The earwigs (Insecta: Dermaptera) form a relatively small, easily recognisable order. They are elongate and dorsoventrally flattened with short leathery forewings (tegmina), which cover only the most anterior part of the abdomen. They look superficially similar to staphylinid beetles, except that earwigs have specialised cerci which are usually modified into forceps. These are used for defence, to aid in copulation, to unfurl and fold their fan-like hindwings and to capture prey in predatory species. The hindwings are also distinctive, with much of the venation in the remigium reduced and a large, well defined anal region with distinctive creases for the unique folding pattern of their wings. The head is triangular and prognathous. Compound eyes are well developed and ocelli are always missing in modern species; however, some fossil species have been found with three ocelli. The mouthparts are mandibular and are similar to those of orthopterans. The pronotum is rectangular with rounded corners and is distinctive enough between species to have its length to width ratio used in identification.
There are approximately 1,900 living species of earwig (Grimaldi & Engel Reference Grimaldi and Engel2005), although they are rare in the fossil record, with 86 described species. Sixty-six extinct species and additional unnamed fossil specimens were listed by Wappler et al. (Reference Wappler, Engel and Haas2005), and an additional 23 extinct species were described or transferred from other orders by Zhang (Reference Zhang1997), Haas (Reference Haas, Martill, Bechly and Loveridge2007), Chatzimanolis & Engel (Reference Chatzimanolis and Engel2010), Zhao et al. (Reference Zhao, Ren and Shih2010a, Reference Brauer, Redtenbacher and Ganglbauerb, Reference Zhao, Shih, Ren and Zhao2011), Engel (Reference Engel2011), Engel et al. (Reference Engel, Ortega-Blanco and Azar2011, Reference Engel, Peris, Chatziamanolis and Delclòs2015, Reference Engel, Huang, Thomas and Cai2016), Perrichot et al. (Reference Perrichot, Engel, Nel, Tafforeau and Soriano2011), Nel et al. (Reference Nel, Aria, Garrouste and Waller2012), Ross & Engel (Reference Ross and Engel2013), Engel & Grimaldi (Reference Engel and Grimaldi2014), Engel & Perrichot (Reference Engel and Perrichot2014), Yang et al. (Reference Yang, Shih and Ren2015) and Xing et al. (Reference Xing, Shih, Zhao and Ren2016).
Their stratigraphic range largely depends on the placement of the Protelytroptera which are known from the early Permian. Originally, these were considered as a separate order preceding the Dermaptera (Tillyard Reference Tillyard1931; Carpenter Reference Carpenter1992). Shcherbakov (Reference Shcherbakov, Rasnitsyn and Quicke2002) placed the Protelytroptera and the Dermaptera, based on the presence of earwig-style wing-folding and metascutal setose ridges, in the order Forficulida with two suborders: Protelytrina and Forficulina, constituting the original Protelytroptera and Dermaptera respectively. Whilst some authors recognise four suborders – Hemimerina, Arixeniina, Forficulina and Archidermaptera – (for example Haas Reference Haas1995), Engel & Haas (Reference Engel and Haas2007) undertook an extensive review of higher earwig taxonomy, although referencing Shcherbakov's suggestion, and regarded Protelytroptera as a separate order. Within the Dermaptera, their classification includes the extinct suborders Archidermaptera and Eodermaptera, with Neodermaptera as the only extant suborder. This classification has been followed by others (e.g., Gullan & Cranston Reference Gullan and Cranston2010; Zhao et al. Reference Zhao, Zhao, Shih, Ren and Wang2010b; Perrichot et al. Reference Perrichot, Engel, Nel, Tafforeau and Soriano2011; Nel et al. Reference Nel, Aria, Garrouste and Waller2012; Kocarek et al. Reference Kocarek, John and Hulva2013) and is followed herein.
The earliest recorded Dermaptera are tegmina from the Late Triassic of England, Australia and Kyrgyzstan (Jarzembowski Reference Jarzembowki, Swift and Martill1999; Wappler et al. Reference Wappler, Engel and Haas2005; Shcherbakov Reference Shcherbakov2008), although it has been suggested that the preservation of the Triassic specimens is too poor and that earwigs originated in the early Mesozoic of Asia (Zhao et al. Reference Zhao, Zhao, Shih, Ren and Wang2010b). Only two species have been described from the Early Jurassic: Brevicula gradus Whalley, Reference Whalley1985 from the Lower Lias (Sinemurian) of England is often cited as the oldest described fossil dermapteran; however, there is another species, Baseopsis forficulina Heer, Reference Heer1865, from the Lower Lias of Switzerland, which Wappler et al. (Reference Wappler, Engel and Haas2005) considered to be Hettangian in age and therefore older. Certainly, from the figure in Heer (Reference Heer1865, plate 7, fig. 5), the shape of the tegmina is consistent with Dermaptera; however, the specimen requires re-examination to confirm it is a dermapteran. Most of the Heer collection is held at the Swiss Federal Institute of Technology (ETZ), but the specimen could not be located for examination (Andreas Müller, pers. comm. 2016). A further species, B. sibirica Brauer et al., Reference Brauer, Redtenbacher and Ganglbauer1889, was named from the Toarcian of Russia. This specimen is probably held at the Geological Museum of the Academy of Sciences in Moscow (PIN), with the rest of the Czekanowski collection. Wappler et al. (Reference Wappler, Engel and Haas2005) list 18 further species from the Middle and Late Jurassic of China, Germany, Kazakhstan and Russia, and four more Middle Jurassic species from China were described by Zhao et al. (Reference Zhao, Ren and Shih2010a, Reference Zhao, Zhao, Shih, Ren and Wangb, Reference Zhao, Shih, Ren and Zhao2011). Given that these early Dermaptera fossils have small tegmina, Haas (Reference Haas2003) hypothesised that they were able to fold their hindwings like modern earwigs, and certainly the species from China described by Zhang (Reference Zhang2002) have the remnants of hindwings, which appears to support this theory.
Herein, we formally describe the specimens of Dermaptera from the Late Triassic of Australia and England (mentioned above), along with additional material from the Jurassic and Cretaceous of England. The material is largely based on isolated tegmina which have not previously been used for dermapteran taxonomy. Many of the specimens were languishing in the collections at the Natural History Museum, London (NHM), but were not recognised as Dermaptera. It was not until a visit to the museum from Dr Dima Shcherbakov (Palaeontological Institute, Moscow) in the 1990s that their true identity was realised, when he informed AJR as to what they were.
1. Material and methods
Two specimens labelled “Insect fragment” are in the Dunstan Collection, purchased by the NHM in 1935, of Late Triassic insects from Denmark Hill, Ipswich, Queensland, Australia, but which are clearly dermapteran tegmina. They are the oldest undoubted Dermaptera in the world.
Most of the English specimens are from the Brodie collection, purchased by the NHM in 1898. Although most of the specimens have locality data, they are labelled ‘Lower Lias'. Thus, it could be assumed that they are Early Jurassic; however, at some of these localities the insect-bearing horizons occur within the Penarth Group of Late Triassic age. Details on the localities and probable ages are provided below. Previously, the position of the Triassic/Jurassic (Tr/J) boundary was unclear, but the establishment of the GSSP for the Tr/J boundary at Kuhjoch (Karwendel mountains, Tyrol, Austria) (Hillebrandt et al. Reference Hillebrandt, Krystyn, Kürschner, Bonis, Ruhl, Richoz, Schobben, Urlichs, Bown, Kment, McRoberts, Sims and Tomãsových2013) has allowed the standardisation of the boundary in the UK. The boundary is set at the base of the Psiloceras tilmani Zone and, although the characteristic species of this zone, P. tilmani, is not present in English deposits, the zone has been correlated with the English Pre-planorbis beds (Page Reference Page, Lord and Davis2010; Cope Reference Cope2012).
Thirty specimens were studied from the Penarth Group (Rhaetian) and Lias Group (Hettangian to Toarcian) of England. Nineteen of these were collected by Brodie, two by Jackson and one by Sole and are held at the NHM (specimens pre-fixed NHMUK); of these, five were too poorly preserved to be identified. One specimen was collected by Hope and is held at the Oxford University Museum of Natural History (OUMNH), one was collected by Moore and is held at the Somerset Heritage Centre for The Museum of Somerset, Taunton (TTNCM) and three were collected by Rob Coram and were donated to the NHM for the current study. Two further specimens are held in the USA, one Brodie specimen is held at Harvard University's Museum of Comparative Zoology (MCZ), which was found using the iDigBio online catalogue, and the other was collected by Lacoe and is held at the Smithsonian Institute in Washington (USNM). From close study of these specimens, it is clear that at least four species are present, three of which are described here as new.
In addition, there are five more specimens from the Cretaceous of England: two from the Durlston Formation (Berriasian) of Dorset, and three from the Upper Weald Clay Formation (Barremian) of Surrey. They represent two new genera and species. The vein terminology of Vishniakova (Reference Vishniakova1980) is generally followed here, except that it is often not possible to distinguish R from Rs or CuA from CuP, in which case only R and Cu (undifferentiated) are recognised.
The specimens were studied using light microscopes (Leica models as available at the different institutes) and photographed using a Nikon D3300 with AF-S Micro Nikkor 40 mm macro lens attached. For specimens with similar morphology, but with a range of sizes, a bivariate plot using tegminal length and width was plotted in the stats program R (R Development Core Team 2016) and was examined for evidence of specimens grouped by size. A formal cluster analysis was not possible, due to small sample sizes and a limited number of variables for measurement.
2. Localities and ages
Figure 1 is a map of the Dermaptera localities in central and southern England. Figure 2 is a general stratigraphic column of the localities described below and Figure 3 is a more detailed stratigraphic log of the probable Dermaptera horizons from near the Tr/J boundary.
