Introduction
The percentage of patients with schizophrenia who demonstrate symptom onset during adolescents may be as high as 25%; however, the proportion of patients with an early-onset diagnosis of schizophrenia appears to be much lower.1–Reference Kelleher, Keeley and Corcoran5 Compared with adult-onset, early-onset schizophrenia occurs more commonly in males and typically is associated with greater illness severity, chronicity, and functional impairment, resulting in a less favorable prognosis.6–Reference Immonen, Jääskeläinen and Korpela11
Because the vast majority of patients with schizophrenia require chronic treatment, long-term safety and tolerability are paramount considerations when choosing an antipsychotic agent. This is especially true in adolescents, a particularly vulnerable population at higher risk for experiencing adverse effects from antipsychotics.12–Reference Al-Dhaher, Kapoor and Saito17
In a recent network meta-analysis based on 28 short-term, randomized controlled trials of schizophrenia in adolescence, the most efficacious antipsychotic (vs placebo) was clozapine, followed by olanzapine, risperidone, and lurasidone.Reference Krause, Zhu and Huhn18 However, the American Academy of Child and Adolescent Psychiatry guidelines (AACAP)Reference McClellan and Stock9 recommend use of olanzapine as a second-line agent due to safety concerns, most notably high risk of weight gain, and metabolic abnormalities (eg, dyslipidemia). The guideline also recommended caution in the use of clozapine. Additionally, a recent meta-review of 78 adverse events of 80 psychotropic medications in children and adolescents with mental disorders found lurasidone to have the best safety/tolerability profile among 15 antipsychotics with data.Reference Solmi, Fornaro and Ostinelli19
Minimal data are available from long-term studies assessing the safety, tolerability, and effectiveness of antipsychotic agents for schizophrenia in adolescent populations.Reference Savitz, Lane and Nuamah20, Reference Correll, Kohegyi and Zhao21 The current study is one of the only large-scale antipsychotic treatment studies we are aware of that prospectively followed adolescent patients with schizophrenia for 2 years.
Lurasidone is an atypical antipsychotic agent with high binding affinity for D2, 5-HT2A, and 5-HT7 receptors (antagonist); moderate affinity for 5-HT1A receptors (partial agonist); and no appreciable affinity for H1 and M1 receptors.Reference Ishibashi, Horisawa and Tokuda22 Lurasidone has demonstrated efficacy and safety in the acute and long-term treatment of adults with schizophrenia in the dose range of 40-160 mg/day23–Reference Tandon, Cucchiaro and Phillips30 and has shown a low propensity for weight gain or metabolic disturbance.Reference Loebel and Citrome31 The minimal effect of lurasidone on weight appears to be largely attributable to its absence of activity at 5HT2C and histamine H1 receptors.Reference Lord, Wyler and Wan32, Reference Kroeze, Hufeisen and Popadak33
In a previously reported double-blind (DB), placebo-controlled, fixed-dose, 6-week trial in adolescents with schizophrenia, patients randomized to lurasidone 40 mg/d or 80 mg/d demonstrated significantly greater improvement in schizophrenia symptoms than placebo-treated patients; and lurasidone treatment was found to be generally safe and well tolerated.Reference Goldman, Loebel and Cucchiaro34 We report here the results of the 2-year, open-label (OL) follow-up of that study designed to evaluate the long-term safety and effectiveness of lurasidone in this adolescent population.
Methods
This was a 104-week, OL extension study (clinicaltrials.gov identifier: NCT01914393) that enrolled patients from 13 to 17 years of age who completed an initial 6-week, DB, placebo-controlled trial evaluating the efficacy and safety of two fixed doses of lurasidone (40 and 80 mg/d) for the treatment of schizophrenia (NCT01911429).Reference Goldman, Loebel and Cucchiaro34
The study was conducted from November 2013 to October 2018 at 65 centers in 14 countries (Bulgaria, Columbia, Spain, France, Hungary, South Korea, Mexico, Malaysia, Philippines, Poland, Romania, Russia, Ukraine, and USA). The study was approved by an Institutional Review Board/ethics committee at each investigational site and was conducted in accordance with the International Conference on Harmonisation Good Clinical Practices guidelines and with the ethical principles of the Declaration of Helsinki. The study was monitored by an independent Data and Safety Monitoring Board. After a full explanation of the study was provided, written informed consent was obtained from a parent or legal guardian, and assent was obtained from each adolescent patient. For patients who became 18 years of age during the course of the study, written informed consent was obtained as soon as possible after their birthday.
