Camino hacia el Rio Tuichi, 14°36′52′′S 68°33′53′′W, 1811 m, 29 v 2011, Gardner et al. (2011): 37 (E). Parque Nacional Madidi (ANMI), Santa Cruz de Valle Ameno, 14°38′23′′S 68°31′30′′W, 1605 m, cultivated, 29 v 2011, Gardner et al. (2001): 38 (E). Santa Cruz: Andrés Ibáñez: “Western slopes of the Andes”, in swampy places about Santa Cruz, 0°–40°S, vii 1860, Pearce s.n. (BM).
Colombia (cultivated). Cundinimarca: Bogotá: Bogotá, Parque de La Independencia; jardines y parques, 2620 m, 20 iii – 20 iv 1946, Duque-Jaramillo 2964 (COL–photo); ibid., Duque-Jaramillo 3108 (COL–photo, NY). Santafé de Bogotá, Parque Nacional, 2650 m, i 1963, Goitia UDBC 2129 (UDBC–image and photos). Avenida de los Cerros Orientales, 2700 m, 1 xi 1973, Mahecha 1391 (UDBC 5985) (UDBC–image and photos). Bogotá, D.E. Calle 74 con carrera 11, 8 vii 1979, E. Forero & J. Forero G. 6207 (NY, COL–photo). [Prov. Sabana Occidente] Subachoque: Vereda Casablanca, finca “California”, 2700 m, plantada junto a una cerca, como ornamental, 12 vi 2003, Hernández Schmidt 1190 (COL–photo). Vereda Tobal, 2800 m, cultivada en un jardín, 9 xi 2003, Hernández Schmidt 1384 (COL–photo). Quindio: Calarcá (introduced): Jardin Botánico del Quindio, Sede principal, Sector del Sendero de los Caobos, 1490 m, 1 iv 1999, Matallana Tobón 523 (COL–photo). Armenia (introduced): City of Armenia, Parque Aborigen, 1550 m, planted as an ornamental, 7 x 1993, Wright 047 (E–3 sheets).
Peru (cultivated).Lima: Lima, botanic garden, Asplund s.n. (S–database); ibid., 1940, Asplund 12027 (S–database).
Scotland.Midlothian: (Cultivated, origin Venezuela, cuttings from Arnold Arboretum via J. Silba). Royal Botanic Garden Edinburgh, accession 19951955*A, Frachon 1337 (E). (Cultivated, origin Colombia, R. Nicholson 348/92). Royal Botanic Garden Edinburgh, accession 20001721*A, Frachon 1340 (E).
Bioregions: Northern Andes, Central Andes
Ecoregions: NT0105 Bolivian Yungas, NT0109 Cauca Valley Montane Forests, NT0118 Cordillera Oriental Montane Forests, NT0121 Eastern Cordillera Real Montane Forests, NT0136 Magdalena Valley Montane Forests (in which it and Podocarpus oleifolius D.Don are two of the most dominant trees), NT0153 Peruvian Yungas, NT0159 Santa Marta Montane Forests, NT0175 Venezuelan Andes Montane Forests (where again it is one of the most dominant trees along with Prumnopitys montana and Podocarpus oleifolius together with assorted dicot trees).
Ecology
Montane forests, cloud forest; (600–)1500–3750 m (generally at higher altitudes than Retrophyllum piresii; the three records from below 1000 m relate to the three atypical specimens from Ecuador and Peru discussed above, all of which have some features of Retrophyllum piresii). Retrophyllum rospigliosii grows best on slightly sloping ground, fertile river beds and small depressions, on wet, deep, fertile acid clay or clay/sand soils with good to slow drainage; it is not tolerant of either swampy or drought conditions. It requires constant humidity and cloudy conditions with an annual rainfall of 1500–2500 mm and an annual average temperature of 10–18°C (Nieto & Rodriguez, Reference Nieto, Rodriguez and Vozzo2003). It plays an important role in intercepting rainfall: a Retrophyllum rospigliosii plantation at La Mucuy (Mérida, Venezuela) intercepted 42% of precipitation, almost the same percentage as the 45% intercepted by nearby montane cloud forest (Ataroff, Reference Ataroff2002). The seeds of Retrophyllum rospigliosii are recalcitrant, with a viability in storage at 12°C of 3–6 months but with a drastic drop in viability after 4 months (Ceballos-Freire & López-Ríos, Reference Ceballos-Freire and López-Ríos2007).
In Cajamarca, Peru, Retrophyllum rospigliosii occurs in undisturbed and fragmented primary forest together with Cecropia polystachya Trécul, Cinnamomum triplinerve (Ruiz & Pav.) Kosterm., Heliocarpus americanus L., Ladenbergia magnifolia (Ruiz & Pav.) Klotzsch, Otoba parvifolia (Markr.) A.H.Gentry, Piptocoma discolor (Kunth) Pruski, Ruagea insignis (C.DC.) T.D.Penn., Virola calophylla Warb., etc. (Vicuña-Miñano & León, Reference Vicuña-Miñano and León2003).
Phytochemistry
Ninety-one compounds have been isolated and identified from the essential oil of the ‘fruits’ of Retrophyllum rospigliosii (Quijano-Celis et al., Reference Quijano-Celis, Gaviria, Vanegas-López, Ontiveros, Echeverri, Morales and Pino2010). The essential oil from the leaves yielded 68 compounds (Quijano-Celis et al., Reference Quijano-Celis, Gaviria, Vanegas-López, Ontiveros, Echeverri, Pino and Morales2013). In both cases, limonene (38% in ‘fruits’, 43% in leaves) and α-pinene (16% in ‘fruits’, 18% in leaves) comprised the largest fractions. Retrophyllum rospigliosii contains several norditerpene dilactones that were previously known only in Podocarpus and/or Nageia, including nagilactones E, F and G, β-sitosterol and a derivative of the latter (Amaro-Luis & Carroz, Reference Amaro-Luis and Carroz1988), as well as a unique compound, rospiglioside (Amaro Luis et al., Reference Amaro Luis, Amesty, Montealegre and Bahsas2006). As well as rospiglioside, Amaro Luis et al. (Reference Amaro Luis, Amesty, Montealegre and Bahsas2006) isolated ferruginol, sugiol, sugiol acetate, totarol, totarol acetate, 4β-carboxy-19-nortotarol and 16-hydroxy-4β-carboxy-19-nortotarol from the leaves of Retrophyllum rospigliosii, but the phenolics of the wood do not appear to have been studied. Its biflavones are similar to those found elsewhere in Podocarpaceae and include sciadopitysin, podocarpusflavone A, podocarpusflavone B, amentoflavone, 7,4′,7′′,4′′′-tetra-O-methyl-amentoflavone, 7,4′,7′′-tri-O-methyl-amentoflavone, 7,7′′-di-O-methyl-amentoflavone, sequoiaflavone and heveaflavone (Chaabi et al., Reference Chaabi, Antheaume, Weniger, Justiniano, Lugnier and Lobstein2007; Amaro-Luis et al., Reference Amaro-Luis, Amesty, Bahsas and Montealegre2008). The biflavones act as phosphodiesterase inhibitors and may therefore have potential as therapeutic drugs for a wide variety of medical conditions (Rahimi et al., Reference Rahimi, Ghiasi, Azimi, Fakhari and Abdollahi2010 and work cited therein). However, the biflavones and other compounds of Retrophyllum rospigliosii assayed were found to lack any antimycotic activity against three pathogenic fungi (Niño et al., Reference Niño, Espinal, Mosquera and Correa2007).
Plant–animal interactions
In Colombia, the seeds of Retrophyllum rospigliosii are eaten by the endangered yellow-eared parrot or loro orejiamarillo, Ognorhynchus icterotis (Massena & Souanc) (Botero-Delgadillo & Páez, Reference Botero-Delgadillo and Páez2011). Whether this activity effects dispersal is not known.
Retrophyllum rospigliosii is the one of the hosts of at least three members of the wood-boring beetle family Scolytidae, namely Araptus impensus (Wood), Platypus secus Wood (Wood & Bright, Reference Wood and Bright1992, as Podocarpus ‘raspigliosii’) and Thamnophthorus impensus Wood (Schedl, Reference Schedl1965). Some of the paratypes of Platypus secus were collected from Retrophyllum rospigliosii logs at the beetle's type locality in La Carbonera Experimental Forest, Mérida, Venezuela (Wood, Reference Wood1971, as Podocarpus ‘raspigliosii’).
Mycological associations
Retrophyllum rospigliosii is the only known host of Tripospora venezuelensis E.Müll. (Coryneliales), which is known only from the type collection from Mérida, Venezuela (Müller & Dennis, Reference Müller and Dennis1965; Benny et al., Reference Benny, Samuelson and Kimbrough1985). The mycorrhiza of Retrophyllum rospigliosii have been investigated by Furman (Reference Furman1970) and Guerrero Forero & Hodson de Jaramillo (Reference Guerrero Forero and Hodson de Jaramillo1988). The latter team found that Retrophyllum rospigliosii had a mixture of vesicular arbuscular mycorrhiza that included several species of Acaulospora Gerdemann & Trappe (Acaulosporaceae) and Glomus Tul. & C.Tul. (Glomeraceae).
Conservation status (global): VU (A2acd) (Gardner & Thomas, Reference Gardner and Thomas2013)
Logging of this valuable timber tree has reduced or fragmented most of the formerly rather extensive stands of this species. Whole mountainsides of the species in Peru were clear-felled in the 1980s for timber, and the species is there now reduced to scattered individuals (M. Gardner, Royal Botanic Garden Edinburgh, pers. comm., 7 Feb. 2012). Yaguana et al. (Reference Yaguana, Lozano, Neill and Asanza2012) have commented that the forest at Numbala (Ecuador), at the edge of Podocarpus National Park, is one of the last remnants of its type. Retrophyllum rospigliosii is regarded as NT in Colombia (Barragan Bedoya & Valdés Torres, Reference Barragan Bedoya and Valdés Torres2011).