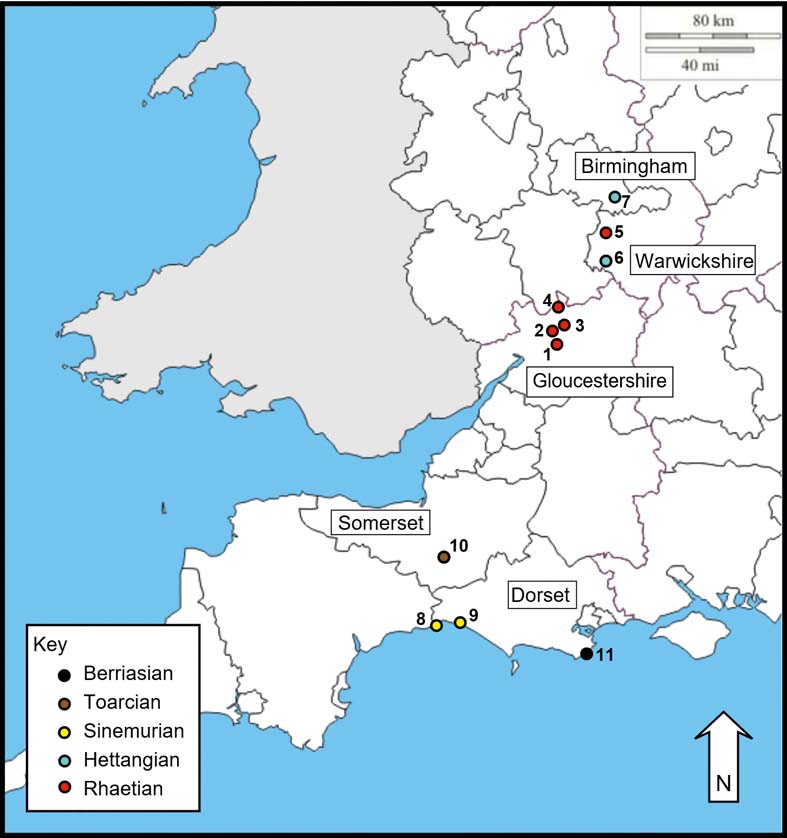
Figure 1 Localities of Mesozoic earwig-bearing horizons in central and southeast England. Rhaetian: 1 = Prior's Norton; 2 = Wainlode Cliff; 3 = Apperley; 4 = Forthampton; 5 = Brown's Wood. Hettangian: 6 = Binton; 7 = Copt Heath. Sinemurian: 8 = Monmouth Beach; 9 = Black Ven. Toarcian: 10 = Ilminster. Berriasian: 11 = Durlston Bay.
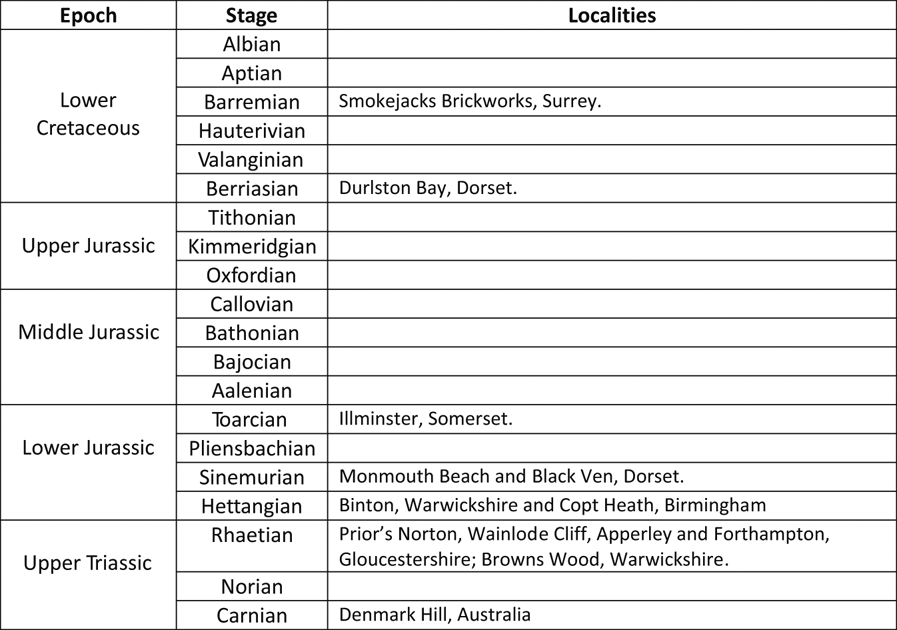
Figure 2 Stratigraphy of all earwig-bearing localities described in this study.
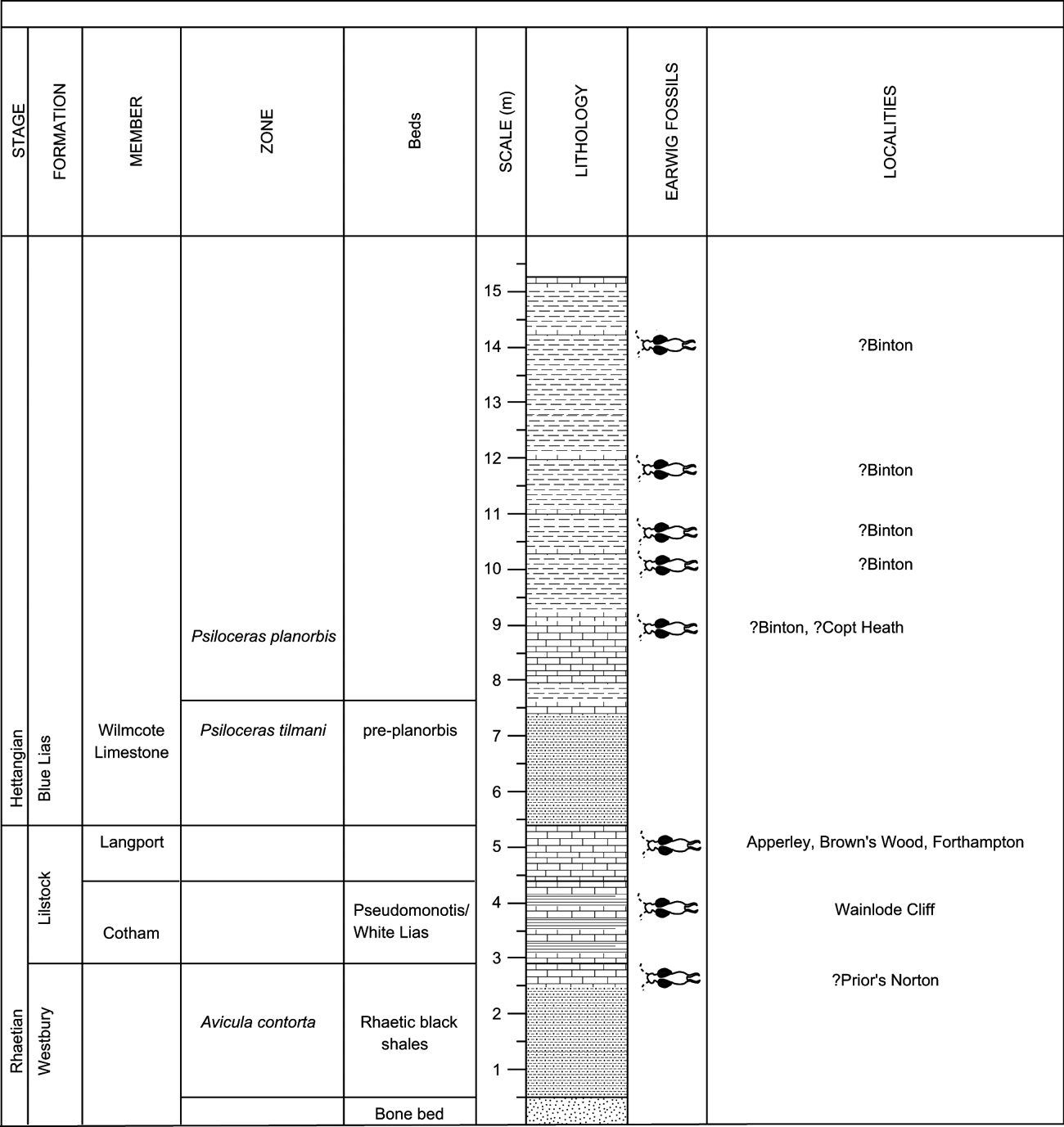
Figure 3 Stratigraphic position of earwig-bearing horizons near the Tr/J boundary in England. Localities are accurately dated to stage and several higher resolution horizons are estimated (indicated by ?). Horizons labelled ‘?Binton' are all horizons from which insects have been collected, but the precise horizon(s) from which earwigs were collected is/are unknown. Image made in SedLog 3.1 (Zervas et al. Reference Zervas, Nichols, Hall, Smyth, Lüthje and Murtagh2009).
Denmark Hill, Australia (Carnian). Two specimens in the Dunstan collection are from Denmark Hill, Ipswich, Queensland, Australia. The Denmark Hill Insect Bed occurs in the Blackstone Formation of the Ipswich Coal Measures Group of Carnian age (Webb Reference Webb1982).
Prior's Norton, Gloucestershire National Grid Reference [SO 855 241] (Rhaetian). Brodie labelled these specimens as ‘Norton'. The exact horizons of Brodie's insect limestone at this locality were never published and Brodie did not mention the locality in his book (Brodie Reference Brodie1845). The section was described by Richardson (Reference Richardson1903, p. 141), which includes a series of dark shales (Rhaetic black shales) overlying the Rhaetic Bone Bed and containing the zonal bivalve Avicula contorta. These shales are similar to the beds of early Rhaetian age described at Charfield [ST 723 919] (Richardson Reference Richardson1904). Although the exact horizon cannot be gleaned from the available literature, it is clear that insect fossils from this locality are Rhaetian, probably from the Westbury Formation.
Wainlode Cliff, Gloucestershire [SO 845 257] (Rhaetian). This section is found approximately 5.5 km north of Gloucester and approximately 3 km southwest of Apperley. The section can be found at a westerly bend in the River Severn near the Red Lion Inn. There is decent exposure of the lower Penarth Group, with the top of the section obscured (Worssam et al. Reference Worssam, Ellison, Moorlock, Barron and Wyatt1989). The stratigraphy of the section is similar to that at Aust Cliff [ST 563 888], with the Westbury Formation and then the Cotham Member of the Lilstock Formation at the top. The insects are found in the Cotham member and several collectors, including Brodie and EAJ, have found other types of insects at Wainlode.