Patients and study design
Entry into the preceding acute treatment studyReference Goldman, Loebel and Cucchiaro34 was limited to patients with a DSM-IV-TR diagnosis of schizophrenia who were experiencing an acute exacerbation (≤ 2 months in duration), with a Positive and Negative Syndrome Scale (PANSS) total score ≥ 70 and ≤ 120, and a Clinical Global Impression-Severity (CGI-S) score ≥ 4 (at least moderately ill). Patients were excluded if they had a history of intellectual disability or any neurologic disorder or an alcohol or substance use disorder diagnosis in the previous 6 months.
Patients were included in the current extension study if they were judged by the investigator to be suitable for participation in a 104-week OL study, were able to comply with the protocol, were not considered by the investigator to be at imminent risk of suicide or injury to self or others, exhibited no evidence of moderate or severe extrapyramidal symptoms, dystonia, tardive dyskinesia, or any other movement disorder, were willing to use medically appropriate contraception if sexually active.
All patients enrolled in the current extension study were started on a dose of 40 mg/d for 1 week, regardless of their treatment group assignment in the original DB study. The dose of lurasidone could be adjusted at regularly scheduled visits (biweekly up to week 8, monthly thereafter) in the flexible dose range of 20, 40, 60, or 80 mg/day. Dose adjustment should occur increments or decrements of one (20 mg) dose level at these scheduled visits; however, dose reductions for tolerability or safety purposes were permitted, based on investigator judgment, between study visits, as early as day 2, and could include dose reductions of a maximum of two dose levels at a time. Lurasidone was taken orally, once daily in the evening with a meal or within 30 minutes after eating.
Concomitant medication
Concomitant treatment with benzodiazepines, antidepressants, and stimulants (for Attention deficit hyperactivity disorder (ADHD)) was permitted; treatment with benztropine (≤ 6 mg/day), or alternative medications, was permitted as needed for movement disorders, and treatment with propranolol (≤ 120 mg/day) was permitted as needed for akathisia. Prophylactic use of medications to treat movement disorders was not permitted. Concomitant use of lorazepam, or equivalent benzodiazepine, was permitted at the discretion of the investigator (≤ 6 mg/day or equivalent dose) for intolerable anxiety/agitation. Benzodiazepine and nonbenzodiazepine sedative-hypnotic agents were also permitted on an as-needed basis for insomnia.
For patients who were hospitalized at the conclusion of the original DB study, continued hospitalization for up to 14 days in the current extension study was permitted. Patients that could not be transitioned to an outpatient setting within 14 days were discontinued from the study.
Safety and tolerability assessments
The presence and severity of adverse events were recorded at each study visit, including any treatment-emergent worsening in preexisting symptoms or conditions that the patient spontaneously reports. As requested by the European Medicines Agency, adverse event reporting was supplemented by administration of the Udvalg for Kliniske Undersogelser (UKU) Side Effect Rating Scale, a clinician-rated scale consisting of 48 adverse effect items (each rated on a 0-3 point scale, 0—no side effects, 1—mild, 2—moderate, 3—severe) divided into four categories (psychic [0-30], neurologic [0-24], autonomic [0-33], and other [0-48]).Reference Lingjaerde, Ahlfors and Bech35 Mean severity scores were calculated for each side effect category and for the total score. Movement disorders were assessed by the Simpson-Angus Scale,Reference Simpson and Angus36 the Barnes Akathisia Rating Scale,Reference Barnes37 and the Abnormal Involuntary Movement Scale.Reference Munetz and Benjamin38 The Columbia Suicide Severity Rating Scale (C-SSRS)Reference Posner, Brown and Stanley39 was used to assess suicidal ideation and behavior. Additional safety evaluations included vital signs, height, weight and body mass index (BMI), laboratory tests (metabolic parameters, hormonal parameters, and other blood chemistry and hematology values), 12-lead electrocardiogram (ECG), Tanner staging, menstrual cyclicity (female patients), and physical examination.
