The emergence of the novel H1N1 influenza virus in the spring of 2009 forced hospitals to accelerate preparations for a potential pandemic and the accompanying surge of ill patients requiring emergency department and hospital care.1 As national, state, and local institutions developed potential estimates of the number of casualties that could arise from a pandemic, it became clear that a shortfall of critical hospital resources for both adults and children was likely.Reference Christian, Devereaux, Dichter, Geiling and Rubinson2Reference Hick and O’Laughlin3 Given this potential shortage of critical care resources, triage plans were developed to prioritize patient admissions to critical care units.
The goal of critical care triage is to provide resources to patients most likely to benefit from them. Triage plans must determine who is too ill to benefit from intensive therapies as well as those likely to survive without critical care. One triage tool, the sequential organ failure assessment (SOFA) score, was incorporated into many adult critical care triage plans.Reference Vincent, Moreno and Takala4Reference Devereaux, Dichter and Christian5Reference Christian, Hawryluck and Wax6 The SOFA score, which relies on six elements of physiologic data, was designed to assess organ function and failure in septic patients and has been validated in adult critical care patients to predict outcome and mortality.Reference Ferreira, Bota, Bross, Mélot and Vincent7 Use of the SOFA to predict mortality made it an attractive method to triage patients out of the ICU in a resource-scarce scenario. Subsequently, the SOFA score was simplified to five variables, including one easily obtainable laboratory value (modified-SOFA, M-SOFA).Reference Grissom, Brown and Kuttler8 The M-SOFA has been suggested for use both as an admission tool for adult patients into a critical care unit and as a tool to allocate daily critical care resources.9
Pediatric critical care resources are also likely to be stressed during a pandemic, as children are predicted to suffer higher infection rates compared to adults.10 As no validated critical care triage tools currently exist for children, planners considered incorporating the M-SOFA for triage into the pediatric intensive care unit (PICU).
Triage of patients into the PICU has both practical and ethical difficulties. Practically, pediatric patients have an overall lower mortality rate than adult critical care patients, making it more difficult to decide who is unlikely to benefit from critical care. In addition, the prediction of death among PICU patients is less certain than in adults, making decisions to limit support for patients no longer expected to benefit from critical care problematic. Ethically, triaging patients in a resource-scarce environment will require a dramatic change in the way pediatricians practice. Triage plans need to be accepted by the medical and general community as fair to preserve the relationship of trust that exists between physicians and patients.Reference Antommaria, Sweney and Poss11
Our objective in this study was to test the M-SOFA as a triage tool for admission to pediatric critical care units and to compare it to two other available pediatric severity of illness scores and to routine clinician judgment. We compared the pediatric M-SOFA, the Pediatric Early Warning System (PEWS) score, and the Pediatric Risk for Hospital Admission score II (PRISA-II) for their ability to discriminate between patients who received and those who did not receive a medical intervention in the PICU.Reference Duncan, Hutchison and Parshuram12Reference Chamberlain, Patel and Pollack13 We hypothesized that one of the three scores would reliably predict the need for a critical care intervention during the first 48 hours of hospitalization among a group of acutely ill hospitalized children.
METHODS
This retrospective cohort study assessed patients transported by a pediatric transport team to the sole tertiary pediatric hospital in Utah between January 1, 2006, and December 31, 2006, via ground or air. The transport team consisted of one to two flight nurses trained in neonatal or pediatric critical care as well as a pediatric respiratory therapist. The transport team discussed the management of children with the physician who directed the transport. The physician director for non-neonatal transports was either a pediatric emergency department or intensive care attending or fellow. The hospital was a regional referral center serving the children of Utah, Idaho, Wyoming, Nevada, and Montana. Children were identified from transport logs. To be included, children had to be aged from 1 day to younger than 19 years and admitted to the hospital. Infants admitted to the neonatal ICU were excluded.
The M-SOFA is a physiology-based score that assigns points for five variables, including the amount of oxygen required to maintain an oxygen saturation of greater than 90%; presence of jaundice; the level of support necessary to achieve a specified blood pressure; the patient's level of consciousness; and creatinine concentration.Reference Grissom, Brown and Kuttler8 The variables in the M-SOFA score were adjusted for pediatric patient age category norms and called the pediatric M-SOFAReference Kliegman, Behrman and Jenson14Reference Custer, Rau and Lee15 (Online Data Supplement 1).