Apperley, Gloucestershire [SO 862 284] (Rhaetian). Brodie (Reference Brodie1845) describes the insect limestone at Apperley as being found in a small quarry on Grey Hill where Worssam et al. (Reference Worssam, Ellison, Moorlock, Barron and Wyatt1989) described a Penarth Group outcrop. The quarry described by Brodie was overgrown by the time Richardson (Reference Richardson1903, p. 140) described a section there. Richardson considered Brodie's ‘Insect Limestone' to be the Pseudomonotis bed, which he thought to be an attenuated form of the Langport Member or White Lias in Gloucestershire, found at the top of the Penarth Group.
Brown's Wood, Warwickshire [SP 116 641] (Rhaetian). The stratigraphy of this locality has been described as being similar to Shelfield [SP 123 624] and Wainlode Cliff (Brodie Reference Brodie1863, Reference Brodie1865). The insect bed at Brown's Wood was located by Brodie (Reference Brodie1865), overlying the Estheria and Pecten beds, which are old terms for the Cotham Member and the Westbury Formation, respectively. Swift (Reference Swift1995) studied the occurrence of the White Lias throughout Britain. The White Lias is absent in Gloucestershire and is replaced by the Pseudomonotis bed; but in Warwickshire, the White Lias is present, so the insect-bearing bed lying above the Cotham Member is probably in the White Lias (Langport Member).
Forthampton, Gloucestershire [SO 857 324] (Rhaetian). Popov et al. (Reference Popov, Dolling and Whalley1994) stated that the section at Forthampton correlates with the Pseudomonotis bed. Brodie (Reference Brodie1845, p.66) described the section at Forthampton, and only a relatively thin bed of brown laminated shale (∼30 cm) separates the Ostrea beds of the Pre-planorbis beds from the underlying insect limestone.
Binton, Warwickshire [SP 142 536] (Hettangian). Brodie (Reference Brodie1845) indicated that there were several localities and horizons that yielded insects at Binton. A section for one of them, Osborne's Pit, was published by Brodie (Reference Brodie1868), but it is uncertain exactly where this site was (Williams et al. Reference Williams, Whittaker, Cornwell, Day, Hawkes, Owens, Warrington and Ivimey-Cook1974, p.39). The insect limestones at Binton all lie above the Pre-planorbis beds (Ostrea beds), so are within the Psiloceras planorbis Zone (Richardson Reference Richardson1912). Wright (Reference Wright1860) details the stratigraphy at Binton and indicates that insects are found in five beds above the Pre-planorbis beds: bed 15, ‘Bottom rock', 0.98 m above; bed 13, ‘paving stone', 1.3 m above, found along with the fish Pholidophorus stricklandi; bed 11, ‘Ribs', a greyish limestone 1.5 m above; bed 5, ‘top liveries', 2.5 m above; and bed 3, ‘top liveries', 4.6 m above, in argillaceous limestone within the Caloceras johnstoni subzone. The most fossiliferous was the ‘bottom rock': “more insects were found here than in all the other beds collectively”. Unfortunately, it is not known from exactly which horizons the dermapteran fossils came.
Copt Heath, near Knowle, Birmingham [SP 175 780] (Hettangian). The insect limestone at this locality was said to lie in detached pieces scattered throughout the field, but with no section exposed (Brodie Reference Brodie1874). Also recorded were the ‘firestones and guinea-bed', which belong to the Wilmcote Limestone Member of the Blue Lias Formation (Old et al. Reference Old, Hamblin, Ambrose and Warrington1991). Brodie also recorded the presence of the zonal ammonite Psiloceras planorbis in these blocks of ‘firestones and guinea-bed', which would suggest a Hettangian age for the insects.
Morton Bagot, Warwickshire [SP 112 646] (Rhaetian). This locality is noted as a separate locality on some of Brodie's labels in NHMUK, but may also be referred to as “Brown's Wood, Moreton Bagot”. In Matley (1912, p. 259, fig. 259), Morton Bagot is labelled as Brown's Wood, even though it is referred to as Morton Bagot in the text. As there is almost no further literature referring to Morton Bagot as a separate locality, we consider this to refer to the same locality as Brown's Wood, 1 km south of the village of Morton Bagot.
Monmouth Beach, Dorset [SY 329 911] (Sinemurian). Isolated tegmina have been found here in ‘Birchi nodules' (bottom of Bed 75, Caenisites turneri Zone) of the Charmouth Mudstone Formation: Black Ven Mudstone Member (see Ross Reference Ross, Lord and Davis2010).
Black Ven, Dorset [SY 355 930] (Sinemurian). Complete dermapteran specimens have been collected from the ‘Birchi nodules' (bottom of Bed 75, Caenisites turneri Zone), ‘Flatstones' and ‘Woodstones' (Bed 83, Asteroceras obtusum Zone) of the Charmouth Mudstone Formation: Black Ven Mudstone Member (see Ross Reference Ross, Lord and Davis2010).
Strawberry Bank, Ilminster, Somerset [ST 361148] (Toarcian). Charles Moore collected many insects from this site in Ilminster. Although he produced a log of this site, he did not mark where the insects were found; however, Matt Williams (pers. comm. Reference Williams, Benton and Ross2015) considers they probably came from his ‘saurian and fish zone' thus from the Harpoceras falciferum Zone of the Upper Lias (Williams et al. Reference Williams, Benton and Ross2015).
Durlston Bay, Dorset [SZ 035 780] (Berriasian). Two specimens were found here. The first was collected by Mrs Burnett and is only labelled ‘Swanage'. The second, belonging to the same species, was collected by Rob Coram from the Corbula Beds within the Durlston Formation: Stair Hole Member (Coram & Jepson Reference Coram and Jepson2012). The ‘Swanage' specimen is preserved in a blue/grey micrite, which is typical of Bed DB175 of the Corbula Beds (see Rasnitsyn et al. Reference Rasnitsyn, Jarzembowski and Ross1998).
Smokejacks Brickworks, Surrey [TQ 113 373] (Barremian). One specimen was found in the Upper Weald Clay Formation at this site, on the north face in a very fossiliferous large phosphatic lens (2 x 1.5 x 0.1 m), collected in 1996. The top of the lens lay 70 cm below the top of the lowest bed of dark grey shale that yields the plant Bevhalstia (see Novokshonov et al. Reference Novokshonov, Ross, Cook, Krzemiński and Soszyńska-Maj2016). Dislodged sideritic concretions in the northeast face have yielded two further specimens, correlated with the Bevhalstia/shale beds in the north face.
3. Systematic palaeontology
Order Dermaptera de Geer, Reference Geer1773 Suborder Archidermaptera Bei-Bienko, Reference Bei-Bienko1936 Superfamily Protodiplatyoidea Martynov, Reference Martynov1925 ?Family Dermapteridae Vishniakova, Reference Vishniakova1980
Type genus. Dermapteron Martynov, Reference Martynov1925 nom. transl. Vishniakova, Reference Vishniakova1980.
Remarks. The family Dermapteridae was originally named as the subfamily Dermapterinae Vishniakova, Reference Vishniakova1980 and was elevated to family status by Engel (Reference Engel2003) without discussion (the family Sinopalaeodermatidae Zhang, Reference Zhang2002 was included as a junior synonym by Engel & Haas (Reference Engel and Haas2007) without discussion). The subfamily name was Latinised based on Dermatopteron Martynov, Reference Martynov1925 originally proposed as a collective group. Nel et al. (2012) restored the Greek spelling, but as this is an unnecessary change, we have followed Vishniakova. The question mark in ‘?Family Dermapteridae Vishniakova Reference Vishniakova1980' above is used to denote uncertainty as to the placement of the genera described below, not to question the authority of the family name.
Genus Phanerogramma Cockerell, Reference Cockerell1915
Emended diagnosis. Tegmina not truncated, tuberculate, with evenly curved anterior margin. M and Cu have a common origin, Cu simple.
Type species. P. heeri (Giebel Reference Giebel1856).
Remarks. Brodie (Reference Brodie1845, p1. 8, figs 15, 17, 18) figured three specimens from the ‘Lower Lias' of England and considered them to “either be the hemelytron of some new genus of Homoptera, or some curiously striated elytron of a beetle” (Brodie Reference Brodie1845, p. 128). Giebel (Reference Giebel1856) named two of Brodie's figured specimens as two new species of the extant orthopteran genus Akicera: A. heeri and A. frauenfeldi. In Brodie (Reference Brodie1845, p. 101), a mistake led to one of the figures (fig. 17) being listed as ‘Blattidae?' from Wainlode and Strensham; however, this should probably have been a reference to figure 13 instead, which is a partial blattodean (cockroach) wing. This led to Scudder (Reference Scudder1891, p. 104) incorrectly citing A. heeri as being from these localities. Cockerell (Reference Cockerell1915) synonymised the two species and placed them in the new genus Phanerogramma in the beetle (Coleoptera) family Tenebrionidae, where the taxon has languished ever since. P. heeri became the senior synonym over P. frauenfeldi.
Of the three specimens figured by Brodie, two are clearly labelled; however, the third cannot be found. Unfortunately, the missing specimen is the holotype of Phanerogramma heeri. A thorough search through the 19 Dermaptera tegmina in the Brodie collection at the NHM has not established if any one of them could be the missing type. They came from several different localities, but unfortunately the location of the type is not known. Either this specimen is present but unlabelled and impossible to recognise, or it is missing. For this reason, a neotype is required for Phanerogramma heeri from Brodie's collection.
All the tegmina are characteristic in having a distinctive tuberculate ornament. Dermapteron incertus (nom. correct. pro incerta Martynov, Reference Martynov1925) (Dermapteridae) and Asiodiplatys speciosus Vishniakova, Reference Vishniakova1980 (Protodiplatyidae) both have a granular ornament within the Protodiplatyoidea; however, Dermapteron has a richer venation (see Vishniakova Reference Vishniakova1980). Phanerogramma is also similar to Sinopalaeodermata neimonggolensis Zhang, Reference Zhang2002 and Palaeodermapteron dicranum Zhao et al., Reference Zhao, Shih, Ren and Zhao2011, both within the Dermapteridae (sensu Engel & Haas Reference Engel and Haas2007). Phanerogramma differs from Dermapteron, Sinopalaeodermata and Palaeodermapteron in having a more evenly curved anterior margin and Cu is simple not forked. Phanerogramma certainly belongs to the Protodiplatyoidea, but given that the body morphology is not known, it is only tentatively placed within the family Dermapteridae based on the venation.
