Introduction
The onset of schizophrenia during childhood (before 13 years of age) is considered rare (Driver et al., Reference Driver, Thomas, Gogtay and Rapoport2020) whereas the prevalence increases during adolescence (prior to 18 years of age) (Pedersen et al., Reference Pedersen, Mors, Bertelsen, Waltoft, Agerbo, McGrath, Mortensen and Eaton2014; Dalsgaard et al., Reference Dalsgaard, Thorsteinsson, Trabjerg, Schullehner, Plana-ripoll, Brikell, Wimberley, Thygesen, Madsen, Timmerman, Schendel, McGrath, Mortensen and Pedersen2020). Recently the incidence rate of early-onset schizophrenia (EOS), defined as onset before 18 years of age, has increased (Okkels et al., Reference Okkels, Vernal, Jensen, Mcgrath and Nielsen2012; Stenstrøm et al., Reference Stenstrøm, Christiansen, Dehlholm-Lambertsen, Nøhr-Jensen and Bilenberg2010), also among 15–19 years old adolescents (Amminger et al., Reference Amminger, Harris, Conus, Lambert, Elkins, Yuen and McGorry2006); however, this is not consistently observed (Hsu et al., Reference Hsu, Lee and Wang2019). The incidence of EOS has increased more in girls than in boys (Kühl et al., Reference Kühl, Laursen, Thorup and Nordentoft2016) but this was not observed in the Hsu et al. (Reference Hsu, Lee and Wang2019) study.
EOS is associated with greater premorbid language, social, and motor impairments than other early-onset psychoses (Hollis, Reference Hollis1995, Reference Hollis2003), and delayed speech milestones and premorbid impairments of reading, spelling, and adjustment have been observed in EOS (Vourdas et al., Reference Vourdas, Pipe, Corrigall and Frangou2003). Worse illness severity later in the course of EOS is associated with worse premorbid adjustment and worse positive and negative symptoms at baseline (Stentebjerg-Olesen et al., Reference Stentebjerg-Olesen, Pagsberg, Fink-Jensen, Correll and Jeppesen2016). Those with EOS show longer duration of untreated psychosis (DUP) (McGlashan, Reference McGlashan1999) than patients with adult onset schizophrenia (AOS) (Coulon et al., Reference Coulon, Godin, Bulzacka, Dubertret, Mallet, Fond, Brunel, Andrianarisoa, Anderson, Chereau, Denizot, Rey, Dorey, Lançon, Faget, Roux, Passerieux, Dubreucq, Leignier and Schürhoff2020; Stentebjerg-Olesen et al., Reference Stentebjerg-Olesen, Pagsberg, Fink-Jensen, Correll and Jeppesen2016). Shorter DUP is associated with greater improvement in schizophrenia symptoms during the first years of EOS (Stentebjerg-Olesen et al., Reference Stentebjerg-Olesen, Pagsberg, Fink-Jensen, Correll and Jeppesen2016) but DUP seems both independent of symptom severity several years after the onset of EOS (Coulon et al., Reference Coulon, Godin, Bulzacka, Dubertret, Mallet, Fond, Brunel, Andrianarisoa, Anderson, Chereau, Denizot, Rey, Dorey, Lançon, Faget, Roux, Passerieux, Dubreucq, Leignier and Schürhoff2020) and of global cognition within the first two years of EOS (Teigset et al., Reference Teigset, Mohn, Brunborg, Juuhl-Langseth, Holmén and Rund2018).
The functional prognosis of EOS is generally unfavorable (Lay et al., Reference Lay, Blanz, Hartmann and Schmidt2000) and heterogeneous, with 60.1 % of patients having poor, 24.5% having moderate, and 15.4% having good outcome (Clemmensen et al., Reference Clemmensen, Vernal and Steinhausen2012). Patients with EOS have more inpatient days than patients with AOS during the first two years but beyond they do not differ (Vernal et al., Reference Vernal, Boldsen, Lauritsen, Correll and Nielsen2020). Earlier age of onset of EOS associates with worse long-term outcome (Eggers and Bunk, Reference Eggers and Bunk1997; Fleischhaker et al., Reference Fleischhaker, Schulz, Tepper, Martin, Hennighausen and Remschmidt2005), although this is not consistently observed (Röpcke and Eggers, Reference Röpcke and Eggers2005; Xu et al., Reference Xu, Guo, Cao, Li, Mei, Ma, Tang, Ji, Yang and Liu2020). Similarly, an earlier age of onset of schizophrenia is associated with worse social/occupational functioning and global outcome (Immonen et al., Reference Immonen, Jääskeläinen, Korpela and Miettunen2017). Shorter DUP is associated with larger improvements in global functioning in EOS, and milder baseline negative symptoms is associated with better later social functioning (Stentebjerg-Olesen et al., Reference Stentebjerg-Olesen, Pagsberg, Fink-Jensen, Correll and Jeppesen2016).
Subjects with EOS show large impairments of intelligence and neurocognitive functions across several cognitive domains with most profound impairments in processing speed and intelligence (Nieto & Castellanos, Reference Nieto and Castellanos2011). These cognitive impairments seem in line with those of youth-onset schizophrenia (<19 years of age) (Rajji et al., Reference Rajji, Ismail and Mulsant2009). Recent studies of adolescents and adults with EOS confirm significant impairments of both executive functions (Han et al., Reference Han, Zhang, Ni, Zhu, Liu, Chen, Lin, Chen and Guan2019; Holmén et al., Reference Holmén, Juuhl-Langseth, Thormodsen, Ueland, Agartz, Sundet, Andreassen, Rund and Melle2012) and intelligence (Han et al., Reference Han, Zhang, Ni, Zhu, Liu, Chen, Lin, Chen and Guan2019). Nearly 39 % of adolescents with EOS have an IQ <1 standard deviation below the normative mean (Hooper et al., Reference Hooper, Giuliano, Youngstrom, Breiger, Sikich, Frazier, Findling, McClellan, Hamer, Vitiello and Lieberman2010). Adolescents with first-episode EOS also show significant attention and non-planning impulsivity traits (Jepsen et al., Reference Jepsen, Rydkjaer, Fagerlund, Pagsberg, Jespersen, Glenthoj and Oranje2018).