Uses
The fruits are reported as edible (Gray & Buchholz, Reference Gray and Buchholz1948; Buchholz & Gray, Reference Buchholz, Gray and Steyermark1957; and several specimen labels). The tree is often cultivated in gardens and parks in Colombia (Nieto & Rodriguez, Reference Nieto, Rodriguez and Vozzo2003) and Peru (Brako & Zarucchi, Reference Brako and Zarucchi1993), and is used in forestry in Colombia (Nieto & Rodriguez, Reference Nieto, Rodriguez and Vozzo2003, who also give details of recommended practices for seed harvesting and silviculture) as well as in Venezuela (Ruiz Terán 918 in sched., K, MO). The wood is of very good quality and is widely used in construction in Venezuela and Colombia. It is easily worked and is used to make furniture, boxes, cabinets, veneer, pencils, paper pulp and other items (Nieto & Rodriguez, Reference Nieto, Rodriguez and Vozzo2003). It is also used to make handcrafted decorative objects (Feuillet Hurtado et al., Reference Feuillet Hurtado, Macias Pinto and Chito Cerón2011). Its characteristics have been described recently by Vásquez Correa & Alcántara Vara (2009) and Vásquez Correa et al. (Reference Vásquez Correa, Alcántara Vara and Herrera Machuca2010). The logging cycle for the species was estimated at 109 years by Becerra-Montalvo & Zevallos-Pollito (Reference Becerra-Montalvo and Zevallos-Pollito2014).
6. Retrophyllum piresii (Silba) C.N. Page, Notes Roy. Bot. Gard. Edinburgh 45: 380 (22 Feb. 1989 [‘1988’]). – Decussocarpus piresii Silba, Phytologia 54: 461, t. 1 (10 Dec. 1983). – Nageia piresii (Silba) de Laub., Blumea 32(1): 211 (3 Feb. 1987). – Type: Brazil: Território de Rondônia, Serra Pacas Novos, Seringal S. Luiz, 50 km east of Rio Pacas Novos, 14 viii 1976, N.A. Rosa, J.M. Pires, W. Rodriques, S. Barros 856 [misprinted in protologue as ‘Rosa & Pires 586’] (holo US, iso K–database, L–image, MG–image, MO, NY, R–2 sheets, images, RB–image). Fig. 9A–C.

Fig. 9. Retrophyllum piresii. A, Branching arrangement (Rosa et al. 856). B, Adult female branch (Rosa et al. 856). C, Mature female cone (Rosa et al. 856). Retrophyllum rospigliosii. D, Young female cone (Vasquez et al. 20459). E, Mature female cone (Bunting 4939). Magnifications: A, B, × 0.67; C, × 1.5; D, E, × 3. Scale bars: A, 6 cm; B, 2 cm; C, D, E, 2 cm. Drawn by Claire Banks.
Etymology
Retrophyllum piresii is named after João Murça Pires (1917–1994), Brazilian botanist, who published (with C. Mainieri) a revision of Podocarpus in Brazil (Silvic. Sao Paulo 8: 1–24, 1973) and who was one of those who collected both the type and the only two other currently known specimens of wild origin, made a few days earlier and on the same day.
Vernacular name: pinheiro-da-amazónia (Secco et al., Reference Secco, Silva do Rosário, Rodrigues, Giulietti, Rapini, Gomes de Andrade, de Queiroz and Cardano da Silva2009)
Distinguishing features
This species shares many characters with its South American congener Retrophyllum rospigliosii but, as was noted in the protologue, it can be distinguished by its elliptic (not lanceolate) leaves with ± parallel sides, and the more ellipsoid female cones that are not narrowed at the base. Additional characters not described by Silba that distinguish it from Retrophyllum rospigliosii are the bracts on the female peduncle all very reduced and scale-like (not foliar), the obtuse leaf apices (misleadingly described in the protologue as ‘bluntly acute’) and the leaves never abruptly narrowed just below the apex (normally so in Retrophyllum rospigliosii). The opposite branching with leaves on penultimate branches opposite and not forming stepped ranks also separates Retrophyllum piresii from the vast majority of specimens of R. rospigliosii, although a few specimens of the latter approach R. piresii in these features, as noted above. Further differences may become apparent when more material is collected (male and juvenile collections of Retrophyllum piresii are particularly desirable to complete the description). The two species also appear to be ecologically separated, with Retrophyllum piresii inhabiting lowland swamp forest at altitudes of less than 1000 m and R. rospigliosii normally occurring in montane and cloud forests, nearly always above 1500 m, although there are one or two low-altitude records as discussed under that species.
Evergreen tree c.30 m tall and up to 2.5 m dbh, adult tree branching at c.15 m above base. Trunk (fide Farjon, Reference Farjon2010) straight and erect, in large trees lacking branches for 15 m or more. Bark (imperfectly known) medium to dark brown outside, purplish to dark brown inside, smooth on branchlets; wood reddish brown. Crown rounded (fide Farjon, Reference Farjon2010: 940). Branchlets of last two orders erecto-patent, slightly curved; ultimate branchlets usually opposite, 50–130 mm with 24–38 pairs of leaves, penultimate ones 180–210 mm. Leaf scars absent. Buds obconical, naked. Leaves on last two orders of branching all of one type (scale-leaves absent). Leaves on penultimate axes normally soon caducous although persistent when subtending branch bases and when axis has not developed further branching, opposite and spreading or orientated so that both leaves of each pair are suberect, not forming stepped ranks. Juvenile foliage not known. Adult foliage leaves unequally amphistomatic, 4–5 mm apart, opposite-distichous, pectinate, not imbricate, subsessile (petiole 0.2–0.5 mm); lamina spreading and forming a shallow ‘V’ along branch, erecto-patent, diverging at 65–80°, mid to deep green, elliptic, oblong-elliptic or obovate-elliptic (never lanceolate), 9.5–11 × 2–2.5 mm in middle of shoot and 5–6 × 1.7–2.5 mm near base, straight, thinly coriaceous, the margin only slightly thickened, both surfaces flat with vein-like striae alternating with the stomatal rows, midrib only slightly raised on both surfaces or not at all, sometimes obscure distally on abaxial surface, the apex obtuse and the blade never abruptly narrowed below it, usually with an indistinct mucro, the base obtuse, the petiole decurrent along the whole length of the internode. Pollen cones unknown. Female cones borne on previous year's growth, terminal on specialised lateral shoots bearing very reduced scale-leaves or bracts. Peduncle c.15–16 mm, about 2/3 length of cone, deflexed-patent to pendulous, its lower portion bearing at least 3 pairs of decussate scale-like, shortly ovate or elliptic bracts, its apical part together with the fertile bract forming an indistinct, cylindrical receptacle that is shed with the cone. Fertile bract with basal part adnate to epimatium, distal part free and navicular. Female cone with epimatium pecan-brown turning reddish when mature, 19–22 × 11–14 mm, distinctly ellipsoid with a shortly conical distal crest, not narrowed at the base, the seed proper slightly smaller.
Taxonomic notes
Silba's protologue, and later description (Silba, Reference Silba1986: 78), are both very brief and give little detail concerning many aspects of its morphology, but no other taxonomic account of this species appears to have been published until now except that of Farjon (Reference Farjon2010), which added some details of trunk and habit. Further collections of the species from the type locality are badly needed, particularly of the unknown juvenile stages and male cones.
Distribution
W Brazil (SW Rondônia: Serra dos Pacaás Novos), near the border with lowland NE Bolivia. More recently recorded from Bolivia and Peru in similar habitats (D.J. de Laubenfels, pers. comm., 24 October 2005). These records require confirmation; one problematic sterile specimen, determined as Retrophyllum piresii by de Laubenfels, is discussed above under R. rospigliosii. For the time being, the species is regarded as endemic to Brazil and known from the two wild gatherings (both female) and one sterile cultivated collection cited here. Map: Fig. 10.

Fig. 10. Global distribution of Retrophyllum piresii.
Specimens seen. Brazil. Rondônia: Guajará-Mirim: Serra Pacaás Novos, Seringal S. Luiz, 50 km E of Rio Pacas Novos, 14 viii 1976, Rosa et al. 856 (holo US, iso K, L–image, MG–image, MO, NY, R–image, RB–image); Serra Pacas Novos, 11°13′S 63°35′W, 250 m, 14 viii 1976, Rosa et al. 856 (iso INPA–image with different label information as indicated). Serra dos Pacos [sic] Novos, 30 min. de vôo de helicopter de S. Luz–Rio Pacos Novos, 11°15′S 23°50′W [sic: presumably an error for 63°50′W], 7 viii 1976, Pires 850 & Rosa (RB–2 sheets both numbered RB253293, images). Região do rio Pacaas Novos, Projeto RADAM/BRASIL, ponto 15 à 250–300 m de altitude, 11°13′S e 63°35′W, 14 viii 1976, Rodrigues et al. 9646 (INPA–2 sheets, images; female; third collector listed as ‘S.N. Rosas’ but probably a misprint for N. Rosa). Brazil (cultivated). Pará: Belém: Belém, Museu Emilio Goeldi, Parque Zoobotânico Emílio Goeldi, procedente de Rondônia, 30 x 2008, Kinupp 3426 (RB–image; also photos of living plant).
Bioregion
Amazonia. Ecoregion: NT0135 Madeira-Tapajós moist forests; perhaps also in NT0166 Southwest Amazon moist forests.
Ecology
Lowland tropical swamp forest; 250–300 m.
Phenology
Reputedly fertile in January (de Oliveira, Reference de Oliveira2012).
Conservation status (global, IUCN 3.1): DD (two independent assessments: de Oliveira, Reference de Oliveira2012; Gardner, Reference Gardner2013)
The only confirmed locality from which wild material has been gathered is within a National Park. Insufficient is known about the extent and area of occurrence, or of potential threats, to award an assessment other than DD and this will not change until further research can be done on the species in the field. The species is in cultivation (see cited specimens).
Uses
None have been reported although it is likely to have wood of high quality similar to that of R. rospigliosii.