The effect of treatment on cognitive function was evaluated with the Cogstate Brief BatteryReference Maruff, Thomas and Cysique40 and will be reported in a separate publication.
Effectiveness assessments
Effectiveness assessments were performed by qualified site-based raters at each monthly assessment visit. In the current OL study, effectiveness measures were considered secondary outcomes and consisted of the following: the PANSS, and the PANSS Positive, Negative, General Psychopathology, and Excitability subscalesReference Kay, Fiszbein and Opler41; the Clinical Global Impression, Severity scale (CGI-S)Reference Guy42; the clinician-rated Children’s Global Assessment Scale (CGAS)Reference Shaffer, Gould and Brasic43; and the Pediatric Quality of Life Enjoyment and Satisfaction Questionnaire (PQ-LES-Q).Reference Endicott, Nee and Yang44 The CGAS is a clinician-administered measure that evaluates global impairment on a scale of 0-100. Higher scores indicate better functioning; scores ≥ 70 are considered in the normal range of functioning.45 The PQ-LES-Q is a quality-of-life measure that has demonstrated reliability and validity in children ages from 6 to 17 and its percentage maximum possible score range from 0% to 100%. A score PQ-LES-Q score of 58% (within 10% of normative community mean score) was the criterion level used to qualify a patient as returning to a normative level of quality of life.Reference Schechter and Endicott46, Reference Rapaport, Clary, Fayyad and Endicott47
Statistical analysis
The safety population consisted of all patients who completed the DB, acute-phase trial, continued into the current extension study, and received at least one dose of lurasidone in the OL phase of the study. All safety and effectiveness summaries were based on the safety population. No inferential statistics were calculated. For continuous variables (including both safety and effectiveness variables), descriptive summary statistics (N, mean, median, 95% confidence interval [CI] etc.) were reported at DB Baseline, OL Baseline, each post-OL visit, week-52 endpoint, and endpoint in the OL study. In addition, changes from DB Baseline and OL Baseline were also reported in a similar way using summary statistics as described above.
For treatment-emergent adverse events, number and percentage of subjects with one or more events were summarized for overall incidence, serious adverse events, and discontinuations due to adverse events.
To analyze the effects of lurasidone on growth parameters, age- and sex-specific z-scores of height and BMI were reported using the World Health Organization (WHO) 2007 growth charts, and age- and sex-specific z-scores for body weight were reported using the Centers for Disease Control and Prevention (CDC) 2000 growth charts.Reference Ogden, Kuczmarski and Flegal48 In this OL extension study, to better interpret growth changes in the absence of a placebo-controlled group, expected value of weight per CDC growth reference and expected value of height and BMI per WHO growth chart were derived for each subject at study visits; mean expected changes relative to DB Baseline were summarized for these growth parameters.
PANSS total and subscale scores (Positive, Negative, General Psychopathology, Excitability), CGI-S score, the CGAS total score, and the PQ-LES-Q score were summarized as continuous variables. Treatment response was defined a priori as ≥ 20% reduction in PANSS total score from baseline (either DB baseline or OL baseline), calculated based on both observed case data and last observation carried forward (LOCF) data. An exploratory analysis was also performed to examine the proportion of patients who met stringent responder criteria, defined posthoc as ≥ 50% reduction in PANSS total score from baseline (DB baseline or OL baseline). The number and proportion of responders relative to baseline by study visit were summarized.
Remission, sustained remission, and recovery
In posthoc analyses, the following criteria were used to define remission (at a given assessment time point): PANSS item scores of ≤ 3 at one assessment visit on all of the following PANSS items (N = 8 items): P1 (delusions), P2 (conceptual disorganization), P3 (hallucinatory behavior), G5 (mannerisms/posturing), G9 (unusual thought content), N1 (blunted affect), N4 (social withdrawal), and N6 (lack of spontaneity).Reference Andreasen, Carpenter and Kane49 Sustained remission required all of these PANSS item severity scores to remain ≤ 3 for at least 6 months.Reference Andreasen, Carpenter and Kane49 Patients met criteria for recovery at a given visit if they met all PANSS item severity criteria for remission at that visit and also had a CGAS score ≥ 70 indicating no clinically significant functional impairment at that visit. Number and proportion of patients who met remission or recovery criteria were summarized, respectively, by study visit. A Kaplan–Meier analysis of time-to the first event was performed to evaluate time-to sustained remission and sustained recovery.