Verbal memory, executive functions, and real-life functioning improved in adolescents with EOS with cognitive remediation therapy (Puig et al., Reference Puig, Penadés, Baeza, De La Serna, Sánchez-Gistau, Bernardo and Castro-Fornieles2014). Mental flexibility can be improved with remediation despite a lack of effect on functioning in adolescents and young adults with EOS (onset <19 years of age); however cognitive improvement was associated with that of social functioning (Wykes et al., Reference Wykes, Newton, Landau, Rice, Thompson and Frangou2007). A relatively small trial observed no association between changes in cognitive functions and those of real-life functioning in adolescents with early-onset psychosis (Ueland & Rund, Reference Ueland and Rund2004).
Adolescents with EOS show impaired Theory of Mind (Bourgou et al., Reference Bourgou, Halayem, Amado, Triki, Bourdel, Franck, Krebs, Tabbane and Bouden2016; Pilowsky et al., Reference Pilowsky, Yirmiya, Arbelle and Mozes2000). Also, younger subjects with first-episode schizophrenia show impairments of emotional facial and prosodic communication recognition (Amminger et al., Reference Amminger, Schäfer, Klier, Schlögelhofer, Mossaheb, Thompson, Bechdolf, Allott, McGorry and Nelson2012a; Reference Amminger, Schafer, Papageorgiou, Klier, Schlogelhofer, Mossaheb, Werneck-Rohrer, Nelson and McGorry2012b). Further, adolescents with schizophrenia (<19 years of age) show lower specificity of facial emotion discrimination and faster identification of sadness (Seiferth et al., Reference Seiferth, Pauly, Kellermann, Shah, Ott, Herpertz-Dahlmann and Habel2009).
The impairments of real-life functioning in adolescents with EOS include domains of social interaction / communication skills, personal living skills, and community living skills (Cervellione et al., Reference Cervellione, Burdick, Cottone, Rhinewine and Kumra2007) and the overall level of real-world functioning (Cohen´s d = −1.8) (Puig et al., Reference Puig, Penadés, Baeza, Sánchez-Gistau, De la Serna, Fonrodona, Andrés-Perpiñá, Bernardo and Castro-Fornieles2012). Processing speed and executive functioning explained 25% of the variance of the concurrent overall real-life functioning in adolescents with EOS, whereas positive and negative symptoms were not significant associates (Puig et al., Reference Puig, Penadés, Baeza, Sánchez-Gistau, De la Serna, Fonrodona, Andrés-Perpiñá, Bernardo and Castro-Fornieles2012). In adolescents with EOS, verbal fluency associated with concurrent psychosocial functioning (Landrø & Ueland, Reference Landrø and Ueland2008). Further, overall adaptive functioning and its subdomains showed small to moderate, cross-sectional associations with intelligence, motor speed, working memory, attention, problem solving, inhibition, and social cognition in children and adolescents with EOS (Hooper et al., Reference Hooper, Giuliano, Youngstrom, Breiger, Sikich, Frazier, Findling, McClellan, Hamer, Vitiello and Lieberman2010). In line with this, attention / vigilance, working memory, and verbal memory, but not intelligence, were found to be associated with personal living skills and community living skills in adolescents with EOS both cross-sectionally and longitudinally (Cervellione et al., Reference Cervellione, Burdick, Cottone, Rhinewine and Kumra2007). Additionally, the functional impairments, for example in social functioning (Cohen´s d = −1.9), of adults earlier diagnosed with EOS were longitudinally associated with two or more neurocognitive functions, for example auditory working memory, verbal memory, and flexibility in their adolescence (Øie et al., Reference Glenne Øie, Ueland and Sundet2011). The observed cognitive associates to functioning in EOS seem in line with those in AOS where functional disability is associated with the severity of several neuro- and social cognitive deficits as well as negative symptoms (Fett et al., Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011; Mucci et al., Reference Mucci, Galderisi, Gibertoni, Rossi, Rocca, Bertolino, Aguglia, Amore, Bellomo, Biondi, Blasi, Brasso, Bucci, Carpiniello, Cuomo, Dell’Osso, Giordano, Marchesi, Monteleone and Maj2021). Negative symptoms, at least partially, mediate the relationship between cognition and functioning (Lipkovich et al., Reference Lipkovich, Deberdt, Csernansky, Sabbe, Keefe and Kollack-Walker2009; Ventura et al., Reference Ventura, Hellemann, Thames, Koellner and Nuechterlein2009).
Characterization of the associations between neuro- and social cognitive functions and adaptive functioning is relevant for identification of potential cognitive intervention targets (Gold, Reference Gold2004). The purpose of the current study was to identify neuro- and social cognitive functions associated with concurrent adaptive functioning in adolescents with EOS, because relatively few studies have focused on cognitive associates to functional outcome in this young clinical group with an immature cognitive development. Based on the above-mentioned literature, working memory, verbal memory, processing speed, and social cognition were hypothesized to be uniquely associated with adaptive functioning, and over and above the potentially mediating role of negative symptoms.
Material and methods
The two studies included in the current work were approved by the Ethical Committee of the Capital Region of Denmark (H-C-2008-076; H-6-2014-068). Informed consent was obtained from the legal holders of custody, and both studies were carried out in accordance with the Helsinki declaration.