The Australian specimens are clearly similar and can be placed in the same genus. Examination of the bivariate plot of the more complete English and Australian specimens resulted in a central group with two satellites (Fig. 4). The central group can be regarded as one species and includes the specimen originally named P. frauenfeldi; thus, a neotype of P. heeri was chosen from this group. The two satellites are significantly far away from the central group, indicating a significant size difference; they also vary from the central group in their venation, colour pattern, stratigraphic age and, in some cases, palaeogeography, and so can be regarded as separate new species. The smallest one is from Australia and the largest is the paired tegmina figured by Brodie (Reference Brodie1845, fig. 15).
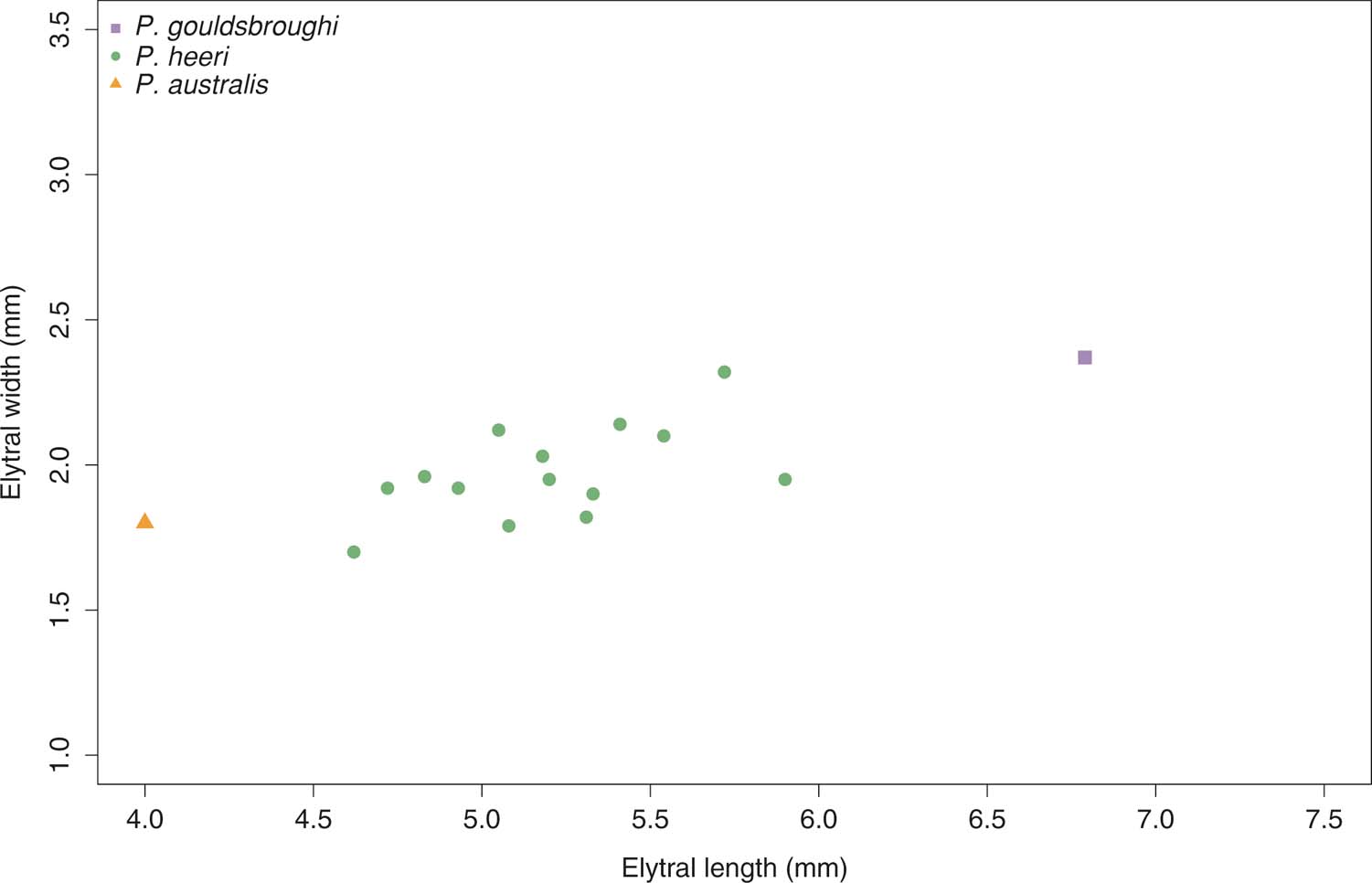
Figure 4 Relationship between length and width of tegmina. Three possible species were identified and this is confirmed by differences in venation and colouration.
Trachopteryx martynovi Carpenter Reference Carpenter1976 (Trachopterygidae) from the Early Permian of Kansas, USA, has a very similar tuberculate ornament to Phanerogramma and it has been suggested (David Grimaldi pers. comm. 2015) that they could be related. However, in Trachopteryx, R1, Rs, MA, MP, CuA and CuP branch pectinately from a common stem, unlike in the Dermaptera specimens, where the veins originate at the base of the wing. In addition, the anterior margin is straight in T. martynovi, but curved in Phanerogramma, and the posterior margin is curved in T. martynovi, but straight in Phanerogramma; thus, it is unlikely that they are closely related.
Phanerogramma heeri (Giebel Reference Giebel1856) (Fig. 5)
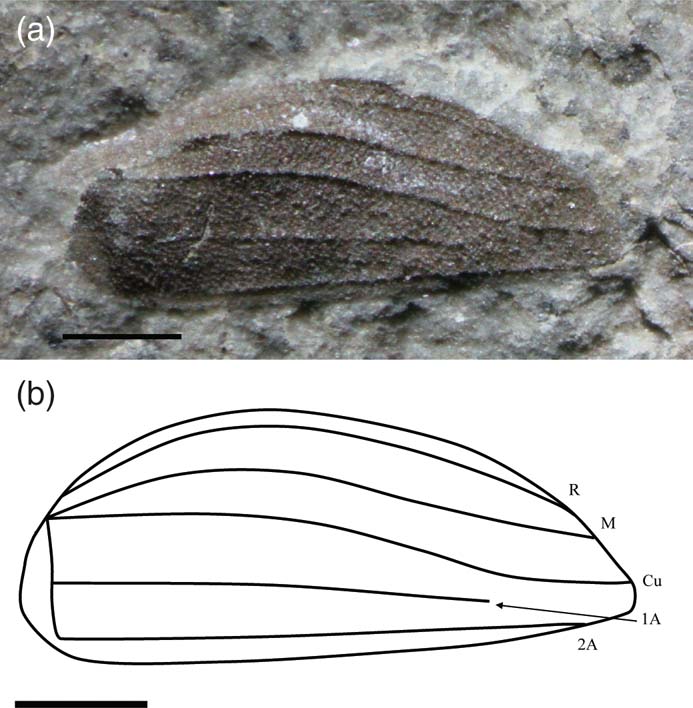
Figure 5 Phanerogramma heeri (Giebel, Reference Giebel1856), neotype NHMUK I.10961, Lilstock Formation (Rhaetian), Forthampton, Gloucestershire, England: (a) photo; (b) line drawing. Scale bars = 1 mm.
Hemelytron of Homoptera or beetle Brodie Reference Brodie1845, p. 128, pl. 8, figs 17, 18.
Akicera heeri Giebel, Reference Giebel1856, p. 310.
Akicera frauenfeldi Giebel, Reference Giebel1856, p. 310.
Akicera heeri Giebel, Goss Reference Goss1879, p. 146.
Akicera frauenfeldi Giebel, Goss Reference Goss1879, p. 146.
Akicera frauenfeldi Giebel, Scudder Reference Scudder1891, p. 104 (no. 611).
Akicera heeri Giebel, Scudder Reference Scudder1891, p. 104 (no. 612).
(?Locustidae) heeri Giebel, Handlirsch Reference Handlirsch1906–08, p. 423.
(?Locustidae) frauenfeldi Giebel, Handlirsch Reference Handlirsch1906–08, p. 423.
Phanerogramma heeri (Giebel) Cockerell Reference Cockerell1915, p. 479, pl. 60, fig. 10.
Phanerogramma heeri (Giebel) Carpenter Reference Carpenter1992, p. 324.
Unnamed dermapteran Jarzembowski Reference Jarzembowki, Swift and Martill1999, p. 150–01, fig. 12B.
Neotype. NHMUK I.10961, Brodie coll., ‘Insect Limestone', Lilstock Formation; Rhaetian; Forthampton, Gloucestershire. Figured by Jarzembowski (Reference Jarzembowki, Swift and Martill1999).
Additional material. NHMUK I.10943, I.11254 Apperley (Rhaetian); USNM 61405 (Lacoe 3452, figured by Cockerell (Reference Cockerell1915)), NHMUK I.10619, I.10870, Brown's Wood (Rhaetian); NHMUK I.11559 Forthampton (Rhaetian); NHMUK I.11020 Copt Heath (Hettangian); NHMUK I.10985 Morton Bagot (Rhaetian); NHMUK I.11002, I.11004 Norton (Rhaetian); NHMUK I.3569 (Holotype of P. frauenfeldi), NHMUK I.10978, I.10981, II.1946 Wainlode (Rhaetian); OUMNH J.55104, MCZ PALE 8667 locality unknown. All Brodie collection, except for: NHMUK II.1946, Jarzembowski coll.; USNM61405, Lacoe coll.; and OUMNH J.55104, Hope coll.
Emended diagnosis. Tegminal length 4.6–6.0 mm, width 1.7–2.3 mm. Evenly pigmented; R running close to C; M and Cu terminate at wing-tip; Cu, 1A and 2A equidistant at base, 2A straight.
Description. Tegmina not truncated, tuberculate, pigmented, with curved anterior margin and straight posterior margin. R faint and simple, runs parallel and close to C, terminating on C just before the tip; M simple, forms a keel at the base, gently curved then straightens out, terminates at tip; Cu simple, originating at same point as M, initially diverges from M then runs parallel to it, converging towards 2A and terminates at tip; A apparently running straight posteriorly with simple 1A branching half-way and fades out, 2A simple, runs parallel to posterior margin and terminates at tip.