Results
A combined total of 285 adolescents completed the 6-week, DB, placebo-controlled trial, of whom 271 patients (95.1%) provided informed consent/assent and continued into the current OL extension study, including 181 patients initially randomized to lurasidone (40 mg/d or 80 mg/d) and 90 patients initially randomized to placebo (Figure 1). Demographic and clinical characteristics at extension phase baseline are summarized in Table 1. In the current extension study, 186 (68.6%) of patients completed 52 weeks, and 156 (57.6%) completed 104 weeks of treatment (Figure 1). Reasons for premature discontinuation were withdrawal of consent (14.0%), adverse event (10.7%), lost to follow-up (4.4%), lack of efficacy (4.1%), and miscellaneous other reasons (9.2%; Figure 1).

Figure 1. Patient disposition.
Table 1. Baseline Demographic and Clinical Characteristics (Safety Population)

Abbreviations: BMI, body mass index; DB, double-blind; OL, open-label; PANSS, Positive and Negative Syndrome Scale; SD, standard deviation.
a Age- and gender-adjusted z-score for weight are based on CDC growth charts for the United States (2000); age- and gender-adjusted z-score for BMI are based on WHO growth charts (2007).
The mean daily dose of lurasidone averaged across the 104-week OL treatment period was 57.0 mg/d. The modal daily dose of lurasidone utilized by patients during OL treatment was 20 mg (by 3.3% of patients), 40 mg (35.4%), 60 mg (24.4%), and 80 mg (36.9%). Mean daily dose of lurasidone across the 104-week OL treatment is similar between patients in the 13 and 14 years and patients in the ≥ 15 years age groups (60.1 mg vs 56.0 mg). Pill counts indicated that adherence was high, with only one patient being nonadherent on three or more visits during 104 weeks of study treatment. The most frequently used, as-needed, concomitant medications were benzodiazepines (23.2%) and anticholinergic medications (9.6%).
Safety
Adverse events with an incidence ≥ 5% are summarized in Table 2. Overall, 20/271 patients (7.4%) reported an adverse event as severe. The incidence of extrapyramidal symptom–related adverse events (excluding akathisia) was 9.6%. The incidence of individual adverse events was similar (< 5% difference), regardless of initial DB treatment assignment (lurasidone or placebo).
Table 2. Adverse Events in at Least 5% of Patients (Safety Population)

a Hypersomnia, sedation, somnolence, hypersomnolence.
b Parkinsonism, dyskinesia, dystonia, extrapyramidal disorder, hypokinesia, salivary hypersecretion, tardive dyskinesia, torticollis, or psychomotor hyperactivity.
At DB baseline, the mean severity score for each Udvalg for Kliniske Undersogelser (UKU) side effect category, and UKU total, were as follows: psychic (4.48), neurologic (.26), autonomic (.34), other side effects (.34), and total (5.42). Change from OL baseline generally showed a gradual improvement over time in UKU total score and psychic side effect subscale score. Changes from DB Baseline were less pronounced for the remaining UKU subscale scores (Table 3).