Participants
To increase the EOS sample size, two studies were combined: The first study took place 2011−14 and the second 2015−17. All patients were recruited from in- and outpatient clinics at the Child and Adolescent Mental Health Center, Copenhagen. Controls were recruited from the same catchment area through an internet advertisement or a letter of invitation by means of the Danish Civil Registration System. The inclusion criteria of the first study for patients with psychosis included schizophrenia, other non-affective psychosis, and affective psychosis according to DSM-IV-TR (APA, 2000). However, the study recruited only patients with non-affective psychotic disorders. Consequently, affective psychoses were excluded in the second study, thus we only included patients with DSM-IV-TR diagnoses of schizophrenia and other non-affective psychosis. The first study also included patients with attention-deficit/hyperactivity disorder (ADHD) (DSM-IV-TR) as a secondary clinical group but these patients were not included in the current study, and the second study excluded patients with a primary diagnosis of ADHD. All other inclusion criteria for patients with psychosis were identical for the two studies: age between 12 and 17 years (both included), a score >4 on minimum one (or >3 on minimum two) of the following items of the Positive and Negative Syndrome Scale (PANSS) (Kay et al., Reference Kay, Fiszbein and Opler1987): Delusions (P1), Conceptual disorganization (P2), Hallucinatory behavior (P3), Grandiosity (P5), Suspiciousness/persecution (P6), or Unusual thought content (G9), and maximum 12 months cumulative psychopharmacological treatment. The inclusion criteria for healthy controls in the two studies were identical: no psychiatric disorders according to DSM-IV-TR, no ongoing medical treatment (except contraceptives), and no history of a psychotic disorder or ADHD in first-degree relatives. The exclusion criteria for all participants in both studies were: a history of significant head injury or neurological illness, alcohol or substance use disorder according to DSM-IV-TR/ICD-10, and hearing impairment (Jepsen et al., Reference Jepsen, Rydkjaer, Fagerlund, Pagsberg, Jespersen, Glenthoj and Oranje2018; Lemvigh et al., Reference Lemvigh, Jepsen, Fagerlund, Pagsberg, Glenthøj, Rydkjær and Oranje2020; Rydkjær et al., Reference Rydkjær, Møllegaard Jepsen, Pagsberg, Fagerlund, Glenthøj and Oranje2017).
Psychopathology
The diagnostic classification was established according to the DSM-IV-TR and ICD-10 (WHO, 1992) and based on the semi-structured Kiddie-Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (K-SADS-PL) (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci, Williamson and Ryan1997) in the patient group. If needed, supplementary information was gathered from medical records. The K-SADS-PL was also used to screen control participants to secure they were mentally healthy. The severity of positive and negative schizophrenia symptoms and general symptoms was rated in patients and controls using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., Reference Kay, Opler and Lindenmayer1988). Two modified PANSS syndrome scales were established to avoid conceptual overlap (statistical redundancy) between some negative and general symptoms and real-life and cognitive functioning. Three negative syndrome scale items relating, at face value, to cognitive or real-life functioning were excluded from the negative syndrome scale: Emotional withdrawal (N2), Passive/Apathetic withdrawal (N4), and Abstraction (N5) (Mohamed et al., Reference Mohamed, Rosenheck, Swartz, Stroup, Lieberman and Keefe2008; Perlick et al., Reference Perlick, Rosenheck, Kaczynski, Bingham and Collins2008). The modified PANSS Negative Syndrome Scale and the full PANSS Negative Syndrome Scale correlated highly in the EOS group (r = 0.924, p < 0.001). Similarly, the modified PANSS General Syndrome Scale excluded Motor Retardation (G7), Poor Attention (G11), and Active Social Avoidance (G16) and correlated highly with the full PANSS General Syndrome scale in the EOS group (r = 0.965 p < 0.001).
The severity of depressive symptoms was assessed using Hamilton Rating Scale for Depression (Hamilton, Reference Hamilton1960) to calculate HAM-D-6 (Bech et al., Reference Bech, Gram, Dein, Jacobsen, Vitger and Bolwig1975; Timmerby et al., Reference Timmerby, Andersen, Søndergaard, Østergaard and Bech2017). DUP was calculated as the time period from the estimated onset of psychosis to the first day of antipsychotic treatment or the first day of testing in the study. The clinical interviews and all psychopathological ratings were performed by a child and adolescent psychiatrist (JR) or a clinical child neuropsychologist (JRMJ); final diagnostic classifications and psychopathological ratings were based on consensus between them. All participants were medically examined to rule out somatic illnesses causing psychopathological symptoms.
Real-life adaptive functioning
Adaptive functioning was assessed using the Vineland Adaptive Behavior Scale, Second Edition (VABS-II) (Sparrow et al., Reference Sparrow, Cicchetti and Balla2011) administered as a semi-structured parental interview with clinician rating. The VABS-II covers three domains: Communication, Daily Living Skills, and Socialization included in an overall adaptive behavior composite score with an age-corrected normative mean of 100 (SD = 15). The VABS-II domains overlap with the major functional domains proposed relevant in Western culture (Harvey & Bellack, Reference Harvey and Bellack2009). The VABS has been widely used (Chatham et al., Reference Chatham, Taylor, Charman, Liogier D’ardhuy, Eule, Fedele, Hardan, Loth, Murtagh, del Valle Rubido, San Jose Caceres, Sevigny, Sikich, Snyder, Tillmann, Ventola, Walton-Bowen, Wang, Willgoss and Bolognani2018) and also in EOS (Hooper et al., Reference Hooper, Giuliano, Youngstrom, Breiger, Sikich, Frazier, Findling, McClellan, Hamer, Vitiello and Lieberman2010). We found very few missing data and thus performed mean imputation (Hemager et al., Reference Hemager, Plessen, Thorup, Christiani, Ellersgaard, Spang, Burton, Gregersen, Søndergaard, Greve, Gantriis, Poulsen, Seidman, Mors, Nordentoft and Jepsen2018). One subject with EOS missed one of the nine Vineland-II subscale scores and the rounded mean of the subject´s two other standardized subscale scores in that domain was imputed. The VABS-II interview was missing for two participants with EOS and here the mean total score of the remaining EOS group was imputed (the VABS-II total score revealed no significant sex or age effects). The interrater agreement on the VABS-II total score between JR and JRMJ was assessed with interclass correlation coefficient; N = 6 VABS-II interviews were administered by one of the two while the other was present and separately rated the parental responses. The average measures interclass correlation coefficient = 0.977, p = .001.