Acknowledgements
Luis Carlos Jiménez (Director of the Herbarium, Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá, Colombia) and Rocio Cortés (Director, Herbario Forestal, Universidad Distrital Francisco José de Caldas, Bogotá, Colombia) are thanked for providing access to herbarium material in order that specimens may be photographed. James Richardson is thanked for taking a large number of digital images of herbarium specimens at COL and UDBC. Chad Husby kindly sent photographs of cultivated material of Retrophyllum piresii. Acknowledgement is made of the use of online images of specimens made publicly available by the herbaria of MG, MOL (via the Botanical Research Institute of Texas's Atrium Biodiversity Information System: atrium.andesamazon.org), P and RB, as well as the Plants of Papua New Guinea web site hosted by the Royal Botanic Gardens and Domain Trust (Sydney) on behalf of these institutions: National and regional Governments of Papua New Guinea; and Federal, State and Territory Governments of Queensland, New South Wales, Victoria, Australian Capital Territory, Commonwealth of Australia, Australian National Botanic Gardens, Commonwealth Scientific and Industrial Research Organisation. Claire Banks drew the excellent illustrations and Vanezza Morales prepared the distribution maps. The Royal Botanic Garden Edinburgh is supported by the Scottish Government's Rural and Environment Science and Analytical Services Division.
Appendix I
Typification of Nageia minor Carrière (basionym of Retrophyllum minus)
As noted by Farjon (Reference Farjon2010: 939), typification of Retrophyllum minus is difficult. First of all, the protologue (Carrière, Reference Carrière1867: 641) clearly implies that the basionym, Nageia minor, as conceived by Carrière, was intended to be based primarily on material from the summits of high mountains, but that “according to Vieillard . . . it is also found on the banks of lac Arnaud”. Clearly, Carrière (Reference Carrière1867), Parlatore (Reference Parlatore and De Candolle1868) and other contemporary workers believed that there was only one Retrophyllum species on New Caledonia, the one based on Nageia minor that we now know as R. minus. As late as the 1920s, Compton (Reference Compton1922) referred to material from Mt. Mou (Compton 607) as Nageia minor, saying that his specimens “match the type perfectly” but were collected from a tree 40–50 ft in height at an altitude of 3500 ft. No specimens from high altitude were, however, cited by Carrière (Reference Carrière1867) in support of his statement that it had been collected at altitude by an ‘English gardener called Richard’ [= N. Richards, collector for F. von Mueller; see main paper]. We have to accept, as Compton (Reference Compton1922) did, that the type of Nageia minor is to be found among the material numbered Vieillard 1275. High-altitude material of New Caledonian Retrophyllum was finally separated from the low-altitude Vieillard collection as Podocarpus comptonii by Buchholz (Reference Buchholz1949) and is now known as Retrophyllum comptonii. The latter, as mentioned in the introduction and relevant species account in the present paper, also descends to low altitudes in the south of the island and sometimes occurs sympatrically with R. minus.
Typification of Nageia minor, the basionym of Retrophyllum minus, is complicated by the existence of several different collections, made from two different localities (‘lac Arnaud’ and ‘Baie du Sud’) and in two time periods (1855–1860 and 1861–1867), all numbered Vieillard 1275. Buchholz (in sched., P00188111), indicates a selection of fragments labelled ‘bord du lac Arnaud’ as representing the ‘type specimen’, and sheet P00118111 at Paris is designated ‘holotype’ of Nageia minor in the Paris Herbarium database. Subsequently, Gray (Reference Gray1962: 77) stated that Vieillard 1275 from “borders of Lac Arnaud” was the ‘holotype’ of Nageia minor, so agreeing with her late coworker's annotation, On the other hand, Farjon (Reference Farjon2010: 938) stated that the ‘holotype’ of the name was a collection of Vieillard 1275 at P from Baie du Sud.
Of the seven sheets at P listed below that bear specimens numbered Vieillard 1275, two sheets (with barcodes P00188106 and P00188107) give the locality only as Baie du Sud and three (barcoded P00118108–110) are labelled only ‘lac Arnaud’, while the remaining two (barcoded P00118111 and P00197538) bear labels indicating both localities. Because there are multiple collections and for other reasons, there cannot be a ‘holotype’ but rather a number of syntypes. Therefore, both indications by Gray (Reference Gray1962) and Farjon (Reference Farjon2010) are incorrect. Because at least six different gatherings (probably more), belonging to two different genera, have been numbered Vieillard 1275 (see below), lectotypification is necessary to fix the application of the names Nageia minor and Retrophyllum minus. Because several of the sheets comprise mixed gatherings, lectotypification has by necessity been a two-stage process. First of all, conventional taxonomic and nomenclatural deductive methods were used to eliminate entire sheets that for a variety of reasons are unsuitable for selecting the actual lectotype (although some bear isolectotypes). These enabled the choice of lectotype to be narrowed down to one particular sheet out of the seven. Then, similar methodology in combination with colour profiling using ImageJ (Rasband, Reference Rasband2014) was used to select the actual lectotype specimen on that sheet and its isolectotypes on others. Finally, because the resulting lectotype and isolectotypes are all sterile, an epitype is designated to further fix the application of the name.
Selection of the lectotype sheet
Élie-Abel Carrière (1818–1896) was a horticulturist at Paris, and there is no trace of a personal herbarium. Williams (Reference Williams2004) believed that he relied on the collections in the Muséum d'Histoire Naturelle, i.e. the herbarium P. There is no evidence that he consulted herbaria elsewhere in connection with his research on gymnosperms. The protologue in fact gives two clues that Paris material formed the basis of his new species Nageia minor: the synonym “PodocarpusSpec. in herb. Mus. Paris.” and the indication of the specimen, “D'apres M. Vieillard (herbier de la Nouvelle-Calédonie no 1275–in Herb. Mus. Par.). . .” Therefore the lectotype must be selected only from among the Paris examples of Vieillard 1275. Seven exist, now barcoded P00188106, P00188107, P00188108, P00188109, P00188110, P00188111 and P00197538. Details of these follow, after a discussion of the elements in Carrière's protologue that are helpful to the task of selecting the choice of lectotype. Individual elements on sheets are designated by adding a suffix letter to the last three digits of the Paris herbarium barcode, e.g. 106-A (also see Appendix table 1, which gives the position of each element on each sheet). It is also important to know some facts about Vieillard's collections and how they were numbered, although it is equally important to remember that Carrière was most probably unaware of these.
Appendix table 1. New Caledonian collections of Retrophyllum minus made by Vieillard, Deplanche and Pancher in the Paris Herbarium, with details of leaf dimensions, reproductive state and colour profiling values obtained using ImageJ. Specimen codes are formed by adding a suffix letter to the last three digits of the P barcode. Colour profile values: mean % red (R), green (G) and blue (B) of 20 × 20 px (c.1.7 × 1.7 mm) squares: for each set of analyses the highest and lowest mean values are shown as a range. The material can be inferred as belonging to nine different collections, six (1–6) by Vieillard and three (I–III) by Deplanche or Pancher, as detailed in the last column

Eugène Vieillard (1819–1896) was a French doctor, naturalist and explorer who was a contemporary of both Émile Deplanche (1824–1874) and Jean Armand Isidore Pancher (1814–1877), two other French doctor–naturalists. All three collected on New Caledonia. Morat (Reference Morat2010) gives biographies and details of their collections. The relationship between Vieillard and Deplanche seems to have been particularly close; they published together concerning New Caledonia (Vieillard & Deplanche, Reference Vieillard and Deplanche1863). Vieillard's numbers refer to taxa, not specimens, so the same number was used for different collections made at different times. The two time periods that appear on Vieillard's printed labels, 1855–1860 and 1861–1867, correspond exactly with Deplanche's two sojourns on the island as given in Morat (Reference Morat2010); indeed, Guillaumin (Reference Guillaumin1911) seems to imply that both collected together during both those periods on the island. Deplanche sometimes used his own number but sometimes a Vieillard number. Deplanche also at times seems to have given material to Vieillard, who would then give this a different number in his own system (de Kok & Mabberley, Reference de Kok and Mabberley1999). These and many other aspects of the hazards associated with specimens collected by Vieillard and/or Deplanche, and their typification, have been carefully set out by Hopkins & Bradford (Reference Hopkins and Bradford2009).
Possibly the most important pieces of information in Carrière's protologue of Nageia minor are:
• the fact that he cited only material from lac Arnaud, which would seem to rule out sheets P00188106 and P00188107, both of which bear labels giving the locality as Baie du Sud, as well as sheets such as P001880111 that bear more than one collection from different localities (sheet P00188011 not only has Vieillard's collections numbered 1275 from both lac Arnaud and Baie du Sud, but also another gathering numbered Deplanche 170, discussed later). However, Farjon (Reference Farjon2010) placed less importance on the locality information than on the appearance and reproductive state of the specimens, restricting candidates for the type to specimens that were either male or sterile. Vieillard's locality information on the labels is known to be unreliable (de Kok & Mabberley, Reference de Kok and Mabberley1999), so Farjon was perhaps acting wisely in putting more importance on morphology. However, it is important to recognise the fact that Vieillard did not describe Nageia minor – Carrière did. Carrière may not have known about the unreliability of Vieillard's locality information, so it seems better to emphasise the importance of the fact that he gave the locality as lac Arnaud and no other.
• the indication of the previous determination of the material he saw: Podocarpus Spec. in herb. Mus. Paris. This rules out sheet P00188109, on which the only determination in Carrière's time was Dacrydium, a completely different genus of Podocarpaceae. It was first realised not to be that genus by Guillaumin, who in 1943 determined that particular sheet as Podocarpus minor. It also rules out all sheets that were not in the P herbarium in Carrière's time. Many of Vieillard's collections now in P were formerly housed at CN (the herbarium of the Université de Caen; Vieillard was director of the Jardin botanique du Caen, 1871–1895), and were transferred to P only in the 1970s. Consultation of the P herbarium database, which gives the origins of all sheets transferred from other sources, shows that none of the sheets discussed here originated from CN but the database and/or labels on the sheets themselves do indicate that some were indeed incorporated into the P herbarium from other sources after 1867 (the date of publication of Carrière's Traité des Conifères) and so have to be rejected as lectotype choices.