The PEWS score was developed to identify hospitalized patients needing resuscitation from impending cardiopulmonary arrest.Reference Duncan, Hutchison and Parshuram12 Because this score is generally applied to children in an inpatient setting, some variables such as the number of consultants were less applicable to transported patients. (Online Data Supplement 2) The PRISA-II score was developed for use in the emergency department to aid in the prediction of needing hospital admission.Reference Chamberlain, Patel and Pollack13 (Online Data Supplement 3)
Trained medical personnel abstracted patient demographics and variables for the three triage tools from each patient's transport record and hospital medical chart. The earliest laboratory values obtained were used. If a laboratory value was not obtained within six hours of admission, it was assumed to be normal. Missing variables were assumed to be normal for age, as instructed by the scores. Every 10th chart was reviewed independently to ensure accuracy of data entry. Pediatric risk of mortality III (PRISM-III) scores, validated scores to predict mortality of children in the PICU, were obtained from an electronic database for patients admitted to the PICU.Reference Pollack, Patel and Ruttimann16 The initial admission disposition (PICU or ward) was decided routinely by physicians without support tools. Any subsequent transfers within 48 hours to the PICU were also examined for possible incorrect physician triage.
The primary outcome was the receipt of a PICU intervention within 48 hours of hospital admission. Qualifying interventions were those that could be provided safely only in an intensively monitored setting and included (1) acute dialysis, (2) treatment for hypotension unresponsive to 60 ml/kg of volume resuscitation, (3) invasive respiratory support exceeding continuous or bilevel positive airway pressure, and (4) monitoring of a depressed level of consciousness (Glasgow Coma Score [GCS] of <13).Reference Reilly, Simpson, Sprod and Thomas17 We excluded patients receiving noninvasive respiratory support, often currently provided in the PICU, as these interventions could likely be applied safely on the ward during resource scarcity. The secondary outcome was the relationship of each tool to the PRISM-III score among patients admitted to the PICU.
Patient demographics are described using summary statistics and reported as medians with interquartile ranges (IQR). Continuous data are compared using the Wilcoxon test for nonparametric data. Logistic regression was performed to calculate the area under the receiver operating curves (AUC) for each tool with 95% confidence intervals (CI). The positive and negative predictive values (PPV and NPV, respectively) of the best performing score and physician disposition for requiring an ICU intervention were calculated. The relationship of the triage tools to the PRISM-III was evaluated using linear regression. Significance was defined as P <. 05.
RESULTS
A total of 1365 children were transported to the hospital from January 1, 2006, to December 31, 2006. Of these children, 752 (55.1%) were admitted to either the general pediatric floor (n = 310, 22.7%) or PICU (n = 442, 32.4%) and constituted the study cohort (Table 1). The remaining patients were admitted to the neonatal ICU (n = 553, 40.5%), sent home (n = 24, 1.7%), died prior to admission (n = 7, 0.5%), were excluded due to age (n = 2, 0.1%), or were taken to a different hospital (n = 2, 0.1%). Twenty-five (1.8%) cases were excluded due to inability to link the transport records with hospital records accurately. Of the study cohort, 482 (64.1%) were transported to our emergency department before being triaged, while 270 (35.9%) were triaged to either the floor or PICU prior to arrival.
TABLE 1 Characteristics of Study Cohort Patients (N=752)
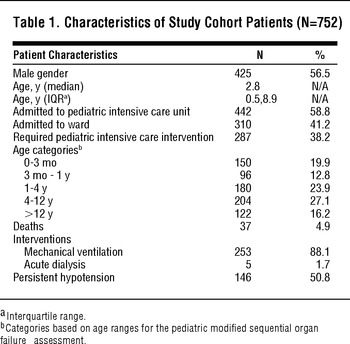
The median patient age was 2.8 years (interquartile range [IQR], 0.5-8.9), with slightly more male (56.5%) than female patients. Thirty-seven patients (4.9%) died during the hospitalization. Of the 442 patients initially admitted to the PICU, 280 (63.3%) received a PICU intervention. Eleven (3.5%) of the 310 patients admitted to the ward were transferred to the PICU in the first 48 hours of hospitalization, 7 of whom received a PICU intervention and were considered incorrectly triaged. Thus, a total of 287 (38.2%) patients received at least one PICU intervention. Of this group, 253 (88.1%) underwent mechanical ventilation, 5 (1.7%) received acute dialysis, 146 (50.8%) had persistent hypotension, and 157 (54.7%) had a GCS less than 13.