Table 3. Change from Double-blind (DB) and Open-Label (OL) Baselines in Udvalg for Kliniske Undersogelser (UKU) Side Effect Rating Scale Scores (Safety Population)
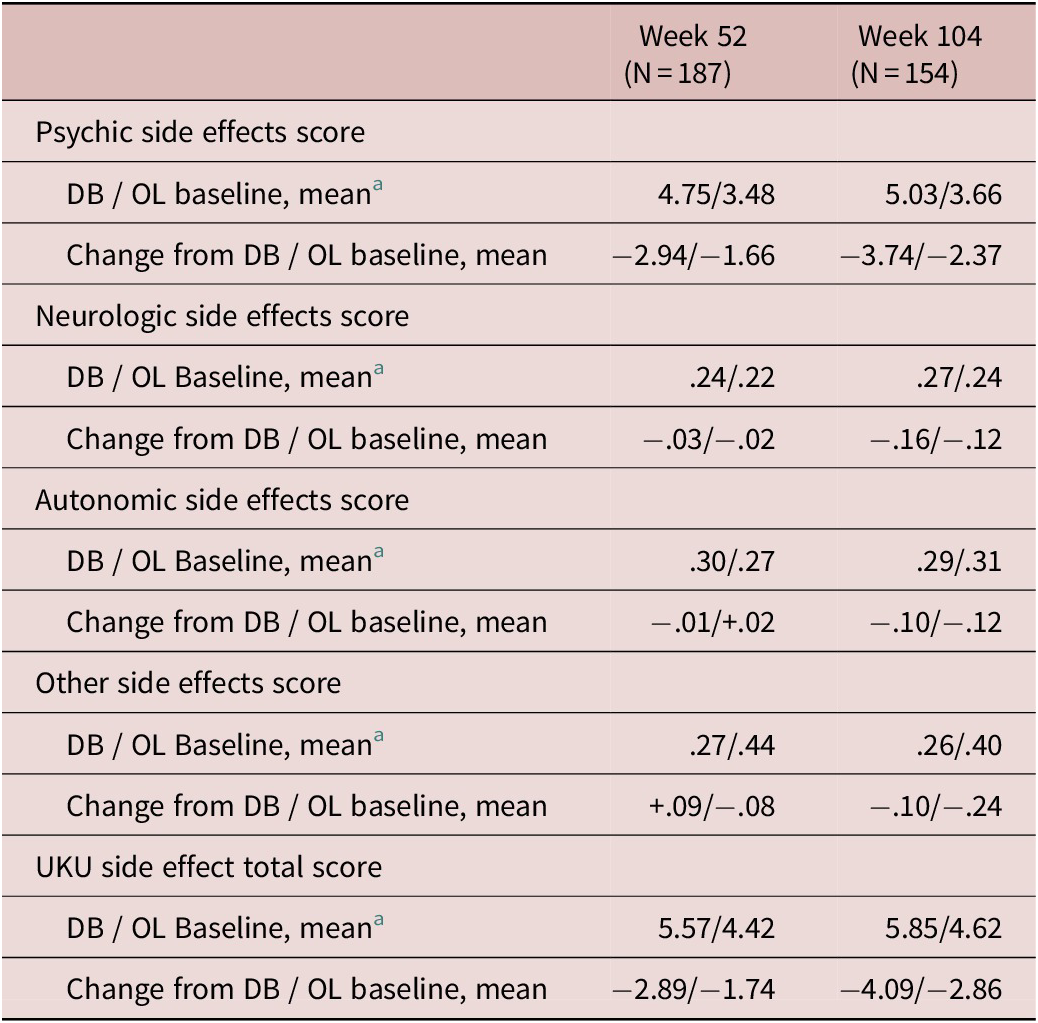
Abbreviations: DB, double-blind; OL, open-label; UKU, Udvalg for Kliniske Undersogelser.
Higher baseline scores indicate greater severity; range of 0 to 30 for psychic, 0 to 24 for neurologic, 0 to 33 for autonomic, and 0 to 48 for other.
a DB baseline means are shown for the subgroup of patients available at weeks 52 and 104, respectively.
The proportion of patients reporting a serious adverse event was 10.3% (28/271), translating into an event rate of .115 events per patient-year of exposure. Specific serious adverse events consisted of fever, appendicitis, hematuria, concussion, foot fracture, soft tissue injury, new-onset type 1 diabetes (n = 1 for each), and the following psychiatric disorders: schizophrenia (n = 11), suicidal ideation (n = 8), psychotic disorder (n = 5), intentional overdose (n = 2), and one each of the following: abnormal behavior, aggression, agitation, confusional state, depression, depressive symptom, suicidal behavior, and suicide attempt. There were no deaths in the study.
A similar proportion of patients in the 13-14 vs ≥ 15-year age group reported at least one adverse event (83.3% vs 77.4%), an adverse event leading to study discontinuation (11.1% vs 10.1%), and a serious adverse event (13.9% vs 9.0%).