Remarks. The figure in Jarzembowski (Reference Jarzembowki, Swift and Martill1999) of the neotype (NHMUK I.10961) is upside down and has been turned the right way up here (anterior margin at top); however, this has resulted in false relief. Cockerell's figure of USNM 61405 appears to show an emarginated tegmen; however, part of the tegmen is missing at that point. The specimens certainly occur in rocks of Rhaetian and Hettangian age and there is nothing to distinguish between them; thus this species crosses the Tr/J boundary.
Phanerogramma gouldsbroughi sp. nov. (Fig. 6)

Figure 6 Phanerogramma gouldsbroughi sp. nov., holotype, NHMUK I.3578, Rhaetian or Hettangian, from “near Bristol”, England: (a) photo; (b) line drawing. Scale bars = 1 mm.
Hemelytron of Homoptera or beetle Brodie, Reference Brodie1845, p. 128, pl. 8, fig. 15.
(Coleopteron) sp. Handlirsch, Reference Handlirsch1906–08, p. 457, pl. 41, fig. 75.
Holotype. NHMUK I.3578, Brodie coll., Rhaetian or Hettangian; ‘near Bristol' (exact locality and age not known).
Etymology. After S. Gouldsbrough, the lead author's partner.
Diagnosis. Tegminal length 6.8 mm, width 2.8 mm. Differs from P. heeri and other species in that it is significantly larger and appears to have a colour pattern. M terminates just before the tip.
Description. Paired tegmina not truncated, tuberculate, pigment pattern, with curved anterior margin and straight posterior margin. R simple, very faint either missing from the left tegmen or mostly obscured on the right; M simple, gently curved then straightens out, terminates just before tip; Cu simple, nearly straight, runs parallel to M, converging towards 2A and terminates at tip; origination of A not visible but assumed to be similar to A. heeri; simple 1A merges with 2A on left tegmen, but fades out on right tegmen, thus demonstrating variation; 2A simple, runs parallel to posterior margin and terminates at tip.
Remarks. Cockerell (Reference Cockerell1915, p. 479) considered this specimen to belong to P. heeri; however, the bivariate plot in Figure 4 places this specimen away from the specimens of P. heeri. There is a distinct colour pattern present, not seen in any specimen of P. heeri. Thus, based on the currently available evidence, it constitutes a new species.
Phanerogramma australis sp. nov. (Fig. 7)
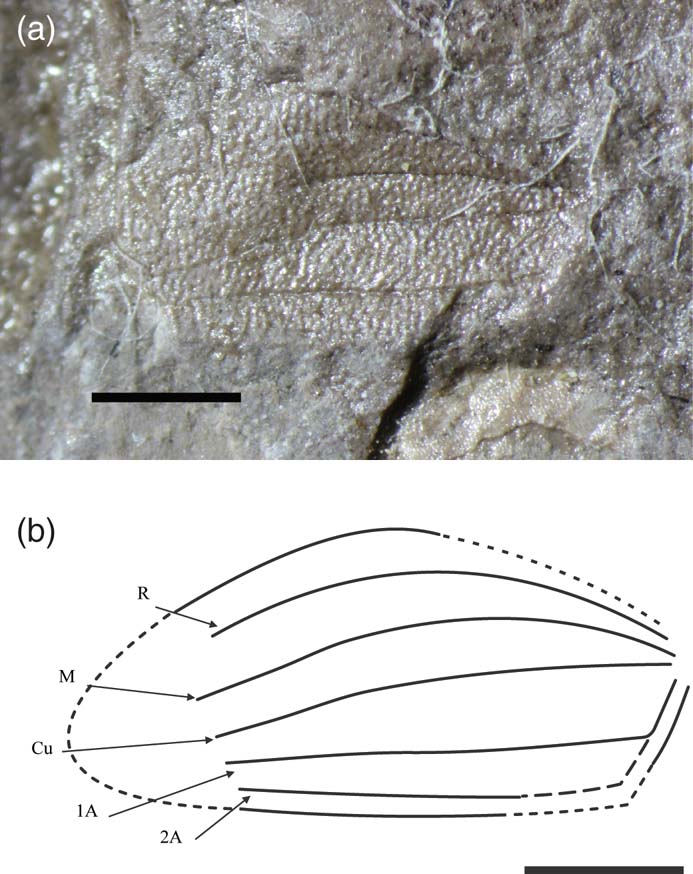
Figure 7 Phanerogramma australis sp. nov., holotype, NHMUK In.35044, Blackstone Formation (Carnian), Denmark Hill, Ipswich, Australia: (a) photo; (b) line drawing. Scale bars = 1 mm.
Holotype. NHMUK In.35044, Dunstan coll., Denmark Hill Insect Bed, Blackstone Formation; Carnian; Denmark Hill, Ispwich, Queensland, Australia.
Etymology. Latin for southern.
Diagnosis. Estimated tegminal length 4.0 mm, width 1.8 mm. Differs from P. heeri and other species in that it is broader, the gap between R and M is wider, and the gap between 1A and 2A is narrower than between Cu and 1A.
Description. Incomplete tegmen missing basal costal margin and tip. Preserved length 3.5 mm, width 1.8 mm. Tuberculate, with curved anterior margin and straight posterior margin. R faint and simple, runs parallel to curved anterior margin and may fade out just before the tip; M simple, gently curved then straightens out, probably terminates at tip; Cu simple, originating at same point as M, initially diverges from M then runs parallel to it, converging towards 2A and probably terminates at tip; A running straight posteriorly with simple 1A branching after half-way and may fade out before tip; 2A simple, runs parallel to posterior margin and probably terminates at tip.
Phanerogramma dunstani sp. nov. (Fig. 8)
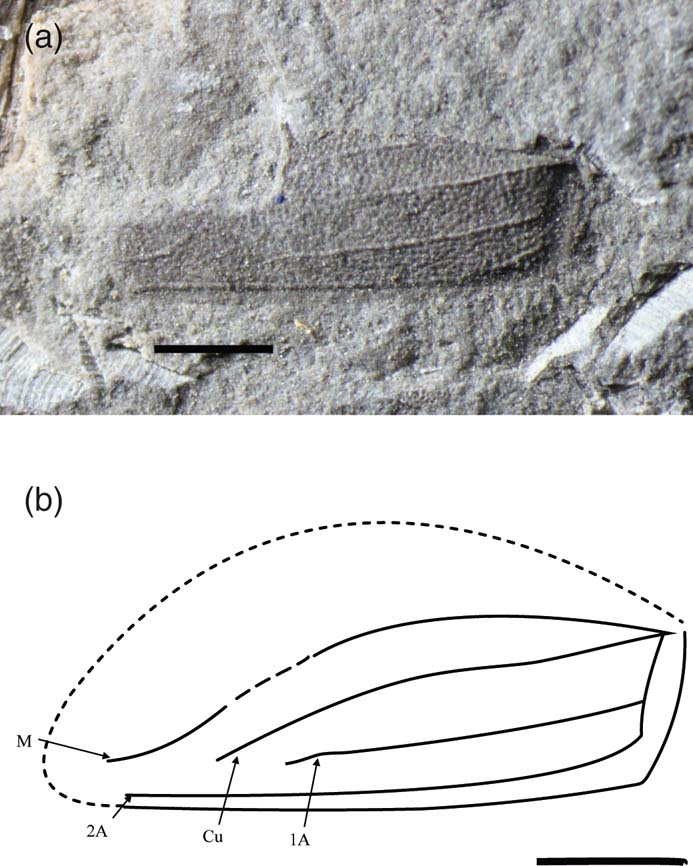
Figure 8 Phanerogramma dunstani sp. nov., holotype, NHMUK In.35041, Blackstone Formation (Carnian), Denmark Hill, Ipswich, Australia: (a) photo; (b) line drawing. Scale bars = 1 mm.
Holotype. NHMUK In.35041, Dunstan coll., Denmark Hill Insect Bed, Blackstone Formation; Carnian; Denmark Hill, Ispwich, Queensland, Australia.
Etymology. After B. Dunstan, as the specimen is from his collection.
Diagnosis. Tegminal length 4.0 mm, estimated width 1.8 mm. Differs from P. heeri and other species in that Cu fades out and does not terminate at the wing-tip, and A2 is curved at base.
Description. Incomplete tegmen missing anterior margin. Preserved width 1.5 mm. Tuberculate, with straight posterior margin. R not visible; M simple, curved and converges with 2A, terminates at tip; Cu simple, originating at same point as M, initially diverges from M then runs parallel to it, fading out just after half-way; A apparently running straight posteriorly with simple 1A branching after half-way and fading out; 2A simple, curved at base and runs parallel to posterior margin and terminates at tip.
Dimapteron gen. nov.
Type species. Dimapteron corami gen. et sp. nov.
Etymology. After Dr Dima Shcherbakov, who informed AJR that this type of tegmen belongs to Dermaptera.
Diagnosis. Punctate, elongated tegmen with emarginate anterior margin. R merging with C at emargination. Cu simple.
Remarks. This tegmen is generally similar in shape to that of Sinopalaeodermata neimonggolensis Zhang, Reference Zhang2002 and Palaeodermapteron dicranum Zhao, Shih & Ren, Reference Zhao, Shih, Ren and Zhao2011, both within the family Dermapteridae. Zhang (Reference Zhang2002) described the ornament of S. neimonggolensis as ‘puncture'. The tegmen described here differs from these taxa in that C and R merge and Cu is simple. Again, given that the body morphology of Dimapteron is not known, it is only tentatively placed in the Dermapteridae.