• the locality being given as in the protologue “au bord du lac Arnaud” and the word “Arbuste” at the beginning of the description: both these texts are found on the labels of specimens now mounted on sheets P00188108, P00188110 and P00188111, but not on P00188109, whose label simply reads “lac Arnaud”. This provides a second reason for excluding sheet P00188109. ‘Arbuste’ is also absent from the labels of specimens collected from Baie du Sud, viz. sheets P00188106 and P00188107.
• the fact that Carrière described only vegetative characters, implying that he did not see either male or female material, or at least that if sexual material was present on whatever sheet(s) (or specimens) he saw, it must have been easily overlooked because of scarcity, small size or both: sheets P00188109 and P00188110 in their present states both include, among several specimens, at least one collection that bears abundant male cones. However, it is possible that at least some of the specimens on both these sheets might not have been present when Carrière examined them. Indeed, the present constitution of at least some of the sheets definitely post-dates his time: the fragments on the sheet now barcoded P00188111 were removed for photography when sent on loan to Buchholz. There is evidence that some damage occurred during or subsequent to this procedure: at the time of photography, the left-hand side branch of Deplanche 170 (here designated 111-J for reference purposes) was longer and possessed a secondary side branch arising from its left side just above the one coming off from the right, and the side branch coming off just below the apex of the specimen here designated 111-F (top right, Vieillard 1275 from lac Arnaud, second from right) was longer; the missing portion is most probably the shoot tip now mounted immediately to the right (here designated 111-G). The two twigs now mounted above the Vieillard 1275 ‘Baie du Sud 1861–67’ label (111-A, 111-B) have both suffered considerable damage, and it is possible that the packet contains some of the detached portions.
• the leaf measurements, which Carrière gave as 12–17 × 4–5 mm.
• the leaf features: very regularly oval-elliptic, flat.
• the branching habit: Carrière's description allows for some variation in branching density (“Rameaux et ramules opposés, plus rarement épars” and in leaf density (“plus rarement épars”), suggesting that the material he had to hand comprised more than one shoot. Plants were described as having erect or suberect branches, the secondary branches and branchlets opposite, more rarely scattered, short. This would seem to rule out sheet P00188108, which consists of a single piece comprising just two side branches, although from the point of view of him overlooking the male cones, which are very few on this specimen, this would perhaps actually be the best choice for lectotype. He described the branches as “dressées ou subdressées” (erect or suberect), a character that is most applicable to the shoots mounted on sheets P118109 and P118110.
The seven sheets are as follows.
1. Barcode P00188106. Left-hand label: VIEILLARD, HERB. DE LA NOUVELLE CALÉDONIE. / No. 1275. / Podocarpus nana, Parl. / Baie du Sud. / 1861–67. Right-hand label: HERB. MUS. PARIS. / Podocarpus minor Parlat. / NOUVELLE-CALÉDONIE. / M. Vieillard, 1861–1867. – Two specimens, the upper one (106-A) male, the lower one (106-B) apparently sterile, with small leaves, many of which, on both specimens, have conspicuous paler margins. Because Carrière gave the locality as ‘lac Arnaud’, the indication ‘Baie du Sud’ would seem to exclude this sheet automatically, but there are also other reasons for doing so, as explained later.
2. Barcode P00188107. Left-hand label: VIEILLARD, HERB. de la Nouvelle Calédonie. / No. 1275. / Podocarpus [nana stroked out] minor Parl. / Baie du Sud. / 1861–67. Right-hand label: HERBIER E. DRAKE. [Otherwise blank]. – Two specimens, the left-hand one (107-A) sterile, the right-hand one (107-B) virtually so but with a single, easily overlooked male cone at the tip of one branch. Leaves of the male specimen larger than in P00118106. – The Drake Herbarium was not gifted to the Paris Herbarium until 1913, 9 years after Drake's death (Stafleu & Cowan, Reference Stafleu and Cowan1976). Therefore, this sheet would not have been available to Carrière before the publication of his 1867 work. For this reason, as well as the facts that Vieillard's label gives the collection period as 1861–1867 and locality as Baie du Sud rather than lac Arnaud, this sheet must be ruled out for lectotypification purposes.
3. Barcode P00188108. Left-hand label: VIEILLARD, HERB. de la Nouvelle Calédonie. / No. 1275. / Arbuste; bord du lac Arnaud. / 1855–1860. Right-hand: HERBIER de l'EXPOSITION COLONIALE / Ministère de la Marine. / Podocarpus minor Parl. / Nlle CALÉDONIE. / M. Vieillard 1862. / Catal. No. 1275. – A single specimen (108-A) that bears a couple of male cones at the tip of one its branches, which could easily have been overlooked by Carrière. Leaves very similar in shape and size to the male specimen of P00118107 and giving the impression that they were once part of the same gathering. Clearly the right-hand label on this sheet was added later, because Parlatore did not describe Podocarpus minor until 1868. Above the right-hand ‘original’ label is a third one that reads HERB. MUS. PARIS. / Herbiers des Colonies françaises donnés par le Ministère des Colonies. / 29 septembre 1894. This would suggest that this sheet did not become part of the P herbarium until 1894, long after Carrière had published Nageia minor, and therefore, like P00188107, it must be excluded for lectotypification purposes even though the specimen is clearly a duplicate of the one Carrière mentioned from lac Arnaud and is designated ‘isotype’ in the Paris Herbarium database. The sheet bears later annotations by Buchholz & Gray in 1950 [Podocarpus minor (Carr.) Parl. type collection] and de Laubenfels in 1972.
4. Barcode P00188109. Label: handwritten, “1275 / Dacrydium / lac Arnaud” (the word ‘Arnaud’ appears more like ‘Afnaud’ or ‘Abnaud’. with what is presumed to be the ‘r’ quite unlike the ‘r’ of ‘Dacrydium’). Seven small pieces of shoot (‘109-A’ to ‘109-G’), the top left (‘109-A’) and bottom left (‘109-E’) ones obviously male (cones particularly numerous on 109-E), the other five shoots all apparently sterile and some fragmentary. There is a later determination slip by Guillaumin in 1943 (Podocarpus minor) and a typewritten one attributed to de Laubenfels in 1972 (Decussocarpus minor, with a second identically typed ‘fide slip’ above it labelled Nageia minor, and with a reference to de Laubenfels in Blumea 32: 211, 1987) but there is no evidence that Buchholz saw the sheet. It is possible that Carrière could have seen this sheet, or at least the sheet lacks evidence that he could not have done. However, the handwritten label lacks the word ‘Arbuste’, found on the labels of some specimens on P00188108, P00188110, P00188111 and P00197538, and the genus assignation ‘Dacrydium’ was not mentioned by Carrière in the protologue, which assigned the specimen he saw to Podocarpus. Therefore the sheet must be excluded for lectotypification purposes even though it is currently labelled ‘isotype’ in the P Herbarium database.
5. Barcode P00188110. Label: design similar to P00188108 but the original text reads “VIEILLARD, Herb. de la Nouvelle Calédonie. / arbuste – bord du Lac Arnaud”. / 1855–1860”. There is a determination in different handwriting: “Podocarpus minor Parl. (nommé par M. Parlatore.” This determination obviously dates from after Parlatore's 1868 publication as well as that of Carrière's of the previous year, but the original label does not preclude it from being considered for typification purposes. – Five shoots (110-A to 110-E) of various sizes, the bottom-centre one (110-E) bearing numerous male cones, the top centre one (110-B) also male but with very few cones, the others either sterile (110-A, 110-C) or possibly bearing very young female cones (110-D). Also a packet containing two small shoot fragments, one bearing c.14 small leaves (110-F) and the other c.9 larger ones (110-G) as well as a detached pollen cone fragment (110-H) and a single leaf (110-J). Again there are later determination labels by Guillaumin in 1943 and de Laubenfels in 1972, but not one by Buchholz. The sheet is currently designated ‘isotype’ in the Paris Herbarium database.
6. Barcode P00188111. This sheet bears three different collections as follows: (Top left) Label: reads VIEILLARD, Herb. de la Nouvelle Calédonie. / Podocarpus nana [sic] Parl. / Baie du Sud. / 1861–1867. – Two sterile shoot fragments here designated 111-A and 111-B. (Top right) Label: very similar to that described in paragraph 1 above, but with minor differences: VIEILLARD, HERB. de la Nouvelle Calédonie. / No. 1275. / arbuste – bord du lac Arnaud. / 1855–1860. Someone else has added a later determination, Nageia minor Carr. – Four shoot fragments (one male: 111-D; others sterile: 111-E, 111-F, 111-G) and a few more or less detached leaves (111-C, 111-H). (Bottom left: 111-J). Label: Dammara [stroked out] / 170 (in pencil) / Podocarpus § Nageia. Also label at bottom right of whole sheet, which clearly pertains to this gathering: HERB. MUS. PARIS. / Nageia minor, Carr. / Podocarpus minor Parlat. / Prodr. t. XVI. p. 509 / No. 170 / Nouvelle Calédonie – M. Deplanche. 1861. – This is male material of a different collection, apparently made by Deplanche. (Packet, top left of sheet) – this contains mixed contents of by now probably in part unidentifiable origin; for example, the detached male cone could have come from either the top right example of Vieillard 1275, 111-D or from Deplanche 170, 111-J. From the prominence of the larger specimen at bottom centre and the two labels at bottom left and bottom right, this sheet appears to have originally comprised Deplanche 170 as either the only or the principal specimen. This example of Deplanche 170 was collected in 1861, the two Vieillard collections in 1855–60 and 1861–67. Because of this, it is likely that both the Vieillard collections, occupying much smaller areas in the upper part of the sheet, could have been added to the sheet later. Buchholz in October 1948 added a long annotation label below the top right specimen, saying, “This is the type specimen of Nageia minor Carr., now known as Podocarpus minor (Carr.) Parl. It was the only specimen of those of Vieillard bearing No. 1275 specifically listed accompanied by the note ‘bord du lac Arnaud’. Also the dates given 1855–1860 indicate that this may be his earliest collection of this entity.” Perhaps on account of this, this sheet is the one currently designated ‘holotype’ of Nageia minor in the Paris Herbarium database. However, sheet P00188111 comprises material collected at three different localities by two different collectors, and the earliest-mounted appears to have been the Deplanche specimen, whereas Carrière only specifically cited Vieillard as collector and lac Arnaud as locality. If Carrière had seen this sheet, in its present composition, he would surely have listed Baie du Sud as another locality in the protologue, but he did not. Furthermore, one of the Vieillard collections (that of 1861–67) would not have been available to him. All this indicates, therefore, that, contrary to Buchholz's annotation, the whole sheet should be excluded for lectotypification purposes. The specimen collected by Deplanche is discussed later.