On the C-SSRS, the proportion of patients with emergent or worsening suicidal ideation (relative to the DB treatment period) was 4.8% (n = 13), and the proportion with emergent suicidal behavior was 1.1% (n = 3).
Mean changes from OL baseline to weeks 52 and 104, respectively, were small and not clinically meaningful for the Simpson–Angus scale 10-item mean score (−.01 and −.01), the Barnes Akathisia Scale total score (−.1 and −.1), and the Abnormal Involuntary Movement Scale total score (−.02 and −.05).
In this population of adolescents, mean change from DB baseline in actual weight (kg) during 104 weeks of OL treatment with lurasidone was very similar to the expected change in weight based on CDC growth charts (Figure 2). Mean change in BMI was also similar to the expected change in BMI based on WHO growth charts (Table 4; Figure 2). The proportion of patients with normal weight (ie, BMI between 5th and 84.9th percentile based on WHO reference values) was 62.7% at DB baseline, and this proportion increased at week 52 (66.1%) and week 104 (73.7%; Table 4).

Figure 2. Change from double-blind (DB) baseline in weight and body mass index (BMI): actual vs expected.
Table 4. Changes in Selected Laboratory Parameters and Growth Parameters (Safety Population)

Abbreviations: BMI, body mass index; DB, double-blind; HDL, high-density lipoprotein; LDL, Low-density lipoprotein; WHO, World Health Organization.
a Age- and gender-adjusted weights are based on CDC growth charts for the United States (2000) and BMI is based on WHO growth charts (2007).
Treatment with lurasidone was associated with small changes from DB baseline at both weeks 52 and 104 in total, high-density lipoprotein (HDL), low-density lipoprotein (LDL), cholesterol, triglycerides, glucose, hemoglobin A1C, and insulin (Table 4), and median changes in prolactin were minimal (≤ 1.2 ng/mL) in both male and female patients (Table 4).
During OL treatment with lurasidone, no clinically meaningful changes were observed in heart rate, electrocardiogram (ECG), orthostatic blood pressure (systolic or diastolic), respiratory rate, or body temperature. On serial ECG assessments during up to 104 weeks of OL treatment, no patient had a QT interval, Fridericia’s Correction (QTcF) value > 460 ms, and no patient had an increase from OL baseline in QTcF that was ≥ 60 ms.
Effectiveness
For the extension study patient population, 6 weeks of initial DB treatment was associated with greater improvement in PANSS total score in patients randomized to lurasidone (n = 181) compared to those randomized to placebo (n = 90: −19.8 vs −12.9). For all patients entering the extension study, mean change in PANSS total score from DB baseline to OL baseline (6 week) was −17.5 resulting in a PANSS total score of 76.0 at OL baseline. After 12 weeks of OL treatment with lurasidone, mean change from DB baseline in PANSS total score was comparable for both patients initially randomized to lurasidone or placebo in the DB phase (−27.8 vs −26.8). For the combined group, continued improvement from DB baseline was observed for PANSS total and subscale scores during up to 104 weeks of treatment with OL lurasidone (Figure 3a). Mean observed change from OL baseline in the PANSS total score was −15.6 at week 52 and −18.4 at week 104 (−12.2 at LOCF-endpoint). Continued improvement from DB baseline was also observed for the CGI-Severity score (Figure 3b).

Figure 3. Change from double-blind (DB) baseline in Positive and Negative Syndrome Scale (PANSS) and CGI-S (OC analyses). (a) PANSS total and factor scores; (b) CGI-severity score.
To verify the impact of early dropouts on change in PANSS total score, two sensitivity analyses were conducted to explore the robustness of change from DB baseline and change from OL baseline in PANSS total score. The results of these sensitivity analyses, summarized in the online Supplemental Appendix A, confirmed the effectiveness of lurasidone therapy in term of change in PANSS total score.
Functioning and quality of life, measured by the CGAS and PQ-LES-Q, respectively, demonstrated progressive improvement across 104 weeks of lurasidone treatment, with the CGAS total score reaching normative levels of functioning at approximately 52 weeks, and the PQ-LES-Q total score reaching normative quality of life levels by week 28 (Figure 4).