Cognitive functioning
Cognitive tests were selected from a comprehensive battery to assess the cognitive domains included in the Measurement and Treatment Research to Improve Cognition in Schizophrenia (Nuechterlein et al., Reference Nuechterlein, Green, Kern, Baade, Barch, Cohen, Essock, Fenton, Frese, Gold, Goldberg, Heaton, Keefe, Kraemer, Mesholam-Gately, Seidman, Stover, Weinberger, Young and Marder2008) plus a motor domain. Verbal memory, verbal working memory, motor speed, planning, fluency, and processing speed were assessed with subtests of the Brief Assessment of Cognition in Schizophrenia (BACS) (Keefe et al., Reference Keefe, Goldberg, Harvey, Gold, Poe and Coughenour2004). Spatial working memory, reaction time, and sustained attention were assessed with the Spatial Working Memory (total errors), Reaction Time (RTI) (mean simple- and five-choice reaction times), and the Rapid Visual Information Processing(A´) from the Cambridge Neuropsychological Test Automated Battery (Fray & Robbins, Reference Fray and Robbins1996; Sahakian & Owen, Reference Sahakian and Owen1992). Visual memory was assessed with the Rey Complex Figure Test and Recognition Trial (RCFT) (Meyers & Meyers, Reference Meyers and Meyers1995). Social cognition (social perception) was assessed with the Awareness of Social Inference Test – Part A2 Social Inference (minimal) (TASIT) (total correct) (McDonald et al., Reference McDonald, Flanagan, Rollins and Kinch2003, Reference McDonald, Bornhofen, Shum, Long, Saunders and Neulinger2006), Danish version (Bliksted et al., Reference Bliksted, Fagerlund, Weed, Frith and Videbech2014). Estimated intelligence was assessed using the Vocabulary, Similarities, Block Design, and Matrix Reasoning subtests from the age relevant Wechsler Intelligence Scale for Children, Fourth Edition (Wechsler, Reference Wechsler2003) or the Wechsler Adult Intelligence Scale, Third Edition (Wechsler, Reference Wechsler2002). One subject with EOS missed the RCFT and the means of the remaining EOS group were imputed. One HC subject missed the TASIT-2 test and the total mean of the remaining controls was imputed. The remaining participants had complete cognitive data sets. Composite scores were constructed (Frazier et al., Reference Frazier, Giuliano, Johnson, Yakutis, Youngstrom, Breiger, Sikich, Findling, McClellan, Hamer, Vitiello, Lieberman and Hooper2012), and for visual memory and reaction time the composite measures were created by averaging two highly correlated outcomes z-scores followed by restandardization (RCFT: Immediate and Delayed recall, r = 0.926, p < 0.001; RTI: mean Simple and mean Five-choice Reaction Time, r = 0.869, p < 0.001).
Statistical Analyses
Chi-square test, independent samples t-test, and Mann–Whitney test were used to compare the groups in terms of sociodemographic, educational, and clinical characteristics for Table 1. The distribution of all cognitive, psychopathological, and adaptive functioning data was evaluated and, when appropriate, logarithmic or square root transformed to approximate a normal distribution. All raw or transformed scores were converted to z-scores based on the mean and standard deviation of healthy controls. For all z-scores lower values designate worse function (for error scores and reaction time scores, z-scores equal the mean raw score of the healthy controls minus the observed raw score divided by the standard deviation of the healthy controls). Potential outliers were checked by visual inspection of histograms and box plots but for final identification an interquartile range outlier labeling rule using a k-value of 2.2 (Hoaglin & Iglewicz, Reference Hoaglin and Iglewicz1987) was applied to the standardized data and identified the following outliers, all within the EOS group: the RTI z-scores of three subjects, BACS Verbal memory z-score for one subject, BACS Token Motor z-score for one subject, BACS Tower of London z-score for four subjects, and Spatial Working Memory total error z-score of one subject which were truncated at each lower interquartile range cutoff. The between-group differences in adaptive and cognitive functioning scores were assessed using multivariate analyses of variance (MANOVA); if significant, this was followed by univariate analyses of variance (ANOVA). Effect size estimates were represented using Cohen´s d.