7. Barcode P00197538. Two labels at bottom of sheet. Left hand label: VIEILLARD, HERB. de la Nouvelle Calédonie. / No. 1275. / Arbuste; bord du lac Arnaud. / 1855–60. Right-hand label: VIEILLARD, HERB. de la Nouvelle Calédonie. / No. 1275. / Podocarpus nana (Parlat.) / baie du Sud. / 1861–67. – Two specimens of different Podocarpus species, the top left specimen determined as P. sylvestris J.Buchholz by R. D. Hoogland in 1992 and the bottom right specimen determined as P. longefoliolatus Pilg. by both Guillaumin in 1943 (also see Guillaumin, Reference Guillaumin1944, who cites examples of Vieillard 1275 under Podocarpus longefoliolatus from both Baie du Sud and Wagap, but none of P. minor) and Hoogland in 1992. It is most likely that the right-hand label applies to the lower-right specimen and the left-hand label to the top-left specimen. This sheet obviously has to be rejected as neither specimen belongs to Retrophyllum.
Of the seven sheets listed above, five can be ruled out for lectotypification purposes for the reasons given, and the choice therefore lies between those barcoded P00188106 and P00188110. Farjon (Reference Farjon2010) mentioned that neither of the toponyms ‘lac Arnaud’ and ‘Baie du Sud’ was in current use and that they might have been written by someone who was not the collector. This may be true of the printed labels but one sheet of Retrophyllum minus at Paris (P00188109) bears an older handwritten label giving the toponym ‘lac Arnaud’. Study of a geological work contemporary with Carrière's Traité de Conifères, by Garnier (Reference Garnier1867), shows that in his time the name Lac Arnaud was used for the larger of the two principal lakes of the Plaine des Lacs, now called Grand Lac, while the other lake, currently known as Lac en Huit, was in Garnier's work called Lac Latour. ‘Baie du Sud’ is the Baie de Prony (Lapie, Reference Lapie1928; McKee, Reference McKee1972). The two localities are therefore geographically close but not identical, and are today in different communes: Grand Lac (Lac Arnaud) is in Yaté while Baie de Prony (Baie du Sud) is in Le Mont-Dore. Because Carrière only cited, very specifically, material collected “au bord du lac Arnaud”, typification must be based on material from that locality alone, rather than from Baie du Sud, the locality of the ‘holotype’ as given by Farjon (Reference Farjon2010). Not only that, but examination of the contemporary botanical literature reveals that no material of the 1861–67 Baie du Sud gathering could possibly have been available to Carrière when preparing his Traité Général des Conifères, which was published on 15 January 1867. It was not cited by Parlatore (Reference Parlatore and De Candolle1868), who cited only one specimen, that from lac Arnaud. More importantly, Brongniart & Gris, who worked at Paris, state (Brongiart & Gris, Reference Brongniart and Gris1869: 326 and 1871: 342) that [in translation] “M. Balansa gives us complete knowledge of another species of Podocarpus . . . belonging to the section Nageia, and which, for many years, had only been represented in our herbaria by two poor specimens, bearing at the top of their short branches small groups of rather poorly developed male spikes” [bold italic emphasis mine]. They cite these two specimens as ‘Deplanche 1861’ and Vieillard “ad ripas lacus Arnaud dicti no 1275”. Consequently, even by 1869 the 1861–67 Baie du Sud collection was not known to Brongniart & Gris, and one must therefore assume that Carrière could not possibly have seen it before publication of Traité des Conifères in 1867. Hence, all sheets bearing Vieillard ‘1861–67’ labels must be excluded for lectotypification purposes. This means that sheet P00188106 must be regarded as unsuitable.
Therefore, out of the seven sheets, only one, P00118110, is suitable for lectotypification purposes. The lectotype must therefore be chosen from among the specimens on this sheet. However, at least one of the shoots now mounted on this sheet bears numerous male cones that it would have been difficult for Carrière to overlook if it had been present on the sheet in his time. Farjon (Reference Farjon2010) considered both male and sterile material when attempting to typify the species. However, I consider that male material should be excluded, for two reasons.
1. Carrière described only vegetative characters. He may have overlooked the male cones on some specimens because of their paucity, but other material now mounted on the same sheets bears abundant cones that could not have been overlooked, as in the case of the sheet here chosen for lectotypification. Male cones were first described by Parlatore (Reference Parlatore and De Candolle1868), but still on the basis of the poorly developed ones on the lac Arnaud gathering of Vieillard 1275. As mentioned above, the Baie du Sud 1861–67 gathering numbered Vieillard 1275, which has more abundant, better developed male cones, was not known either to Carrière (Reference Carrière1867) or Brongniart & Gris (Reference Brongniart and Gris1869, Reference Brongniart and Gris1871).
2. Farjon (Reference Farjon2010: 939) wrote that “the sterile/male shoots all seem to have been gathered from the same plant”. However, careful examination of the material on the six Paris sheets of Vieillard 1275 that belong to Retrophyllum minus, and indeed of the one sheet from which I have deduced the lectotype must be chosen, shows that this is not true. The male specimens currently found among some collections of Vieillard 1275 (supposedly) from lac Arnaud, i.e. specimens 108-A, 109-A, 109-E, 110-B, 110-E and 111-D, actually fall into two distinct groups, here regarded as different collections from different plants. Specimens 109-E and 110-E have small dark leaves (much smaller than the 12–17 mm mentioned in the protologue) and abundant cones, while specimens 108-A, 109-A, 110-B and 111-D have larger, paler leaves and mostly very few cones. Unfortunately, analysis of the colour profiles generated by ImageJ reveals that these latter four specimens are identical in their profile characteristics to Deplanche 170 (here designated P00118111 specimen 111-J: Appendix table 1), which also bears few male cones. Therefore it is possible that either: i, all these shoots may actually be duplicates of Deplanche 170 111-J; or ii, Deplanche 170 111-J and the male specimens numbered Vieillard 1275 108-A, 109-A, 110-B and 111-D were collected at the same time from the same plant but numbered separately by Deplanche (as 170) and Vieillard (as 1275). (Hopkins & Bradford, Reference Hopkins and Bradford2009, describe a similar practice by the two collectors in gatherings of the genus Pancheria Brongn. & Gris). Therefore they, and similar sterile specimens (109-F, 110-D, 111-A, 111-C, 111-F, 111-G and 111-H), are all considered unsafe for lectotypification purposes, partly because Vieillard may not have collected them and partly because Carrière could not have seen either gathering numbered Deplanche 170, as discussed later.
Among the material currently mounted on sheet P00188110, the fragment at top left (here designated 110-A) has leaves measuring 12–17 mm long, exactly the range given in the protologue, and it is sterile. This fragment is accordingly here designated lectotype of Nageia minor and therefore of Podocarpus minor, Decussocarpus minor and Retrophyllum minus. Its colour profile is shown in Fig. 11L, M. It is curious that another specimen now mounted on this sheet and (by analysis of its colour profile characteristics: data not shown) clearly part of the same gathering, 110-C, has leaves measuring 21–24 mm, well outside the range quoted in the protologue. However, similar objections can be raised about any of the sheets numbered Vieillard 1275 in their current states (except P00118108, which comprises only one specimen), and it can only be assumed that their current compositions post-date Carrière's examination of the material.

Fig. 11. ImageJ colour profiles of Retrophyllum minus leaves. Lines represent colour channels, always from top to bottom: red (R), green (G), blue (B). A, C, E, G, J, L, N, P, R, 20 × 20 px squares; B, D, F, H, K, M, O, Q, S, transverse lines across leaves at midpoint. A & B, Vieillard 1275 106-A, Baie de Sud. R channel separate from G and B, which are very close together. A unique profile not found in any other gathering, indicating that this gathering from Baie de Sud (‘1’ in the last column of Appendix table 1) is different not only all from the lac Arnaud gatherings of Vieillard 1275 but also from the other gathering from Baie de Sud. C & D, Vieillard 1275 107-A, Baie de Sud. The three channels are all separate from one another. This profile (gathering ‘2’, Appendix table 1) is similar to Vieillard 1275 gathering 3 (E, F) and to Deplanche 170 111-J (G, H). E & F, Vieillard 1275 108-A. All three channels well separated, the B even more so than the R and G. Leaves rough textured (many irregularities in the lines in Fig. 11B). G & H, Deplanche 170 111-J. Profiles E–H are typical of Vieillard 1275 gathering 3 from lac Arnaud and Deplanche 170 gathering I. They are also very similar to Vieillard 1275 107-A and 107-B (‘Baie de Sud’, gathering 2), indicating that the three gatherings, or at least the two from lac Arnaud, were most likely made from the same plant at the same time. J & K, Vieillard 1275 111-E, typical of gathering 4. R and G channels scarcely separated and sometimes merging; B channel clearly separated from the other two. Leaves rather rough. L & M, Vieillard 1275 110-A, chosen as lectotype and with profiles typical of Vieillard 1275 gathering 5. Channel separation similar to J & K but leaves much smoother. N & O, Vieillard 1275 110-E, typical of gathering 6 with small dark leaves and abundant male cones. R & G channels close but separate. Rough texture. P & Q, Deplanche 170 146-A. All channel values low but channels all separated clearly, G closer to R than B. Compare with G & H – the profiles are different. R & S, Pancher s.n. with number ‘Vieillard 1275’. G channel closer to B than to R but all separated. The profiles do not match any of the Vieillard 1275 gatherings and this is a unique, female gathering by Pancher.