Figure 4. Mean value over time in Children’s Global Assessment Scale (CGAS) total score and Pediatric Quality of Life Enjoyment and Satisfaction Questionnaire (PQ-LES-Q) score (OC).
Response, remission, and recovery
Responder rates (≥ 20% reduction from DB baseline in PANSS total score) increased during long-term treatment with lurasidone until reaching an asymptote of approximately 90% at week 28 (Figure 5a). Notably, the responder rates at week 104 were only modestly lower for patients in the observed case analysis versus LOCF-endpoint analysis (82.8% vs 91.0%). More stringent responder rates (≥ 50% reduction in PANSS total score from DB baseline) at OL baseline, weeks 52, and 104 were 18.5%, 58.2%, and 58.3%, respectively. Remission rates also reached an asymptote of approximately 65% at week 28 (Figure 5a).

Figure 5. Response and remission during open-label (OL) treatment with lurasidone. (a) Responder and remitter rates; (b) Kaplan–Meier plot of time to the earliest sustained (for 6 months) remission.
During long-term treatment with lurasidone, 52.8% (143/271) of patients met a stringent definition of sustained remission, requiring patients to meet remission criteria continuously for 6 months. The Kaplan–Meier estimate of median time to first onset of sustained remission was 64.1 weeks (95% CI: 40.4, 76.1 weeks) (Figure 5b).
Patients who met remission criteria (cross-sectional) and who also met CGAS criteria for normative levels of functioning (total score ≥ 70) were considered to be recovered. Recovery rates at week 52 and week 104 were 43.9% (83/189) and 51.3% (80/156), respectively.
Discussion
The results of this multicenter, OL study in adolescent patients with schizophrenia found lurasidone to be generally well-tolerated during 2 years of OL treatment, with a safety profile consistent with results from previously reported short- and long-term studies in adult patients with both schizophrenia and bipolar depression and with results of short-term studies in children and adolescents with bipolar depression.23–31, Reference Goldman, Loebel and Cucchiaro34, 50-52 No new or unexpected adverse events were reported, no deaths occurred, and no serious drug-related effects were observed. Results from the structured UKU side effect rating scale yielded tolerability findings similar to spontaneous adverse event reporting, with reductions in adverse event severity noted across all assessment domains during the course of treatment, resulting in a 70% overall reduction in severity for the UKU side effect total score. The incidence of extrapyramidal symptoms and akathisia was low, and movement disorder assessments showed no clinically meaningful changes. Overall, 58% of patients completed 2 years of lurasidone treatment, with only 11% discontinuing prematurely due to an adverse event. These rates compare favorably to rates in other prospective, long-term studies of atypical antipsychotics in adolescents with schizophrenia.Reference Savitz, Lane and Nuamah20, Reference Correll, Kohegyi and Zhao21, Reference Findling, Cavuş and Pappadopulos53, Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann54
Consistent with the results of long-term studies in adults with schizophrenia, the present study found 2 years of treatment with lurasidone to have minimal effects on physiologic growth-adjusted weight, lipids, and glycemic indices.Reference Citrome, Cucchiaro and Sarma25, Reference Loebel, Cucchiaro and Xu27, Reference Loebel and Citrome31 This finding is in contrast to previously reported effects of selected atypical antipsychotics on weight and metabolic parameters.Reference Correll12, Reference Correll, Manu and Olshanskiy13, Reference Krause, Zhu and Huhn18 A network meta-analysis of short-term treatment studies in children and adolescents with schizophrenia found lurasidone to have a lower risk of weight gain than risperidone, quetiapine, olanzapine, clozapine, and paliperidone.Reference Krause, Zhu and Huhn18 The current results extend the findings of the previous short-term study.Reference Goldman, Loebel and Cucchiaro34 During long-term lurasidone therapy, no clinically meaningful changes were noted in prolactin levels, and no prolactin-related adverse effects occurred (eg, galactorrhea, amenorrhea). In addition, no meaningful changes were observed in vital signs or ECG parameters. These results are consistent with the finding of a recent meta-review in youth with psychiatric disorders, in which among 15 different antipsychotics, lurasidone was the one with the fewest adverse events that were significantly greater than placebo relative to adverse events not dissimilar from placebo.Reference Solmi, Fornaro and Ostinelli19
Continued reduction in symptom severity on standard efficacy measures (PANSS total and factor scores; CGI-S) occurred during 2 years of OL treatment with lurasidone. Improvement in schizophrenia symptom severity was substantial, with the magnitude of improvement that occurred during OL treatment being comparable to what was observed during initial 6-week DB treatment. The magnitude of improvement during extension phase treatment was not solely attributable to attrition of patients, as illustrated by a comparison of summary results over time between observed and LOCF values, and by further comparison with sensitivity analysis results based on multiple imputations of missingness.