Table 1. Sociodemographic, educational, clinical, global functioning, and antipsychotic medication characteristics

a Number of years in school excluding preschool year; EOS: N = 57.
b Placement in special education class ever; EOS: N = 57.
c N = 27 participants with EOS and N = 31 healthy control participants were old enough to have taken the final school graduation examination. EOS: 21 of N = 27 passed; HC: 31 of N = 31 passed.
d Parental household income (if parents lived separately, the primary home for the participants was selected): high income > 500.000 DKK; 500.000 DKK < moderate income < 300.000 DKK; low income < 300.000 DKK.
e Tetrahydrocannabinol (THC) urine testing (Rapid response, Jepsen HealthCare); EOS: N = 56; HC: N = 71.
f Psychotic disorder Not Otherwise Specified.
g Comorbid Depressive Disorder Not Otherwise Specified.
h The modified PANSS negative syndrome scale excludes N2: Emotional withdrawal, N4: Passive/Apathetic withdrawal, and N5: Abstraction.
i The modified PANSS general syndrome scale excludes G7: Motor retardation, G11: Poor attention, and G16: Active social avoidance.
j The 6-item Hamilton Depression Rating Scale (HAM-D6) includes item 1 (depressed mood), item 2 (guilt feelings), item 7 (work and interests), item 8 (psychomotor retardation), item 10 (psychic anxiety), and item 13 (somatic symptoms, general) from the HAM-D17.
k The Children´s Global Assessment Scale (CGAS).
l Number of subjects in current treatment with antipsychotic medications at the time of the cognitive assessment. N = 1 patient with EOS began treatment after the psychophysiological assessment and before the cognitive assessments: N = 18: Aripiprazole; N = 1: Aripiprazole + Olanzapine; N = 1: Aripiprazole + Paliperidone; N = 1: Aripiprazole + Chlorprothixene; N = 1: Risperidone; N = 9: Quetiapine; N = 4: Quetiapine Prolong; N = 1: Aripiprazole + Quetiapine Prolong; N = 1: Olanzapine; N = 1: Ziprasidone; N = 1: Clozapine + Ziprasidone.
m Chlorpromazine equivalents (mg. per day) according to Gardner et al., (2010) Am. J. Psychiatry. Median: 300,00 and range: 40,00-700,00.
n Duration of untreated psychosis (DUP): median = 428 days; range: 17 – 1883 days.
o Comorbid disorders included: Depressive Disorder Not Otherwise Specified (N = 16), Attention-Deficit / Hyperactive Disorder, Combined Type or Predominantly Inattentive Type (N = 8), Obsessive-Compulsive Disorder (N = 6), Specific Phobia (N = 5), Eating Disorder Not Otherwise Specified (N = 4), Asperger´s Disorder (N = 3), Posttraumatic Stress Disorder (N = 2), Panic Disorder with Agoraphobia (N = 2), Panic Disorder without Agoraphobia (N = 2), Autistic Disorder (N = 1), Mild Mental Retardation (N = 1), Generalized Anxiety Disorder (N = 1), Oppositional Defiant Disorder (N = 1), Tourette´s Disorder (N = 1), Tic Disorder Not Otherwise Specified (N = 1), Chronic Motor or Vocal Tic Disorder (N = 1), Anorexia Nervosa (N = 1), Adjustment Disorder with Mixed Anxiety and Depressed Mood (N = 1), Agoraphobia Without History of Panic Disorder (N = 1), Adjustment Disorder Unspecified (N = 1).
The selection of demographic variables, cognitive functions, and illness characteristics to be included in the multiple regression models was based on the significance of their bivariate associations with the VABS-II total z-score (Puig et al., Reference Puig, Penadés, Baeza, Sánchez-Gistau, De la Serna, Fonrodona, Andrés-Perpiñá, Bernardo and Castro-Fornieles2012). We used Pearson´s product-moment correlations (illness characteristics and cognitive functions z-scores), independent samples t-test (gender), and ANOVA (household income). The primary analysis was a multiple regression analysis (Enter method) including significant demographic variables and neuro- and social cognitive functions z-scores as independent variables and VABS-II total z-score as the dependent variable. Because of the mediating role of negative symptoms between cognition and functioning, a second multiple regression analysis (Enter method) included the significant cognitive associates observed in the primary analysis plus the illness characteristics that were significantly associated to the VABS-II total z-score. The terms "predictor variable" and "explained variance" are used in a strictly statistical sense and do not imply causality. For the explorative comparisons of adaptive functioning z-scores between the EOS subgroup with comorbidity versus that without and between the EOS subgroup treated with antipsychotic medication versus the antipsychotic unmedicated subgroup, ANCOVA with age and gender as the covariates were performed; in the case that covariates were nonsignificant, ANOVA was then performed. Alpha level was set at 0.05 due to the specific hypothesis (Parellada et al., Reference Parellada, Fraguas, Bombín, Otero, Castro-Fornieles, Baeza, Gonzalez-Pinto, Graell, Soutullo, Paya and Arango2009; Perneger, Reference Perneger1998). All analyses were performed in SPSS, version 25.
Results
The demographic, educational, and psychopathological characteristics of the EOS and healthy control group are presented (Table 1). A relatively high percentage of the EOS group were females. Significantly fewer participants with EOS than healthy controls had been in regular education classes throughout their school years.
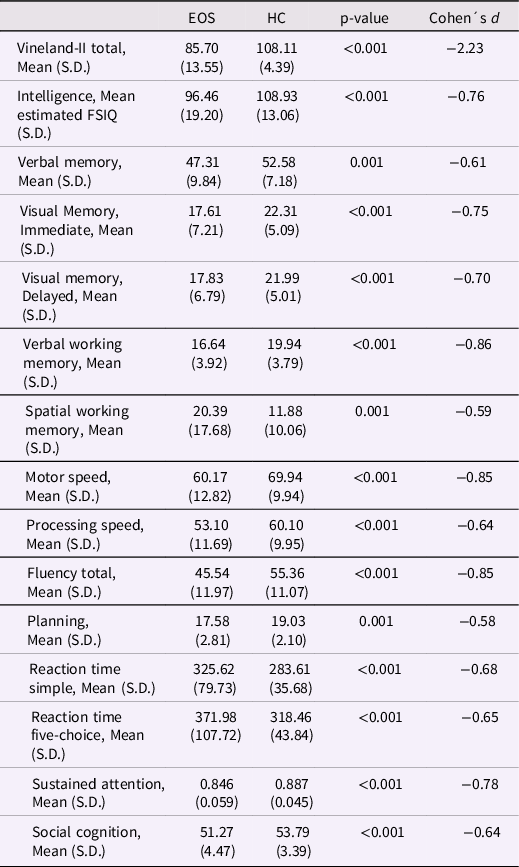
The mean VABS-II total scale score, estimated FSIQ, and neuro- and social cognitive functions raw scores in the groups are presented (Table 2). The MANOVA indicated an overall significant effect of group (F (15,115) = 13.55, p < 0.001; Wilks’ Lambda = 0.361). The following ANOVAs revealed significantly lower VABS-II total and all cognitive functions scores in the EOS than HC group; effect sizes ranged: −2.23 to −0.58.