All three tools had scores that were significantly different for patients who received a PICU intervention compared to those who did not (Table 2). The pediatric M-SOFA had the best discriminant ability (AUC 0.826; 95%CI: 0.794, 0.857), followed by the PRISA-II (AUC 0.812; 95%CI: 0.781,0.842), although these tools were not statistically different. The PEWS had the least discriminant ability (AUC 0.756; 95%CI: 0.721, 0.792). No tool had high correlation with PRISM-III scores: pediatric M-SOFA (R2 = 0.0018), PRISA-II (R2 = 0.0462), and PEWS (R2 = 0.0222).
TABLE 2 Scores of the Three Triage Tools with Receiver Operating Curve Characteristics
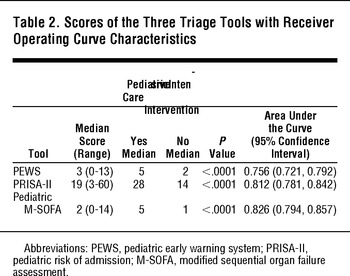
Figure 1 shows the ROC curve for the Pediatric M-SOFA with the sensitivity and specificity labeled for each potential cutoff point. The predictive value of the model varies with the chosen cutoff point. A cutoff point of 1 for the pediatric M-SOFA score, which maximizes sensitivity (93%) but has low specificity (34%), has a PPV of 47% and an NPV of 89%. Therefore, using a cutoff point of 1 would mean that more than one-half of the children admitted to the PICU would not need an ICU intervention, but few patients sent to the ward would require an intervention. In contrast, setting a cutoff point of 8 gives a low sensitivity (27%) and a higher specificity (99%), resulting in a PPV of 98% and an NPV of 69%. This would result in nearly one-third of patients admitted to the ward subsequently requiring transfer to the PICU. A cutoff point of 3, which maximizes both sensitivity (76%) and specificity (72%), resulted in a PPV of 64% and an NPV of 84%. Routine clinician triage to the ward or PICU achieved a sensitivity of 98% and a specificity of 65%. The PPV and NPV of physician triage were 63% and 98%, respectively.

Figure. Receiver Operating Curve for Pediatric Modified Sequential Organ Failure Assessment.
Receiver operating curve demonstrating the trade-off between sensitivity (SENS) and specificity (SPEC) for each pediatric modified sequential organ failure assessment score.
COMMENT
We compared three pediatric scores and physician judgment for their ability to predict the receipt of intensive care resources. To be useful for triage, a tool should have high positive and high negative predictive values. A low PPV may inappropriately triage low-risk patients to the ICU, resulting in lack of bed availability. A low NPV may put a child at risk by inappropriately triaging the patient to the ward. No tool exceeded both the PPV and NPV of routine clinician decisions.
The potential need to triage pediatric patients is a real one. The capacity of current pediatric critical care resources may be inadequate during a pandemic. Of the estimated 350 pediatric ICUs in the United States, more than 50% have fewer than eight beds, with most PICUs normally operating at a high census.Reference Randolph, Gonzales, Cortellini and Yeh18Reference Odetola, Clark, Freed, Bratton and Davis19 Currently, ill patients are frequently admitted to the PICU for monitoring and to prevent the need for an intervention; however, this may not be possible in a pandemic or disaster setting. Our results showed that approximately one-third of patients admitted to the PICU did not require a PICU intervention. This finding suggests that intensive monitoring of some patients outside of the PICU may be a way to safely increase bed capacity during a patient surge. Ventilators and personnel are thought to be the limiting critical care resources.Reference Hick, Rubinson and O’Laughlin20 Of note in our study, 88.1% of patients who received a PICU intervention received mechanical ventilation.
A triage tool is desirable in a resource-limited environment, as it ideally removes the individual clinician from making potentially contentious decisions, can be equally applied to all patients, and can be rapidly used in a time when capacity is overwhelmed. The ideal triage tool would be easy to apply from readily available clinical data and have both high sensitivity and high specificity to assign patients who require hospital admission to the pediatric ward or to the PICU. Our results showed that the PEWS score is fair in its ability to distinguish moderately ill patients from those who are critically ill. The PRISA-II and pediatric M-SOFA performed better than PEWS, but still discriminated inadequately among patients. The M-SOFA was attractive for its ease of use with five easily obtainable variables. However, when assessing its predictive value, it became clear that there was no cutoff point score at which the predictive values both placed children correctly into the PICU and relieved the burden on the PICU of unnecessary admissions.