Dimapteron corami gen. et sp. nov. (Fig. 9)
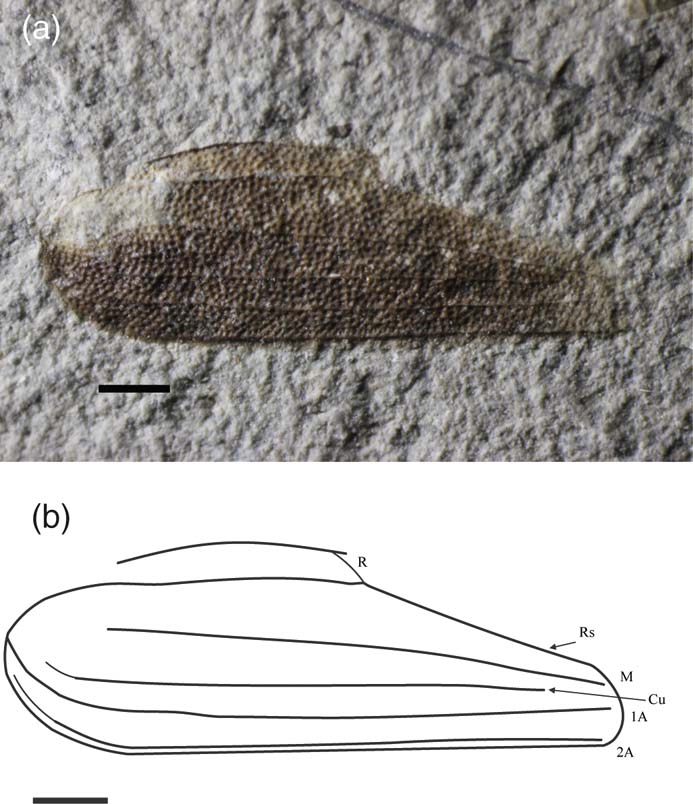
Figure 9 Dimapteron corami gen. et sp. nov., holotype, NHMUK I.15008/In.59187 (part and counterpart), Durlston Formation (Berriasian), Durlston Bay, Dorset, England: (a) photo; (b) line drawing. Scale bars = 1 mm.
Dermapteran forewing, Coram et al. Reference Coram, Jarzembowski and Ross1995, p. 150, fig. 15.
Dermapteran (earwig) forewing, Coram & Jepson Reference Coram and Jepson2012, p. 29, fig. 26.
Holotype. NHMUK I.15008/In.59187 (part and counterpart), Burnett coll.; Corbula beds, Durlston Formation; Berriasian, Durlston Bay, Dorset.
Paratype. NHMUK II.3089 Coram coll., Corbula beds, Durlston Formation; Berriasian; Durlston Bay, Dorset.
Etymology. After Rob Coram, who collected the paratype.
Diagnosis. Tegminal length 8.3 mm, tegminal width 4.0 mm. Pigmented with pale spot at anterior base. M, A1 and A2 terminate at tip, Cu fades out just before tip.
Description. Punctate, elongate tegmen, pigmented with pale spot at anterior base, emarginated at two-thirds of length. C curved, merging with R at emargination, then continues straight towards tip; M simple and straight terminating at tip; Cu simple and straight, closer to A1 than M, fades out just before tip; M, Cu and 1A converge towards tip; A1 and A2 simple and straight running parallel to each other and the posterior margin, terminating at tip.
Valdopteron gen. nov.
Type species. Valdopteron woodi gen. et sp. nov.
Etymology. Latin for Weald and wing.
Diagnosis. Tegmen punctate, neither truncate nor emarginate, with curved anterior margin straightening towards tip; Rs close to anterior margin; M and Cu almost merge with M running straight towards anterior margin before curving posteriorly; Cu straight basally, gently curved apically; 1A straight along most of its length only slightly curving at the apex; 2A more prominent, straight apically, basal third not known.
Valdopteron woodi gen. et sp. nov (Fig. 10)
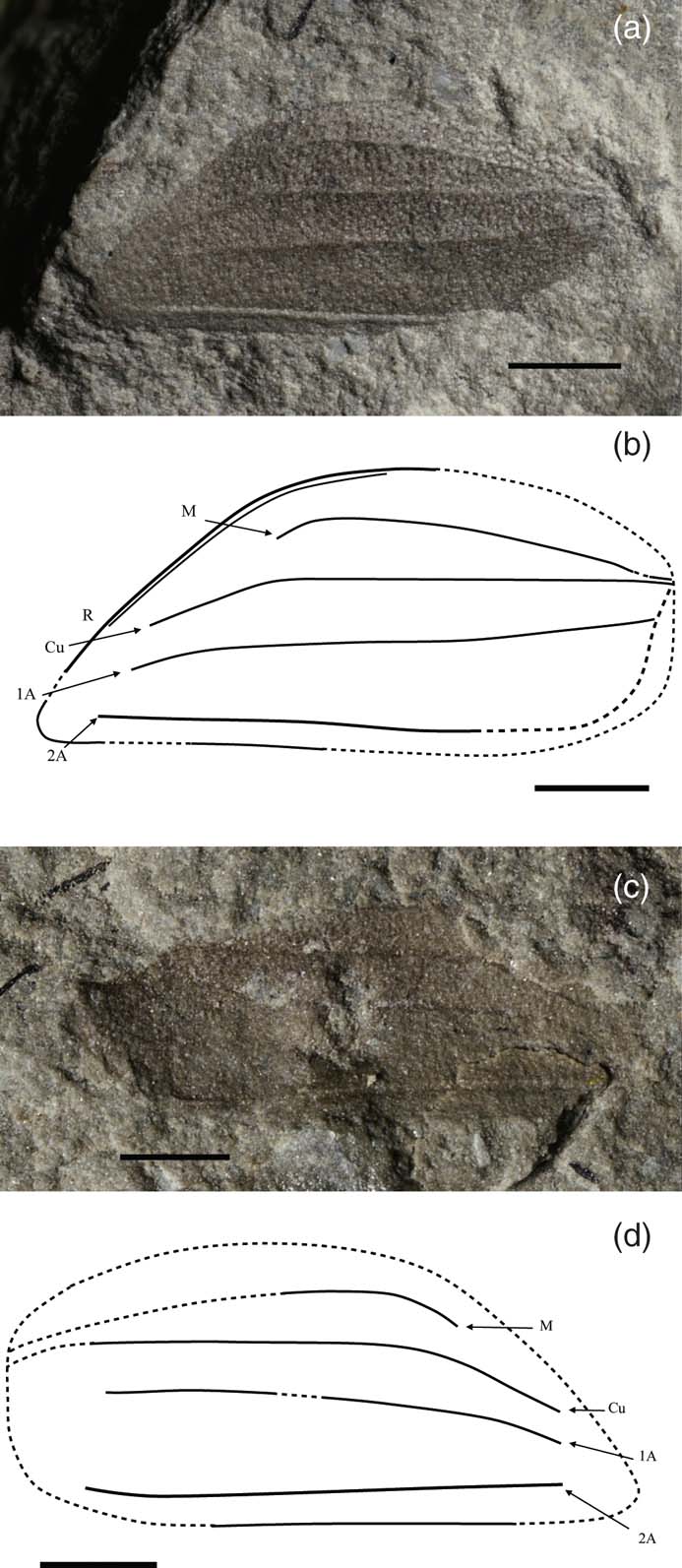
Figure 10 Valdopteron woodi gen. et sp. nov., Upper Weald Clay (Barremian), Smokejacks Brickworks, Surrey, England. (a–b) holotype, NHMUK II.3099: (a) photo; (b) line drawing. (c–d) paratype, NHMUK II.3100: (c) photo; (d) line drawing. Scale bars = 1 mm.
Dermaptera Novokshonov et al. Reference Novokshonov, Ross, Cook, Krzemiński and Soszyńska-Maj2016, p. 47.
Holotype. NHMUK II.3099 [S. 3022a, b] (part and counterpart; Fig. 10 A–C), Jarzembowski coll.; Upper Weald Clay; Barremian; Smokejacks Brickworks, Surrey, England.
Paratype. NHMUK II.3100 [S. 3020a, b] (part and counterpart), Jarzembowski coll.
Additional material. NHMUK II.1856 (1) (part and counterpart), Upper Weald Clay; Barremian; Smokejacks Brickworks, Surrey, England. Coll. 12/06/1996. Incomplete tegmen, tentatively placed in this species.
Etymology. After the late Chris Wood, Cretaceous stratigrapher and journal editor.
Diagnosis. As for genus.
Description. Holotype incomplete, left tegmen with punctate ornament, maximum tegminal length 5.7 mm, tegminal width 2.1 mm (as preserved). Cuticle unpatterned, brown coloured. Anterior margin curved, posterior margin straight. Veins simple except M; Cu and M approaching anteriorly. 2A prominent, slightly flexed anteriorly. 1A less strongly developed, slightly sinuous. Rs submarginal. Veins Rs–1A curved apically at their distal ends.
Family Protodiplatyidae Martynov, Reference Martynov1925
Brevicula Whalley, Reference Whalley1985
Type species. Brevicula gradus Whalley, Reference Whalley1985.
Emended diagnosis. Punctate tegmina without venation, reaching third abdominal segment. Posterior margin straight to slightly convex and the anterior margin emarginate. Coxae short, rounded. Ovipositor present in females.