Table 2. Mean real-life, adaptive functioning VABS-II total score, estimated FSIQ, neurocognitive, and social cognitive raw scores between the EOS and the healthy control group
In terms of bivariate associations within the EOS group, the parental household income, age, and gender were not associated with the VABS-II total z-score (Table 3). The social cognitive and four neurocognitive functions z-scores (visual memory, verbal working memory, reaction time, and processing speed), the modified PANSS Negative, and modified PANSS General z-score were significantly associated with the VABS-II total z-score.
Table 3. Associations between the real-life, adaptive functioning VABS-II total z-score and the psychopathological dimensions z-scores, cognitive functions z-scores, DUP, and demographic variables in the EOS group

a Very small VABS-II total z-score effect size between males (N = 13) and females (N = 46) (Cohen´s d = 0.08).
The primary multivariate regression analysis revealed verbal working memory z-score as the single unique associate of concurrent VABS-II total z-score in a model that included five cognitive functions, and this model explained 22.7 % of the variance of the VABS-II total z-score (Table 4). The second multiple regression analysis included the verbal working memory, modified PANSS Negative, and PANSS General z-scores as independent variables, and this model revealed only verbal working memory z-score and the modified PANSS General z-score as significant associates of the VABS-II total z-score; this model explained 34.3 % of its variance (Table 4). The bivariate correlations between cognitive functions, DUP, and psychopathological dimensions are shown in Table 5.
Table 4. Multiple regression analysis of associations between the selected cognitive functions z-scores and the real-life, adaptive functioning VABS-II total z-score as the dependent variable and a second multiple regression analysis including the observed significant cognitive associate and two psychopathological z-scores
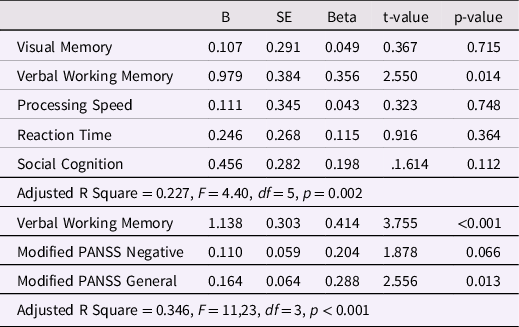
Table 5. Pearson´s product-moment correlation estimates between cognitive functions z-scores, duration of untreated psychosis, and psychopathological dimensions z-scores within the EOS group

*p < 0.05 ** p < 0.01 *** p < 0.001.
The following analyses are explorative. Reanalyzing the data including N = 57 subjects without imputation of the VABS-II total z-score did not change the results substantially (data not shown). An ANOVA revealed no significantly lower VABS-II total z-score in the EOS subsample currently treated with antipsychotic medications than the antipsychotic unmedicated subgroup (F (1,57) = 2.529, p = 0.117) (Cohen´s d = 0.43). An ANOVA comparing the EOS subgroup with any comorbidity versus the EOS subgroup without showed comorbidity to be associated with a significantly lower mean VABS-II total z-score (F (1,57) = 4.525, p = 0.038) (Cohen´s d = 0.61). Any comorbidity versus no comorbidity was thus exploratively added as a predictor variable in addition to verbal working memory, modified PANSS Negative, and modified PANSS General z-scores in a multiple regression analysis with the VABS-II total z-score as the dependent variable. Comorbidity was not a significant predictor (p = 0.426) in this model, and the results did not change substantially (data not shown). For information, age did not correlate significantly with any cognitive function (0.178 < ps < .875) except spatial working memory (r = 0.339, p = .009) or with any PANSS dimension (0.310 < ps < 0.773). The chlorpromazine equivalent was not significantly associated with adaptive functioning, and with regard to cognitive functions it was only significantly associated with reaction time and planning. The chlorpromazine equivalent was included as an additional predictor in the primary and second multiple regression analysis with the VABS-II total z-score as the dependent variable. The chlorpromazine equivalent was a nonsignificant predictor in both models and the reported results did not change substantially (data not shown).
Discussion
This cross-sectional study aimed to identify cognitive functions associated with adaptive functioning in adolescents with EOS. Adolescents with EOS showed a large adaptive functioning deficit and a generalized profile of moderate to large neuro- and social cognitive deficits. Visual memory, verbal working memory, processing speed, reaction time, and social cognition were associated with their concurrent adaptive functioning, when assessed in a bivariate perspective. Contrary to our hypothesis, only verbal working memory emerged as being uniquely associated with adaptive functioning in the multivariate analysis and explained 22.7 % of the variance of adaptive functioning. Secondarily, verbal working memory remained uniquely associated with adaptive functioning in the context of modified negative and general symptoms dimensions and only the general symptoms dimension associated with adaptive functioning, and this model explained 34.6 % of its variance.