The following three specimens at P match 110-A in their colour profile characteristics and in their morphology: 109-C, 109-G and 110-C. These four specimens match most closely the characters as given in the protologue and are selected as lectotype (110-A) and isolectotypes (109-C, 109-G and 110-C). These four specimens are the only ones here considered to be suitable as lectotypes. All other specimens numbered Vieillard 1275, whether labelled from lac Arnaud or Baie du Sud, are not regarded as type material, because morphologically they show evidence of having been collected from plants other than the one from which the lectotype was gathered.
Material collected by Deplanche
Vieillard and Deplanche collected much material at the same time in the field. The collection referred to by Brongniart & Gris (Reference Brongniart and Gris1869, Reference Brongniart and Gris1871) as ‘Deplanche 1861’ is clearly the specimen in the Paris herbarium (P) numbered Deplanche 170. This collection, according to its printed label, was made in 1861 and is mounted on the sheet barcoded P00188111, which now also bears two fragmentary assemblages numbered Vieillard 1275, as discussed above. The first mention of Deplanche 170 in the literature is by Brongniart & Gris (Reference Brongniart and Gris1869), who mention it as one of the only two collections of Podocarpus minor known to them. As mentioned earlier, Carrière (Reference Carrière1867) did not cite it; therefore we must assume that it became available for consultation sometime between 1867 and 1869. If that is so, Carrière could not have seen it before publication of Nageia minor.
A second example of Deplanche 170 exists at P, namely the sheet barcoded P00188146. According to its printed label, this was collected in 1863, but another label indicates that it was not donated to the P herbarium until 29 September 1894, long after Brongniart & Gris (Reference Brongniart and Gris1869, Reference Brongniart and Gris1871) published their note. Because the two collections were made in different years, it seems that, like Vieillard, Deplanche may have used the number 170 as a catalogue number rather than a field collection number. (In fact, the number Deplanche 170 has also been used for a collection of Parasitaxus usta (Vieill.) de Laub. from Pic de Pouébo in the north of New Caledonia, corresponding to Vieillard 1269 of that species from the same locality: Gray, Reference Gray1960, as Podocarpus ustus.) Obviously Carrière could not have seen this sheet. Colour profile analysis of the leaves of the two sheets numbered Deplanche 170 (Appendix table 1; Fig. 11G, H, P, Q) reveals that they represent collections from two different plants and that the two shoots on the sheet barcoded P00188146 (Fig. 11P, Q) both came from the same plant. As noted above, the colour profiles of the leaves on Deplanche 170 111-J (Fig. 11G, H) on the other sheet (P00188111) are identical to 111-A, C, D, F, G, H on the same sheet that are assumed to have been collected by Vieillard, almost certainly from the same plant.
Alleged female specimens of Vieillard 1275
Farjon (Reference Farjon2010) also claimed that male and female shoots, on different sheets, had been given the number Vieillard 1275. It is not clear from his work what was the basis for this statement; de Laubenfels (Reference de Laubenfels, Aubreville and Leroy1972) indicated that Vieillard 1275 from both lac Arnaud and Baie du Sud was male, and cited no female material under the number. Likewise, I have not seen any female material numbered Vieillard 1275 in the Paris Herbarium that genuinely belongs to any one of the specimens in that assemblage of gatherings. Farjon (Reference Farjon2010) mentioned a sheet at K numbered ‘Pancher 1275’ that was female and, implicitly at least, regarded it as belonging to Vieillard 1275. Pancher typically did not give numbers to his specimens. However, de Laubenfels (Reference de Laubenfels, Aubreville and Leroy1972) cites a female specimen of Retrophyllum minus at P collected by Pancher in 1864 at Prony, which is on Baie du Sud. One of the labels on this sheet (now barcoded P00188118, and comprising seven mostly small fragments) is headed ‘Vieillard 1275’, but a later curator has pencilled before that number, “C'est la même plant que”. There are two possible interpretations of this comment: (1) whoever wrote it was indicating that it was not being regarded as part of Vieillard 1275; and (2) it was meant to indicate that it was a duplicate of Vieillard 1275 in Pancher's herbarium (apparently, Pancher did ‘appropriate’ specimens from both Vieillard and Deplanche, according to letters of Deplanche mentioned by Hopkins & Bradford, Reference Hopkins and Bradford2009). Careful examination of the Paris example of the Pancher collection reveals that all seven fragments on it are homogeneous other than three being female and the others sterile (sometimes on account of being such small pieces). Image analysis of the colour profiles of the various fragments supports both the homogeneity of the fragments forming this collection (Appendix table 1; Fig. 11R, S) and that it is a unique gathering, by Pancher, unconnected with Vieillard 1275; this is also supported by the fact that three of the fragments are female, whereas only male material has been seen among the fertile specimens on the Paris sheets of Vieillard 1275.
Epitype designation
Because Vieillard 1275 is a mixture of sterile and male material and all of the male material has had to be excluded for typification purposes for the reasons given above, an epitype is designated here to fix the application of the name in relation to Podocarpus palustris so that the latter name might be used correctly if in future it were segregated from Retrophyllum minus at any rank. Because Buchholz distinguished Podocarpus palustris from P. minor on the basis of female sexual characters rather than male ones, his specimen Buchholz 1729, on whose labels he sets out the differences he perceived, is here chosen as epitype of Nageia minor and therefore also of Podocarpus minor and Retrophyllum minus. Unfortunately, the labels on the NY and P duplicates of Buchholz 1729 do not give as much information on the seed characters as that on a photograph at A of what is here assumed to be the example at ILL. This label reads “This specimen shows the seeds [of Podocarpus minor] not previously described. They are deep maroon red, crested, having kernels with straight point. The cone bearing two seeds at left below are rare, not present in any duplicate of this collection. The leaf anatomy of this collection agrees with the Vieillard types. They have wide midveins (not visible externally) and similar loaf-like sclereids”. The locality information (“Plaine des Lacs, along Riv. des Lacs a western tributary of Yate river”) is, however, abbreviated on this label compared with those of the NY duplicate specimen and the photographs of the ILL specimen and dissected seeds at P, and there is no date. Consequently, the NY specimen, whose label is dated and gives a more precise locality, is here designated as epitype. Buchholz 1729 was the only collection made by him of Podocarpus minor sensu stricto, as opposed to P. palustris, according to a list of his collections posthumously published (Buchholz, Reference Buchholz1955), although, with respect to P. palustris at least, that list is incomplete because it does not list the holotype and paratypes of P. palustris that are duplicated at Paris, nor does it include the photograph and fragment of Buchholz 1719, another specimen of P. minor sensu stricto.