Physician judgment outperformed all of the tools for pediatric patient triage; however, in this routine setting physicians prefer not to miss any child who might need an intervention (high sensitivity) contributing to the low PPV; thus, while only 7 of the 310 (2.2%) patients triaged to the ward by physicians subsequently required a PICU intervention, 36.7% who were admitted to the ICU did not receive an intervention. In a resource-scarce environment, when filling an ICU bed might involve the need to deny another patient access to ICU care, physicians would likely change their behavior to obtain higher specificity. However, clinician-driven triage could prove to be ethically challenging for medical providers. To be perceived as fair, triage decisions need to be consistent among physicians, which may be difficult.Reference Christian, Hamielec and Lazar21
The M-SOFA and SOFA have both been shown to correlate with mortality in adult patients and were proposed as tools to triage adult patients into the ICU as well as to palliative care or ongoing care on the hospital ward due to high risk of death.Reference Devereaux, Dichter and Christian5Reference Christian, Hawryluck and Wax6Reference Grissom, Brown and Kuttler89 Use of the pediatric M-SOFA would be inappropriate for deciding that a child could no longer benefit from ICU care, given its lack of correlation with a validated predictor of mortality, the PRISM-III.Reference Pollack, Patel and Ruttimann16 The overall low rate of mortality in pediatric ICUs compared to adult ICUs limits the use of mortality as a primary outcome in pediatric studies and would make decisions to triage to palliative care based on a physiologic scoring system problematic.Reference Macrae22
LIMITATIONS
This study has several limitations. First, none of the three tools tested was developed for triage into the PICU. The M-SOFA is an inherently problematic tool, as some variables are also outcomes. For example, a patient receiving a vasoactive medication is both a predictor that a patient needs the ICU and the intervention outcome. We chose to evaluate the M-SOFA because it was under active consideration for pediatric triage in some state plans.
Second, the population of patients transported to the hospital is not representative of the general emergency department population requiring hospital admission. The higher acuity of patients transported to a tertiary care center would tend to increase the prevalence of children requiring a PICU intervention, thus increasing the PPV and decreasing the NPV of the scores studied. Therefore, results of this analysis may be an overestimate of the predictive ability of the tested tools when applied to a less acutely ill sample of hospitalized patients. However, the studied cohort did yield a large range of patient illness severity, as well as patient age, and similar mortality rate to the overall PICU population.
During a pandemic, patient triage will be carried out by practitioners with varying levels of expertise in pediatrics. In our study, patient triage was ultimately decided by a board-certified pediatric emergency department or pediatric critical care physician. It is unclear if practitioners with less pediatric experience would also outperform these scoring tools. The ability to appropriately triage patients also depends on the information provided to the physician by the transport personnel. Our transport team nurses have specific training in pediatric and neonatal transports and a minimum of three years of prior nursing experience, and thus represent a very highly qualified transport team, which is not available in many locations.
Finally, our study had 162 patients admitted to the PICU who did not receive an intensive care intervention. An argument could be made that these children were appropriately triaged, and the care they received in the PICU allowed them to avoid receiving an intervention. Avoiding patient deterioration is a goal of PICU care and will make it very difficult to triage patients when resources are scarce. Indeed, in a very severe pandemic, only children requiring an intervention might be admitted to the hospital. This last point emphasizes the need for a rational approach to triage. Clinicians may differ on the benefit of avoiding interventions for some children that will cost the availability of resources for other children who need to receive an intervention. Balancing these competing demands is not in the clinical experience of most physicians in developed countries.
CONCLUSION
This study describes the ability of three different tools to triage children transported to a tertiary care center to the PICU or the ward. None of the three tools had all the characteristics of an ideal triage tool: a high PPV and high NPV with simplicity of use. Thus, none can be recommended for triage into the PICU. Even though a simple and accurate method of triaging pediatric patients is greatly needed, no scoring system is currently validated. At present, physician discretion appears to be the best method of pediatric patient triage to the PICU.
Funding/Support: This study was performed at Primary Children's Medical Center, Salt Lake City, Utah. Internal funds from the Division of Pediatric Critical Care at the University of Utah were used in support of this study.