The generalized impairments of neurocognition in our EOS group is generally consistent with earlier meta-analytic findings (Nieto & Castellanos, Reference Nieto and Castellanos2011) and a recent report (Smelror et al., Reference Smelror, Johannessen, Wedervang-Resell, Jørgensen, Barth, Andreou, Ueland, Andreassen, Myhre, Rund Børn and Agartz2021). The observed social cognitive impairment is in line with earlier findings in EOS (Bourgou et al., Reference Bourgou, Halayem, Amado, Triki, Bourdel, Franck, Krebs, Tabbane and Bouden2016; Pilowsky et al., Reference Pilowsky, Yirmiya, Arbelle and Mozes2000).
The level of adaptive functioning observed in our EOS group is relatively similar to earlier findings in EOS (Cervellione et al., Reference Cervellione, Burdick, Cottone, Rhinewine and Kumra2007) and its large effect size is in line with an earlier result in EOS (Cohen´s d = 1.80) (Puig et al., Reference Puig, Penadés, Baeza, Sánchez-Gistau, De la Serna, Fonrodona, Andrés-Perpiñá, Bernardo and Castro-Fornieles2012).
In a bivariate perspective relatively few cognitive functions were associated with the adaptive functioning of the current EOS group compared to previous results in EOS where the majority of cognitive functions were cross-sectionally associated with real-world living skills (Hooper et al., Reference Hooper, Giuliano, Youngstrom, Breiger, Sikich, Frazier, Findling, McClellan, Hamer, Vitiello and Lieberman2010; Puig et al., Reference Puig, Penadés, Baeza, Sánchez-Gistau, De la Serna, Fonrodona, Andrés-Perpiñá, Bernardo and Castro-Fornieles2012). One possible explanation may be the larger sample size in the Hooper et al. (Reference Hooper, Giuliano, Youngstrom, Breiger, Sikich, Frazier, Findling, McClellan, Hamer, Vitiello and Lieberman2010) study. Another may be the aggregation of scores from different tests in creation of predictors in that study which was also the case for two cognitive variables in the Puig et al. (Reference Puig, Penadés, Baeza, Sánchez-Gistau, De la Serna, Fonrodona, Andrés-Perpiñá, Bernardo and Castro-Fornieles2012) study. Further, our early recruitment strategy may have led to more instability in both adaptive functioning, psychotic symptoms, and psychopharmacological treatment than in the stabilized patients with EOS in the Puig et al. (Reference Puig, Penadés, Baeza, Sánchez-Gistau, De la Serna, Fonrodona, Andrés-Perpiñá, Bernardo and Castro-Fornieles2012) study.
Neither in the current nor in the Puig et al. (Reference Puig, Penadés, Baeza, Sánchez-Gistau, De la Serna, Fonrodona, Andrés-Perpiñá, Bernardo and Castro-Fornieles2012) study were attention and intelligence associated with adaptive functioning; however, processing speed, verbal working memory, and visual memory were associates in both studies. In the Hooper et al. (Reference Hooper, Giuliano, Youngstrom, Breiger, Sikich, Frazier, Findling, McClellan, Hamer, Vitiello and Lieberman2010) study, working memory was also associated with adaptive functioning in children and adolescents with EOS, as were attention and intelligence. In the Cervellione et al. (Reference Cervellione, Burdick, Cottone, Rhinewine and Kumra2007) study, working memory as well as verbal memory and attention were also cross-sectionally associated with adaptive functioning in adolescents with EOS. The currently observed pattern of weak to moderate bivariate associations between cognitive and adaptive functioning seems in line with earlier findings in EOS (Cervellione et al., Reference Cervellione, Burdick, Cottone, Rhinewine and Kumra2007; Hooper et al., Reference Hooper, Giuliano, Youngstrom, Breiger, Sikich, Frazier, Findling, McClellan, Hamer, Vitiello and Lieberman2010; Puig et al., Reference Puig, Penadés, Baeza, Sánchez-Gistau, De la Serna, Fonrodona, Andrés-Perpiñá, Bernardo and Castro-Fornieles2012).
Our finding of verbal working memory as a unique associate of adaptive functioning of adolescents with EOS seems in line with meta-analytic findings in AOS where immediate verbal memory shows a pooled estimated r of 0.40 to functional outcome (Green et al., Reference Green, Kern, Braff and Mintz2000). Verbal working memory correlates with community functioning (r = 0.22) and with social problem solving (r = 0.25) (Fett et al., Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011). Additionally, only working memory differed between high- and low-functioning adults with AOS (Alden et al., Reference Alden, Cobia, Reilly and Smith2015). Finally, working memory improvements were found to be associated with functional improvements in a cognitive treatment trial (Rispaud et al., Reference Rispaud, Rose and Kurtz2016). Working memory allows for storage and manipulation of the information that is necessary for complex cognitive tasks such as comprehension and reasoning. This may explain why better verbal working memory associates with better adaptive functioning and why the other cognitive functions included in this study were significantly and positively associated with verbal working memory.
Contrary to our significant bivariate association between social cognition and adaptive functioning, the multivariate analysis did not confirm social cognition as a unique associate. This contrasts with results in adults with AOS, where aspects of social cognition are more strongly associated with community function than neurocognition (Fett et al., Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011). In our sample, verbal working memory shared variance with social cognition which may have caused it not to reach statistical significance. This may suggest our measure of social cognition acts as a mediator between neurocognitive functioning and adaptive functioning (Schmidt et al., Reference Schmidt, Mueller and Roder2011). Similarly, the shared variance between verbal working memory and processing speed, reaction time, and visual memory may have led them not to reach statistical significance in the multivariate perspective. This pattern of findings questions an unequivocal interpretation of the significant bivariate correlations between these neuro- and social cognitive functions and adaptive functioning because their correlations with verbal working memory suggest that executive functioning was involved in the performance on these tests. Our results suggest verbal working memory as the primary cognitive function to be targeted in future cognitive remediation trials to improve cognitive and adaptive functioning in adolescents with EOS.