Appendix II
List of accepted names and synonyms
Decussocarpus de Laub. sect. Decussocarpus – Retrophyllum C.N. Page
Decussocarpus comptonii (J.Buchholz) de Laub. – 3
Decussocarpus minor (Carrière) de Laub. – 4
Decussocarpus piresii Silba – 6
Decussocarpus rospigliosii (Pilg.) de Laub. – 5
Decussocarpus vitiensis (Seem.) de Laub. – 1
Nageia Gaertn. sect. Polypodiopsis (C.E.Bertr.) de Laub. – Retrophyllum C.N. Page
Nageia comptonii (J. Buchholz) de Laub. – 3
Nageia minor Carrière – 4
Nageia piresii (Silba) de Laub. – 6
Nageia rospigliosii (Pilg.) de Laub. – 5
Nageia vitiensis (Seem.) Kuntze – 1
Podocarpus L'Hér. ex Pers. sect. Polypodiopsis C.E.Bertr. – Retrophyllum C.N. Page
Podocarpus comptonii J.Buchholz – 3
Podocarpus filicifolius N.E.Gray – 2
Podocarpus minor (Carrière) Parl. – 4
Podocarpus montanus sensu Knuth (1926: 95) non (Humb. & Bonpl. ex Willd.) Lodd. – 5
Podocarpus palustris J.Buchholz – 4
Podocarpus rospigliosii Pilg. – 5
Podocarpus vitiensis Seem. – 1
Retrophyllum C.N. Page
Retrophyllum comptonii (J.Buchholz) C.N. Page – 3
Retrophyllum filicifolium (N.E.Gray) R.R.Mill, comb. nov. – 2
Retrophyllum minus (Carrière) C.N. Page – 4
Retrophyllum piresii (Silba) C.N. Page – 6
Retrophyllum rospigliosii (Pilg.) C.N. Page – 5
Retrophyllum vitiense (Seem.) C.N. Page – 1
[Torreya bogotensis Linden ex K.Koch, nom. utique rejic.] – ?1 or ?5
Appendix III
List of exsiccatae
anon. 5536 (Department of Agriculture, Fiji) – 1; Archer, W.A. 541 – 5; Asplund, E. s.n. (no date; Lima botanic garden) – 5; 12027 – 5; Aubréville, A. & Heine, H. 130 – 4; 170 – 4; 229 – 3; 241 – 4; Aymard, R. (MacKee 16325) – 4
Balansa, B. 186 – 4; 1381 – 3; Barets, R. 8 – 3; Barrabé, L. 365 (Rigault, F. & Barrière, R. s.n.) – 3; Barrera, E. 45 – 5; 46 – 5; 50 – 5; Baudouin, A. 542 – 3; Baumann-Bodenheim, M.G. 5654B – 3; 6370 – 4; 6378 – 4; 6580 – 4 (juvenile; adult specimen numbered Guillaumin & Bodenheim 6580, q.v.); 13923 – 4; 14057 – 3; 15028 – 3; 15178 – 3; 15197 – 3; 15393 – 3; 15411 – 3; Baumann-Bodenheim, M.G. see also Guillaumin & Baumann-Bodenheim with same numbering sequence; Bernardi, L. 335 – 5; 1959 – 5; 9369 – 4; 9445 – 3; 9520 – 3; 9879 – 3; 10131 – 3; 10149 – 3; 10347 – 3; 12754 – 3; Bernier, J. s.n. (barcode P00188269) – 3; s.n. (7 iii 1948) – 4; 203 (2 sheets) – 3; 204 – 4; 245 (all 4 sheets) – 4; 246 – 4; 247 – 4; 248 – 4; 249 – 4; 250 – 4; 251 – 4; 267 – 3; 268 – 3; 269 – 3; 270 – 3; 271 – 3; Betancur, J. et al. 1817 – 5; Blanchon, J.P. 208 – 4; 341 – 3; 399 – 3? (confirmation needed); 736 – 4; 1160 – 4; 1246 – 3; Bohorquez R., P. 475 – 5; Boisseau, P. (MacKee 12725) – 3; Boorman, J.R. s.n. – 1; Brass, L.J. 12787 – 2; 12787a – 2; 12912 – 2; Brass, L.J. & Versteegh, C. 12534 – 2; Breteler, F.J. 4495 – 5; Brinon, H. 1359 – 3; Brousmiche, E. s.n. (Pic de Na Kado) – 3; 697 – 3; Buchholz, J.T. 1085 – 3 (paratype and isoparatypes); 1222 – 3; 1347 – 4 (paratype & isoparatypes, Podocarpus palustris); 1348 – 4 (paratype & isoparatypes, Podocarpus palustris); 1350 – 3; 1350a – 3; 1359 – 3; 1359a – 3; 1421 – 4 (holotype of Podocarpus palustris ILL, isotypes of Podocarpus palustris A–photo, ILL–2 sheets, K, MO, NY, P, RSA, S, TEX, WIS); 1447 – 3 (paratype); 1449 – 3 (paratype and isoparatypes); 1452 – 3; 1474 – 4 (paratypes of Podocarpus palustris, ILL00010015, ILL00010022 [photo of latter, A], isoparatype of Podocarpus palustris, S); 1578 – 3; 1648 – 3; 1684 – 3 (holotype ILL with photos at A and P, isotypes K, MO, NY, S); 1684s – 3; 1697 – 3 (paratype and isoparatypes); 1697a – 3 (paratype and isoparatypes); 1697s – 3 (paratype and isoparatypes); 1705 – 4; 1719 – 4; 1729 – 4 (epitype); 1791 – 3; Bunting, G.S. 4898 – 5; 4939 – 5
Campos, J. & Rodríguez, E. 2825 – 5; Cardenas, C.d.l.A. & Oliveros, S.E. 198 – 5; Carr, C.E. 14160 – 2; 15666 – 2; Cherrier, J.-F. (MacKee 39235) – 3; (MacKee 39255) – 3; (MacKee 44895) – 4; Compton, R.H. 607 – 3; 608 – 3; 1524 – 3; 1527 – 3; 1587 – 3; Corbasson, M. (MacKee 13037) – 3; Cox, P.A. 1360 – 1; Cretinon, L. & Gardner, M.F., ICCP New Caledonia Exped. (1999) 17 – 4; 26 – 4; 44 – 4; 50 – 4; 50A – 4; 73 – 3; 90 – 3; Cribs, L. 1493 – 4; 1752 – 4; Cuatrecasas, J., Schultes, R.E. & Smith, E. 12768 – 5; Curran, H.M. 2147 – 5
Dagostini, G. et al. 1340 – 4; Damanu, E. G7 – 1; KU22 – 1; L10 – 1; L12 – 1; NH15 – 1; NL10 – 1; R10 – 1; R15 – 1; R32 – 1; Däniker, A.U. 228 (6 x 1924) – 4; 228 (11 x 1924) – 4; 228a – 4; 2901a – 3; 2902 – 3; Daza, A. & Pennington, T.D. 16464 – 5; de Laubenfels, D.J. s.n. (3 xii 1957) – 4; P 112 – 4; P 115 – 4; P 129 – 3; P 153 – 3; P 160 – 4; P 309 – 1; P 360 – 3; P 361 – 3; P 415 – 3; P 508 – 1; 754 – 5; 755 – 5; 756 – 5; Degener, O. 14483 – 1; 14484 – 1; 14496 – 1; Deplanche, É. 170 (1861) – 4; 170 (1863) – 4 (transitional to 3); d'Espeissis, J.C. 1460 – 1; Dickison, W.C. 223 – 4; Dobson, F.H. III 1277 – 5; Dugand, A. & Jaramillo, R. 3962 – 5; Duque-Jaramillo, J.M. 2964 – 5; 3108 – 5
Ehrendorfer, F. 6600-138-38 – 3; Erdtman, H. s.n. (8 ix 1960) – 3; s.n. (9 ix 1960) – 3; Erdtman, H. & Chevalier, L. s.n. (8 ix 1960) – 3; Espejo, E.L. 29ELE – 5; Espinal-T., S. & Delgado-F., A. 1790 – 5; Esposto, N. 556 – 5 (holotype B, fragment S, isotypes GH, NY); Eyma, P.J. 5155 – 2
Forero, E. & Forero G., J. 6207 – 5; Foster, A.S. 160 – 3; 200 – 4 (topotype, Podocarpus palustris); Frachon, N. 1337 – 5; 1340 – 5; Franc, I. 207 (i 1914, Plaine des Lacs) – 4; 207 (no date, Prony) – 4; 207 (x 1913, Prony) – 4; 207 (i 1914, Prony) – 4; 207 (Série A, no date, Prony) – 4; 207 (Série A, x 1913, Prony) – 4; Franco, P., Jaramillo, R. & Uribe, J. 2430 – 5; Frodin, D.G. NGF 26292 – 2; 26917 – 2; Funck, N. & Schlim, L.J. 1208 – 5
García-Barriga, H. 11038 – 5; 11041 – 5; 11586 – 5; 12492 – 5; 15490 – 5; 17259 – 5; 17584 – 5; 17585 – 5; 18014 – 5; 20159 – 3; 20468 – 5; García-Barriga, H. & Jaramillo-Mejía, R. 19866 – 5; Gardner, M.F. et al., New Caledonia Exped. 2001 1014 – 3; 1026 – 3; Gardner, M.F. et al., Third New Caledonia Araucaria Exped. TNCA 2036 – 3; TNCA 5000 – 4; TNCA 5001 – 4; TNCA 5045 – 3; Gardner, M.F., Gaudeul, M. & Hollingsworth, P.M. 02 – 4; 04 – 4; 68 – 3; 85 – 3; 108 – 3; 246 – 3; 256 – 3; Gardner, M.F., Nieto, E., Alanes, D. & Zenteno, F. (2011) 30 – 5; 37 – 5; 38 – 5; Gentry, A., Diaz, C. & Blaney, C. 61207 – 5; 77310 – ?5 (see notes under that species); Gibbs, L.S. 674 – 1; Gideon, O. & Obedi, S. LAE 77181 – 2; Gillespie, J.W. 3865 – 1; Godefroy, C.Footnote * s.n. – 3; Goitia, D. s.n. (UDBC 2060) – 5; (UDBC 2129) – 5; (UDBC 10797) – 5; Graeffe, E.O. s.n. (unloc.) – 1; s.n. (‘Albizzi Levu’) – 1; Graham, J.G. 5179 – 5; Green, P.S. 1187 – 4; 1216 – 3; 1268 – 3; 1783 – 3; Grignon, C. et al. 470 – 3; 559 – 3; 584 – 3; Guillaumin, A. 8339 – 4; 8345 – 4; Guillaumin, A. see also Baumann-Bodenheim, and Guillaumin & Baumann-Bodenheim, both with same number sequence; Guillaumin, A. & Baumann-Bodenheim, M.G. 6511 – 4; 6580 (adult) – 4 [see also Baumann-Bodenheim 6580, juvenile]; 6582 – 4; 6594 – 4; 6766 – 4; 11257 – 3; 11261 – 3; 11282 – 3; 11299 – 3; 11301 – 3; 11749 – 4; 11811 – 4; 12717 – 3; 12725 – 3; 12727 – 3; 12815 – 3; 12843 – 3; 12861 – 3; 12910 – 3; 12960 – 3