Brevicula gradus Whalley, Reference Whalley1985 (Figs 11, 12)
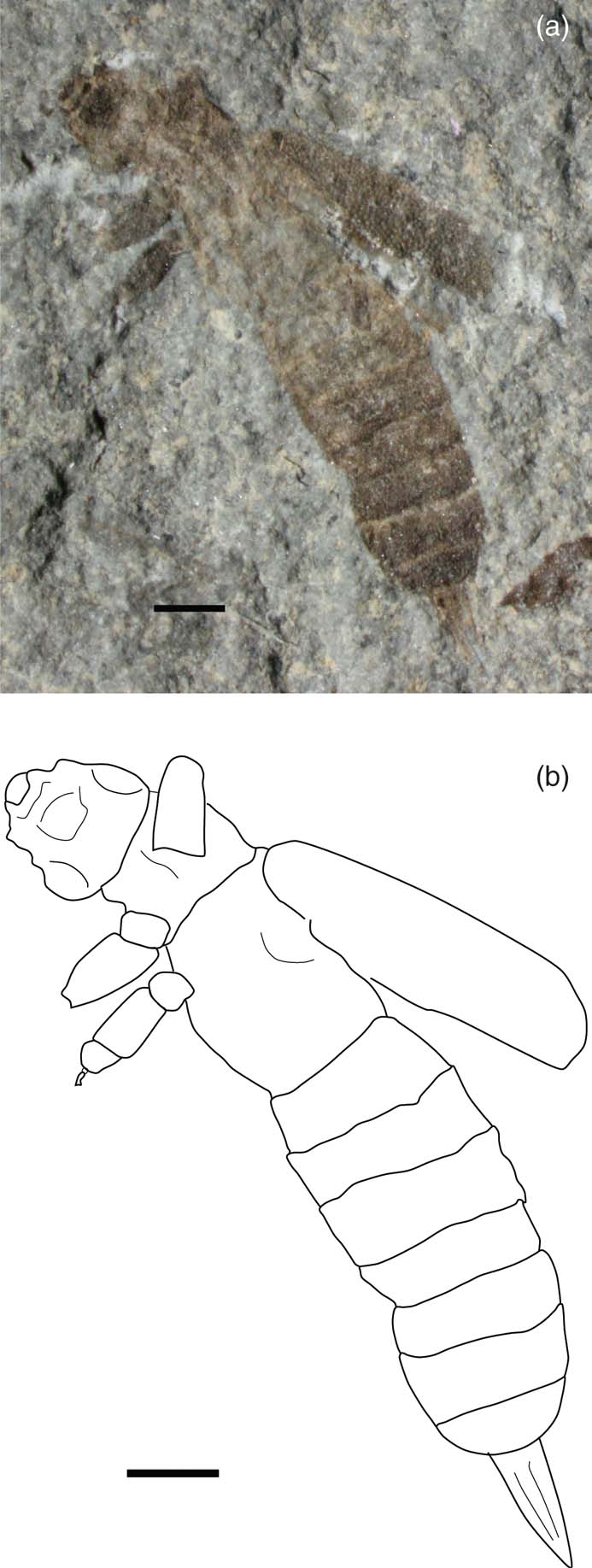
Figure 11 Brevicula gradus Whalley, Reference Whalley1985, holotype, NHMUK In.53993, Charmouth Mudstone Formation (Sinemurian), Black Ven, Dorset, England: (a) photo; (b) line drawing. Scale bars = 1 mm.
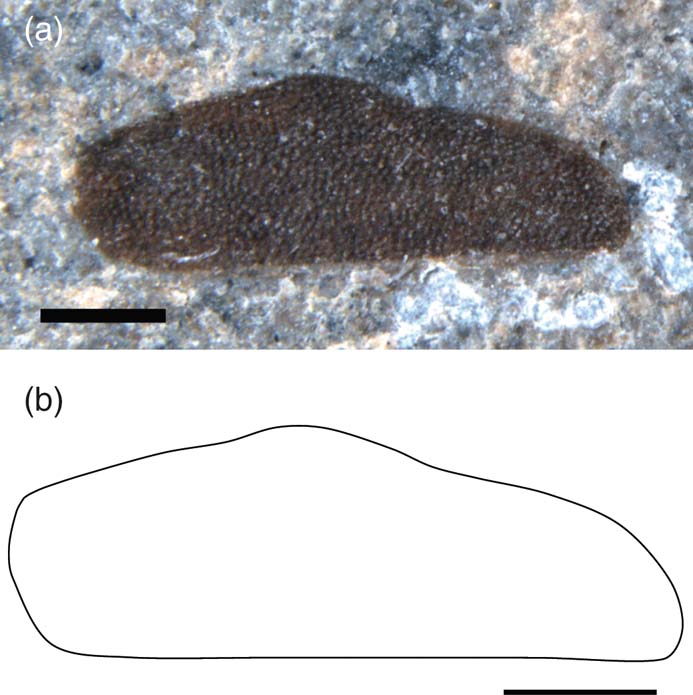
Figure 12 B. gradus, isolated tegmen, NHMUK II.3087, Charmouth Mudstone Formation (Sinemurian), Monmouth Beach, Dorset, England: (a) photo; (b) line drawing. Scale bars = 1 mm.
Brevicula gradus Whalley Reference Whalley1985, pp. 116–17, figs 3–4.
Brevicula gradus Whalley; Ross & Jarzembowski Reference Jarzembowki, Swift and Martill1993, p. 376.
Brevicula gradus Whalley; Zhang Reference Zhang2002, p. 355.
Brevicula gradus Whalley; Ross Reference Ross, Lord and Davis2010, p. 278, pl. 49, fig. 1.
Brevicula gradus Whalley; Jarzembowski & Palmer Reference Jarzembowski, Palmer, Jarzembowski, Siveter, Palmer and Selden2010, p. 174, fig. 4.25.
Brevicula gradus Whalley; Nel et al. 2012, p. 191.
Holotype. NHMUK In.53993 (part and counterpart), female with ovipositor, Jackson coll., Flatstones, Black Ven Marls; Sinemurian; Black Ven, Dorset.
Paratype. NHMUK In.51036 (part and counterpart), Jackson coll. Female with ovipositor from Woodstones of Black Ven (Sinemurian).
Additional material. NHMUK II.2181, Sole coll. Female with ovipositor from Woodstones of Black Ven (Sinemurian). NHMUK II.3087, II.3088, both Coram coll., Monmouth Beach (Sinemurian).
Emended diagnosis. As for genus, with tegminal posterior margin straight.
Description. (Emended from Whalley Reference Whalley1985). Length (tip of head to tip of ovipositor) 10–12 mm, width of abdomen 2–2.2 mm. Head prognathous; only base of filiform antennae preserved; prothorax rounded; tegmina strongly punctate, elongate; probably reaching third abdominal segment; abdomen parallel sided, with at least six visible segments, terminal segment rounded; legs cursorial, slender, femora slightly thickened, tibia thin, four or five segmented tarsi. Pointed ovipositor in females. Cerci not visible, probably not preserved, which may indicate they were fine and filiform.
Remarks. Whalley (Reference Whalley1985) considered this taxon possessed two slender forceps; however, this structure does not appear to be divided and is much more likely to be an ovipositor, as suggested by Nel et al. (2012). Although originally described as a dermapteran, Zhang (Reference Zhang2002) considered this taxon could “bear more primitive features” than those of other fossil Dermaptera, and considered it could be a staphylinid instead. The presence of the ovipositor of B. gradus indicates it is certainly a dermapteran. The presence of older Dermaptera (Phanerogramma) indicates that they had certainly appeared by the late Triassic, and more derived forms with no venation could have evolved by the Sinemurian. The presence of an ovipositor and the absence of distinct forceps indicates that this taxon probably only had primitive, simple, filiform cerci (not preserved), typical of the family Protodiplatyidae, such as in Longicerciata mesozoica Zhang, Reference Zhang1994 and Sinoprotodiplatys zhangi Nel et al., 2012.
Brevicula maculata sp. nov. (Fig. 13)
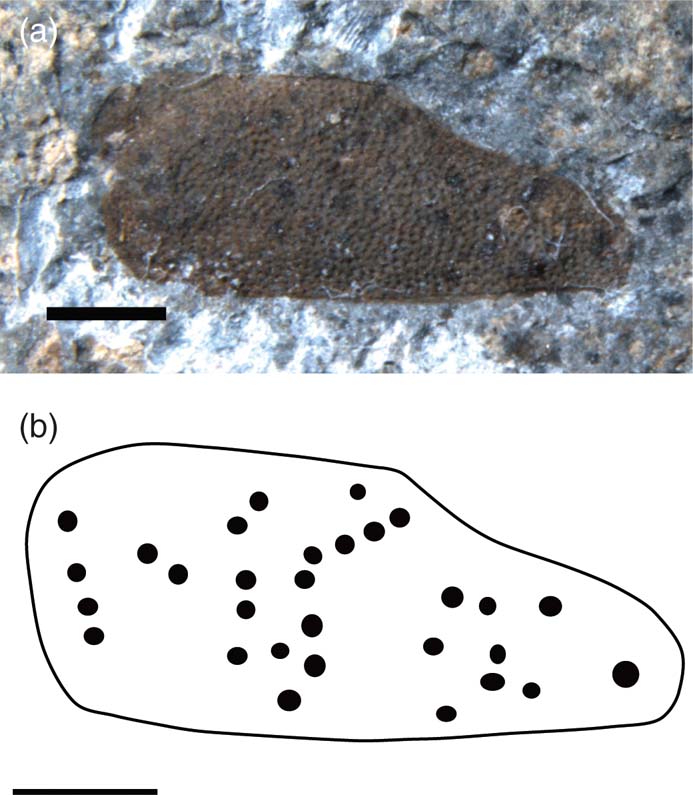
Figure 13 Brevicula maculata sp. nov., holotype, NHMUK II.3086, Charmouth Mudstone Formation (Sinemurian), Monmouth Beach, Dorset, England: (a) photo; (b) line drawing. Scale bars = 1 mm.
Holotype. NHMUK II.3086, Coram coll., Caenisites turneri Zone, Sinemurian, Charmouth Mudstone Formation, Monmouth Beach, Dorset.
Etymology. After the presence of spots on the tegmina.
Diagnosis. Tegmina as for genus, with slightly convex posterior margin. Anterior margin straight to just over half of tegminal length then sigmoidally curved to blunt apex. Venation absent; distinct spots present.
Description. Isolated tegmen, length 4.5 mm, width 1.5 mm. Many dark spots visible over tegminal surface.
Remarks. Holotype donated to the NHM by Rob Coram for the purpose of this study. This is the only known fossil earwig specimen with spots.
Dermaptera incertae sedis
Remarks. There is one more isolated tegmen known from the Mesozoic of England. It consists of a faint impression, but certainly appears to be different from other fossil taxa; however, in the absence of more characters, it cannot be placed in any family.
Trivenapteron gen. nov.
Type species. Trivenapteron moorei gen. et sp. nov.
Diagnosis. Differs from other fossil dermapteran tegmina in that it has three prominent, diverging veins (probably R, M and Cu), plus a simple A vein.
Etymology. Latin for three veins and wing, gender masculine.