In the current study, the modified negative symptoms dimension associated with adaptive functioning in the bivariate but not the multivariate perspective. This may reflect a Type II error, however, negative symptoms were also not significantly associated to functioning in the multivariate perspective in another study of adolescents with EOS (Puig et al., Reference Puig, Penadés, Baeza, Sánchez-Gistau, De la Serna, Fonrodona, Andrés-Perpiñá, Bernardo and Castro-Fornieles2012), but were associated to functioning in adults earlier diagnosed with EOS (Röpcke & Eggers, Reference Röpcke and Eggers2005). Also longitudinally, negative symptoms relate inversely to later functioning in EOS (Maziade et al., Reference Maziade, Gingras, Rodrigue, Bouchard, Cardinal, Gauthier, Tremblay, Côté, Fournier, Boutin, Hamel, Roy, Martinez and Merette1996; Vyas et al., Reference Vyas, Hadjulis, Vourdas, Byrne and Frangou2007). Additionally, Digit Span performance, an often-used verbal working memory measure, explained 41% of the predicted variance of negative symptoms in the acute phase of AOS (Goldman et al., Reference Goldman, Axelrod, Tandon, Ribeiro, Craig and Berent1993). The currently observed association between the modified general symptom dimension and adaptive functioning is in line with a similar finding in adults with EOS (Röpcke & Eggers, Reference Röpcke and Eggers2005).
Comorbidity was found to be associated with lower adaptive functioning in our adolescents with EOS but this effect did not survive in our multivariate model. In line with this, psychiatric comorbidity was found not to predict future functioning in AOS (Mucci et al., Reference Mucci, Galderisi, Gibertoni, Rossi, Rocca, Bertolino, Aguglia, Amore, Bellomo, Biondi, Blasi, Brasso, Bucci, Carpiniello, Cuomo, Dell’Osso, Giordano, Marchesi, Monteleone and Maj2021).
In terms of limitations, the cross-sectional nature of the current study does not allow for causal inferences nor prediction of functional prognosis. The uneven gender distribution of the EOS sample did not allow for assessments of potential gender differences; the results may have less applicability to males. We included the total adaptive functioning score and the functional subdomains may have differential associations to the cognitive functions. The testers (JRMJ; CKL) were not blinded to the clinical and adaptive functioning data. Finally, the level of adaptive functioning may vary with differential parental support (Couture et al., Reference Couture, Penn and Roberts2006) which was not assessed in the current study.
We observed no significant difference in adaptive functioning in the antipsychotic medicated versus non-medicated participants. The medication dose associated with few cognitive functions but not with adaptive functioning; taking medication dose into account did not change the results. Only a modest improvement in community functioning with antipsychotic medication has been reported in AOS (Swartz et al., Reference Swartz, Perkins, Stroup, Davis, Capuano, Rosenheck, Reimherr, McGee, Keefe, McEvoy, Hsiao and Lieberman2007). Females with EOS were highly prevalent in our sample, which is inconsistent to the male predominance in earlier studies of youths with EOS, for example (Frazier et al., Reference Frazier, McClellan, Findling, Vitielle, Anderson, Zablotsky, Williams, Mcnamara, Jackson, Ritz, Hlastala, Pierson, Varley, Puglia, Maloney, Ambler, Hunt-Harrison, Hamer, Noyes and Sikich2007). There are indications that the EOS incidence rate for females has become higher than for males (Dalsgaard et al., Reference Dalsgaard, Thorsteinsson, Trabjerg, Schullehner, Plana-ripoll, Brikell, Wimberley, Thygesen, Madsen, Timmerman, Schendel, McGrath, Mortensen and Pedersen2020; Kühl et al., Reference Kühl, Laursen, Thorup and Nordentoft2016; Okkels et al., Reference Okkels, Vernal, Jensen, Mcgrath and Nielsen2012); however, there is inconsistency in this finding (Hsu et al., Reference Hsu, Lee and Wang2019; Stenstrøm et al., Reference Stenstrøm, Christiansen, Dehlholm-Lambertsen, Nøhr-Jensen and Bilenberg2010).
Conclusions
Visual memory, verbal working memory, processing speed, reaction time, and social cognition are in the current study cross-sectionally associated with adaptive functioning in adolescents in the early illness phase of EOS. Of these cognitive functions, only verbal working memory was uniquely associated to adaptive functioning in a multivariate perspective. In contrast to general symptoms, neither the severity of modified negative nor of positive symptoms dimensions was associated with that of adaptive functioning. We suggest verbal working memory as the primary target in future cognitive remediation therapy trials to improve cognitive and adaptive functioning in adolescents with EOS.
Acknowledgements
The authors would like to thank the participants and their parents in addition to the students Rókur av Fløtum Jespersen, Nanna Pagsberg and Andreas Elleby from the Center for Neuropsychiatric Schizophrenia Research for assistance.
Funding statement
This work was supported by a Post. Doc. scholarship from the Research Foundation of Mental Health Services in the Capital Region of Denmark (JRMJ); a Ph.D. scholarship from the Research Foundation of Mental Health Services in the Capital Region of Denmark (JR); Lundbeck Foundation Center for Clinical Intervention and Neuropsychiatric Research(JR, R25-A2701); Læge Gerhard Linds Legat (JR); Fru C. Hermansens Legat (JR; JRMJ); Slagtermester Wörzners og Hustru Inger Wörzners Mindelegat (JR); and Psykiatrisk Forskningsfond af 1967 (JR); Tømrermester Jørgen Holm og Hustru Elisa F. Hansens Mindelegat (JRMJ); Jascha Fonden (JRMJ). No funding source was involved in the study.
Conflicts of interest
None.