Hammer, P. s.n. – 1; Hammermaster, E. & Sayers, D. NGF 21842 – 2; Hance, H.F. 17247 (BM, = Vieillard 1275) – 4; Hartley, T.G. 15064 – 4; Henao, J.E. 237 – 5; Henty, E.E. & Frodin, D.G. NGF 27359 – 2; Hernández Schmidt, M. 1190 – 5; 1384 – 5; Hollingsworth, P.M. et al. 2008: 90 – 3; 153 – 3; 194 – 3; Huamán, M., Mateo, J.L., Rojas T., C. & Hurtado, D. 313 – 5; Humbert, H. 25588 – 5 (notPrumnopitys harmsiana or Podocarpus harmsianus); 26148 – 5; Hürlimann, H. 931 – 3; 1062 – 3; 1220 – 3; 1573 – 3; 1832 – 3; 1842 – 3; 1964 – 3; 1966 – 3; 3113 – 4; 3157 – 4; 3173 – 3
Ingle, H.D. I.44 – 4; I.66 – 3
Jaffré, T. 352 – 4; 2585 – 3; Jahn, A. 99 – 5; Jérémie, J. & Tirel, C. 1572 – 4
Kinupp, V.F. 3426 – 6; Kostermans, A. s.n. (Morotai, 1949) – 2 (lecto L, foliage only; isolecto A, K); Kostermans s.n. (Morotai, 1949) p.p. (L, detached fruits) – Nageia wallichiana (C.Presl) Kuntze; Kuria, T. 87216 & Oliver, P. – 2
Lam, H.J. 6932 – 1; Lauri, P.E. 45 – 3; Lauri, P.E. & Gay, H. 165 – 4; Lavoix, L. (MacKee 19303) – 4; (MacKee 19304) – 4; (MacKee 19305) – 4; Le Rat, A. 71 – 3; 149 – 4; 253A – 4; 607 – 4; 751 – 4; 1040 – 4; 2587 – 4; 2621 – 4; Little Jr., E.L. 15245 – 5; Lowry, P.P. II 5747 – 3; Lowry, P.P. II et al. 4683 – 4; 6821 – 3
MacDaniels, L.H. 2323 (P-1041) – 3; 2544 – 4; MacKee, H.S. (a.k.a. McKee) 1118 – 4; 1119 – 4; 2373 – 4; 3377 – 4; 3382 – 4; 3516 – 3; 9886 – 3; 12725 (coll. P. Boisseau) – 3; 13037 (coll. M. Corbasson) – 3; 13492 – 3; 15594 – 3; 15639 – 3; 16325 (coll. R. Aymard) – 4; 17032 – 3; 17056 – 3; 17354 – 3; 17357 – 3; 17358 – 3; 17670 – 3; 17908 – 3; 18694 – 3; 18807 – 3; 18808 – 3; 19303 (coll. L. Lavoix) – 4; 19304 (coll. L. Lavoix) – 4; 19305 (coll. L. Lavoix) – 4; 20244 – 3; 20928 – 3; 21219 – 3; 32355 (coll. L. Viratelle) – 4; 33961 – 3; 39997 (coll. R. Nasi) – 3; 35577 – 3; 39235 – 3; 39255 (coll. J.-F. Cherrier) – 3; 39724 – 3; 40011 (coll. R. Nasi) – 3; 40185 – 3; 40376 – 3; 40377 – 3; 43169 – 4; 44895 (coll. J.-F. Cherrier) – 4; 46368 (coll. B. Suprin) – 3; Mahecha, S. s.n. (UDBC 5436) – 5; s.n. (UDBC 11190) – 5; 1391 (UDBC 5985) – 5; Matallana Tobón, G. 523 – 5; Mauriasi, R. et al. BSIP 17025 – 1; 17794 – 1; McKee, H.S. (see MacKee); McMillan, C. 5015 – 3; 5120 – 4; 5139 – 4; McPherson, G.D. 1867 – 3; 2132 – 4; 2191 – 3; 2748 – 4; 2996 – 4; 3066 – 3; 3958 – 3; 4020 – 3; 4121 – 3; 5477 – 3; McPherson, G. 19209 & Mouly, A. – 3; McPherson, G. & Munzinger, J. 18100 – 3; 18149 – 1; 18254 – 3; McPherson, G. & van der Werff, H. 17834 – 3; McPherson, G., Swenson, U. & Mouly, A. 19070 – 3; 19073 – 3; Mead, J.P. 1964 – 1; 1974 – 1; 1982 – 1; Monteagudo, A., Peña, A., Francis, R. & Mateo, J.L. 13230 – 5; 13353 – 5; Monteagudo, A., Peña, A., Mateo, J.L. & Francis, R. 12942 – 5; 12986 – 5; 13078 – 5; 16407 – 5; Morales, C., Suárez, D. & Endara, S. 867 – 5; Morat, P. 5676 –3; 7621 – 3; Mosén, H. 4393 – Holocalyx balansae Micheli (Fabaceae), fide Lindman (Reference Lindman1898); Munzinger, J. 4830 – 4; Munzinger, J. & McPherson, G. 764 – 3; Munzinger, J. 1200, Suprin, B. & Carriconde, F. – 3; 1307, Suprin & Carriconde – 3; Munzinger, J. 1661, Tronchet, F., Le Borgne, T. & Oddi, A. – 3; 1680 – 3; Musselman, L.J. 5280 – 4; 5415 – 3
Nasi, R. s.n. (MacKee 39997) – 3; (MacKee 40011) – 3; Neill, D. & Jost, L. 15337 – 5; Neill, D. & Salinas, J. 7202 – 5 (atypical); Nicholson, R. 348/92 (wild collection source of Frachon 1340) – 5
Page, C.N. 22030 – 4; 22031 – 4; 22032 – 4; 22033 – 4; 22034 – 4; 22041 – 3; Palacios, W. & Tirado, M. 13026 – 5; 13159 – 5; Pancher, J.A.I. s.n. (barcode P00188209) – 3; s.n. (unloc.) – 4; s.n. (“C'est la même plant que Vieillard 1275”, material from Prony) – 4; s.n. (“C'est la même plant que Vieillard 1275”, high-altitude material from Cougui and Mt. Mou) – 3; Pearce, R. s.n. (vii 1860) – 5; Peni, T. 636 – 1; Pennington, R.T. 1422 – 5; 1426 – 5; 1427 – 5; 1431 – 5; 1433 – 5; Perea, J. 381, Mateo, C., Francis, R. & Ortiz, G.– 5; Pérez-Arbeláez, E. 3150 – 5; 4798 – 5; Petit, G. 138 – 4; Piaito, W. BSIP 7061 – 1; Pintaud, J.C. 51 – 3; Pires, M. & Rosa, N. 850 – 6; Pittier, H. 12756 – 5; Pullen, R. 2840 (LAE 96292) – 2
Quirós Quirós, B. 75 – 5; Quizhpe, W., Medina, B., Aguirre, C. & Prado, M. 1092 – 5; Quizhpe, W. & Wisum, A. 2461 – 5
Raoul, N. s.n., no date (Plaine des Lacs, barcode P00188116) – 4; Redrodro, M. K110 – 1; Résineux no. 8, coll. R. Virot – 3; Rigault, F. & Barrière, R. s.n. (Barrabe, L. 365) – 3; Rodrigues, W., Pires, J.M. & ‘Rosas, S.N.’ [sic: = Rosa, N.?] 9646 – 6; Rodríguez-C., Á., Sarmiento, H. & Cruz, P. 505 – 5; Rohrdorf, O. 178 – 4; Roldán, F.J. et al. 3168 – 5; Roncancio, D. 23 – 5; 024 – 5; 055 – 5; Rosa, N.A. et al. 856 – 6 (holotype US, isotypes K, L, MG, MO, NY, R, RB; protologue has number misprinted ‘Rosa & Pires 586’); 856 – 6 (iso INPA, information on label differs from other sheets); Ruiz Terán, L. 918 – 5; 1148 – 5
Sands, M.J.S. 2381 et al. – 2; Sarlin, P. 73 – 4; 228 – 3; 238 – 3; Schlechter, R.F.R. 15331 – 3; 15332 – 3; Schmid, R. 137 – 3; 1422 (wrongly in NOU–database as ‘M. Schmid’) – 3; 4838 (wrongly in NOU–database as ‘M. Schmid’) – 3; Schmidt-Mumm, U. 324 – 5; Schneider, M. 905 – 5; Schnell, R. 10647 – 1; Seemann, B. 576 sheet 1/2– 1; Seemann 576 sheet 2/2 – 1 (lectotype K, isolecto BM–2 sheets, E, S); Selling, O.H. 78 – 4; 202 – 3; 208 – 3; Service des Eaux et Forêts, Nouméa 225 – 4; 226 – 4; Sévenet, T. 958 – 3; Silba, J. B-640 – 3; B-654 – 4; Skottsberg, C. 202 – 3; Smith, A.C. 1796 – 1; 7076 – 1; Smith, D.N. & Brack, W. E. 7634 – 5; Smith, D., Brack, W. & Palomino, J. 1752 – 5; Sobel, G.L. & Strudwick, J. 2130 – 5; Soukup, J. 1801 – 5 (but loose seed in packet, NY, belongs to Archer 541); 4401 – 5; Staufer, H.U. & Blanchon, J.P. 5807 – 4; Stergios, B.G., Caracas, R. & Zambrano, L. s.n. – 5; 20755 –5; Steyermark, J.A. 55999 – 5; 98706 et al. – 5; Stone, B.C. 14836 – 4; Suprin, B. 693 – 4; 1407 – 3; 1612 – 3; 1636 – 3; 1830 – 3; 2628 – 3; s.n. (MacKee 46368) – 3
Terán, L. Ruiz see Ruiz Terán, L.; Teulon, W. s.n. (J.H. & B.H. Tothill 844) – 1; Thorne, R. 28565 – 4; Tothill, Mr & Mrs. (J.D. & B.H.) 844 (coll. W. Teulon) – 1; Triana, J.J. 665 – 5; 1800 – 5
Uribe Uribe, L. 1734 – 5
van Royen, P. & Sleumer, H. 6073 – 2; Vásquez, A.M. 3 – 5; 5 – 5; Vásquez, R. & Jaramillo, N. 20459 – 5; Vaughan, J.H. 3254 – 1; Veillon, J.M. 120 – 3; 122 – 3; 281 – 3; 949 – 3; 2962 – Falcatifolium taxoides (Brongn. & Gris) de Laub.; 3305 – 3; 3514 – 3; 5659 – 3; 6062 – 3; 6747 – 3; 6783 – 3; Vélez, J.G. 6946 – 5; Vélez-Puerta, J.M. 1999 – 5; Versteegh, C. B.W. 913 – 2; Vieillard, E. 1265 – 3; 1275 (unloc., coll. Pancher) – 4; 1275 (Baie du Sud, 1861–1867) – 4; 1275 (BM, also numbered Hance 17247) – 4; 1275 (lac Arnaud, 1855–1860) – 4 (lectotype and 4 isolectotypes, P, plus other non-type material as indicated in Appendix I); 3064 – 3; 3264 p.p. (Mont Mou) – 3; 3264 p.p. (Wagap) – 3; Villamizar, H. & Guevara, F. 63 (UDBC 2223) – 5; Vink, W. & Schram, F.A.W. BW 8730 – 2; Viratelle, L. (MacKee 32355) – 4; Virot, R. s.n. (19 vi 1938) – 3; 38 – 3; 658 – 4; Résineux no. 8 – 3
Walker, F.S. BSIP 212 – 1; Watt, A. 519 – 3; Webster, G.L. 19205 – 4; Webster, G.L. & Hildreth, R. (with Kuruvoli, I.) 14270 – 1; 14277 – 1; Weigend, M., Diané, N., Gottschling, M., Hilger H. H. & Skrabal, J. 5795 – 5; White, C.T. 2033 – 3; 2120 – 3; 2261 – 4; Whitmore, T. BSIP 1580 – 1; Woods, P.J.B. 241 – 2; 345 – 2 (epitype E, isoepitype K); Wright, J.A. 047 – 5
Zlarnik, W.G. 30 – 3