Trivenapteron moorei gen. et sp. nov. (Fig. 14)
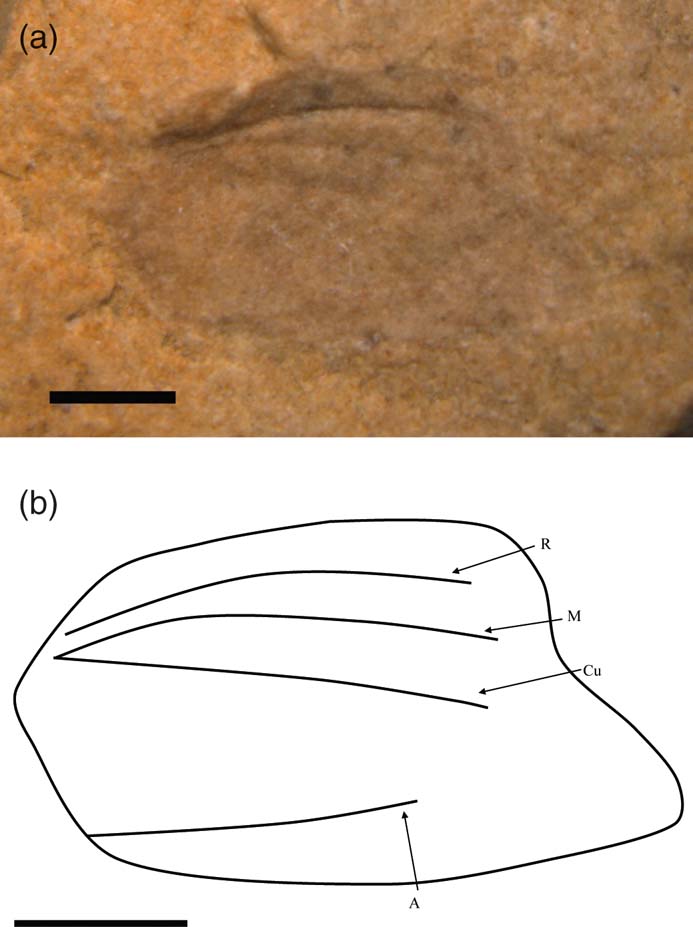
Figure 14 Trivenapteron moorei gen. et sp. nov., holotype, TTNCM 489, Upper Lias (Toarcian), Ilminster, Somerset, England: (a) photo; (b) line drawing. Scale bars = 1 mm.
Earwig tegmen, Williams et al. Reference Williams, Benton and Ross2015, p. 686.
Holotype. TTNCM 489 Moore coll., Upper Lias; Toarcian; Strawberry Bank, Ilminster, England.
Etymology. After Charles Moore.
Diagnosis. As for genus.
Description. Tegminal length 3.9 mm, tegminal width 2.3 mm. Sub-quadrate, truncate tegmen, pointed apically and without ornament. Three diverging veins, with M and Cu having a common origin, plus a simple A vein running near to the posterior margin.
4. General discussion
This study provides a taxonomy of earwig specimens primarily from UK collections, based on isolated tegmina. Phanerogramma is the oldest named dermapteran genus, with P. australis and P. dunstani the oldest known described species (Carnian).
Previously, the range of the Dermapteridae was within the Jurassic: Callovian to Oxfordian (Nicholson et al. Reference Nicholson, Mayhew and Ross2015); however, if Phanerogramma, Dimapteron and Valdopteron do belong to this family, then the range is extended from the Triassic: Carnian to Cretaceous: Barremian.
There are vague similarities in the appearance of the tegmina described here and the tegmina of a new proposed order of insects, Alienoptera, described from Cretaceous amber of Myanmar (Bai et al. Reference Bai, Beutel, Klass, Yang and Wipfler2016). When attached to a whole specimen, the differences are clear, but there may be some confusion if isolated tegmina are found. There is, as yet, only one specimen of this order known, so current knowledge is limited. There are some key differences that distinguish the orders. Although Bai et al. (Reference Bai, Beutel, Klass, Yang and Wipfler2016) do not give any specific measurements, the tegmina appear much smaller than those described herein (∼2 mm in length). Additionally, these tegmina are more equilaterally sided, appearing more like a triangle, whereas those of earwigs are more elongate. It is not entirely clear in the images provided, but the tegmina do not appear to be ornamented or have venation, and although there are specimens of earwigs without venation, they usually have some form of ornamentation. Therefore, isolated tegmina which are more elongate and which possess ornamentation and/or venation, and especially paired tegmina which evidently come together at the posterior margin, should still be referred to the Dermaptera. Also, the clavus of some heteropterans can look superficially similar to an earwig tegmen when isolated. They are much more elongate than those found on the Alienoptera specimen and more triangular than those found on earwigs.
Today, many earwigs are ground dwelling, living most of their lives in leaf litter, and have low dispersion rates compared with other arthropods (Moerkens et al. Reference Moerkens, Leirs, Peusens and Gobin2010). It is therefore interesting to find the same genus in both the UK and Australia. Of course, this assumes that the body morphology of the English and Australian species is similar, but this morphology is not known – hopefully, complete specimens will be found one day.
In the late Triassic, the supercontinent of Pangaea started to break up, though at that time Laurasia and Gondwana were still connected, so Phanerogramma could have had a wide distribution. Some modern taxa do have extensive natural ranges, even though they do not distribute readily. For example, Labidura is found in Africa, East Asia, East Indies, Australia, South America and southern Europe today (Popham Reference Popham2000). Even some species have wide distributions, for example, Forficula auricularia is found throughout Europe, North Africa and West Asia without human intervention (Crumb et al. Reference Crumb, Eide and Bonn1941). Popham (Reference Popham2000) traced the origin of forficuline earwigs to the area which now comprises northeastern South America and northwestern Africa, using current species distribution and continental drift theory, and proposed several distribution routes. In the Late Triassic, South America and Africa lay in between Europe and Australia. Interestingly, Riek (Reference Riek1974) noted the similarity between Late Triassic fossil insect genera of South Africa and Australia, demonstrating that other taxa had wide distributions at that time. It is likely that more Dermaptera tegmina, perhaps related to Phanerogramma, are in fossil insect collections elsewhere, but have not been recognised, so they may yet turn up in collections from South America and Africa.
5. Palaeobiogeography of the British earwigs
During the Rhaetian, much of Britain was above sea level, with the North British landmass reaching south to Birmingham, the Welsh massif covering most of Wales, and the Anglo-Brabant massif reaching out towards Swindon in the west and Luton in the north (Poole Reference Poole1979; Benton et al. Reference Benton, Cook and Turner2002; Fischer et al. Reference Fischer, Voigt, Franz, Schneider, Joachimski, Tichomirowa, Götze and Furrer2012), as shown in Figure 15a. Insects were deposited in the Rhaetian sea, under which lay the English Midlands. This sea was often isolated, with marine incursions resulting in fluctuating salinities, and was a generally hostile environment that supported an impoverished fauna (Swift Reference Swift1995). Insects collected from deposits of this age may have come from any of these landmasses and it is likely that populations were spread across the islands. Some of the insects are complete and probably drowned in the sea, although many specimens are fragmentary and were probably washed into the basin. Insects collected from the marine Hettangian deposits of the Midlands could have come from the Welsh massif, as assumed by Whalley (Reference Whalley1985), or more likely from the Anglo–Brabant massif which extended towards the Severn Basin. By this time, the Welsh massif had receded to the west, due to marine transgression, and the North British landmass became a Scottish landmass, reaching just north of Newcastle (Bradshaw et al. Reference Bradshaw, Cope, Cripps, Donovan, Howarth, Rawson, West and Wimbledon1992; Simms Reference Simms, Simms, Chidlaw, Page and Morton2004), as shown in Figure 15b. Hettangian-age insect deposits are relatively few compared to Rhaetian insect deposits, and are mostly only found in Warwickshire. It is interesting that only this relatively small area has produced insects, especially given the effort put into the collection of fossil insects in this part of the country. Perhaps the water currents of the time concentrated insect debris in the Binton–Grafton–Copt Heath area and deposited them there, or perhaps only in that area were conditions right for the preservation of fossil insects. Insects collected from the Sinemurian deposits of Dorset probably lived on the small Cornubian island which now makes up Devon and Cornwall.

Figure 15 Palaeogeographic maps of the United Kingdom: (a) Rhaetian, modified from Fischer et al. (Reference Fischer, Voigt, Franz, Schneider, Joachimski, Tichomirowa, Götze and Furrer2012) and Benton et al. (Reference Benton, Cook and Turner2002); (b) Hettangian, modified from Simms (Reference Simms, Simms, Chidlaw, Page and Morton2004); (c) Toarcian, modified from Arias (Reference Arias2007) and Stumpf (Reference Stumpf2016). Coloured dots represent insect-bearing localities for each stage.
In the Late Triassic, Phanerogramma was probably more prevalent on the North British landmass, with smaller populations on the Welsh massif and the Anglo–Brabant massif. During the Hettangian, the receding of the North British landmass to the north, coupled with a dearth of fossil insects collected in the north of England, could explain the apparent decrease in number of Phanerogramma specimens from the Hettangian as compared with the Rhaetian.
During the Pliensbachian to Toarcian, it seems that the Welsh massif was larger, extending towards the Midlands, and the Anglo–Brabant massif receded to the east (Arias Reference Arias2007), as shown in Figure 1c, meaning that insects from this period (including Trivenapteron moorei from Ilminster, Somerset) are more likely to have come from the Welsh massif.
6. Acknowledgements
Special thanks to Michael Engel from the University of Kansas, USA, for help and advice with the initial stages of the project, and to Mike Benton, the lead author's PhD supervisor at the University of Bristol, for advice and support throughout the project. Many thanks to Claire Mellish for access to the specimens in the NHM collection; to Rob Coram for donating specimens from his private collection for study and for assistance in the field; to Mark Florence for supplying a photograph of the Smithsonian specimen of P. heeri; to Matt Williams and Dennis Parsons for access to the Charles Moore Collection at the Somerset Heritage Centre; to Eliza Howlett for access to the Hope Collection at the Oxford University Museum of Natural History; and to Terry Keenan and Pete Austen for Wealden photos. Also thanks to the Natural Environment Research Council (NERC) for funding the lead author's PhD project (studentship number: NE/L002434/1), from which came this research. EAJ acknowledges the Chinese Academy of Sciences President's International Fellowship Initiative (2011T2Z04). This is a Leverhulme Emeritus fellowship contribution for EAJ.
Maps were adapted from d-maps.com:
Figure 1: http://www.d-maps.com/carte.php?num_car=5596&lang=en;
Figure 12: http://www.d-maps.com/carte.php?num_car=2554&lang=en.

