In posthoc analyses to test the durability of improvement during the OL long-term treatment, stringent criteria were applied to evaluate the proportion of patients who met a consensus definition of sustained remission, requiring patients to meet remission criteria continuously for 6 months.Reference Andreasen, Carpenter and Kane49 Altogether, 53% of patients met criteria for sustained remission, with a Kaplan–Meier analysis indicating that the median time to first onset of sustained remission was 64 weeks. After approximately 28 weeks of OL treatment, mean levels of functioning (CGAS) and quality of life (PQ-LES-Q) returned to normative levels, providing further corroboration of the effectiveness of longer-term therapy with lurasidone.
Several important study limitations should be noted. This was an OL trial that was not randomized or blinded and had no placebo or active comparator control. Eligibility for enrollment in the DB trial was based on inclusion and exclusion criteria for the initial DB efficacy study that may reduce the generalizability of the results. In addition, entry into the current OL extension study was limited to patients who completed the DB study. This may have introduced a selection bias resulting in more severely ill patients electing not to continue in the current study. Several of the efficacy analyses (sustained remission using Kaplan–Meier analysis) were posthoc analyses. Finally, the UKU Side Effect Rating Scale was specifically required by the European Medicines Agency; however, the scale has not been validated for use in adolescent populations.
Conclusion
In this study, one of the longest and largest prospective schizophrenia trials in a pediatric population, 2 years of treatment with lurasidone, was found to be safe and well tolerated, with a high rate of patient retention. These safety results are consistent with previous long-term lurasidone studies in adults and short-term studies in children. Long-term treatment with lurasidone was also associated with continued improvement in the symptoms of schizophrenia, resulting in a majority of patients achieving symptomatic remission. The effectiveness of lurasidone was supported by observed improvement across multiple outcome parameters. Taken together, the current results suggest that lurasidone has a favorable benefit–risk profile that places it as a potential first-line treatment of schizophrenia in adolescent patients.
Acknowledgments
Dr. Edward Schweizer of Paladin Consulting Group provided editorial and medical writing assistance, which was funded by Sunovion Pharmaceuticals Inc.
Funding
This was funded by Sunovion Pharmaceuticals Inc.
Disclosures
Dr. Correll has been a consultant and/or advisor to or have received honoraria from: Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Merck, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Rovi, Supernus, and Teva. He has received grant support from Janssen and Takeda. He is also a stock option holder of LB Pharma.
Dr. Findling receives or has received research support, acted as a consultant and/or has received honoraria from Acadia, Adamas, Aevi, Akili, Alcobra, Alkermes, Allergan, Amerex, American Academy of Child & Adolescent Psychiatry, American Psychiatric Press, Arbor, Axsome, Daiichi-Sankyo, Gedeon Richter, Genentech, KemPharm, Luminopia, Lundbeck, MedAvante-ProPhase,Merck, NIH, Neurim, Noven, Nuvelution, Otsuka, PCORI, PaxMedica,Pfizer, Physicians Postgraduate Press, Q BioMed, Receptor Life Sciences, Roche, Sage, Signant Health, Sunovion, Supernus Pharmaceuticals, Syneos, Syneurx, Takeda, Teva, Tris, TouchPoint,and Validus.
Dr. Tocco, Pikalov, Deng, and Goldman are employees of Sunovion Pharmaceuticals Inc.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1092852920001893.











