Introduction
Daytime sleepiness (also known as daytime somnolence or sedation) associated with antipsychotic treatment may adversely impact functional performance and quality of life.Reference Kane and Sharif 1 Medications with significant antihistaminergic effects, such as diphenhydramine and certain antipsychotic agents, are commonly associated with daytime sleepiness. Previous studies have suggested that daytime sleepiness can impair cognition, including concentration and alertness, and may interfere with daily work and task performance.Reference Kane and Sharif 1 – 3 In addition, daytime sleepiness may impair driving skills and increase risk for accidents. The American Academy of Sleep Medicine (AASM) estimated that on average, 80,000 U.S. drivers fall asleep while driving every day, and up to 20% of all serious car crash injuries are associated with driver sleepiness, independent of the other major factor of driving under the influence of alcohol. 3 The adverse impact of daytime sleepiness on personal health, performance, and well-being is therefore substantial, and translates into significant economic loss for both individuals and society.Reference Kane and Sharif 1 – Reference Colten and Altevogt 4
Despite awareness of the significant health and economic burden posed by daytime sleepiness in the general population,Reference Colten and Altevogt 4 and the propensity for antipsychotic agents to induce sedation, the effects of daytime sleepiness on patient outcomes in schizophrenia have not been adequately studied. In particular, few schizophrenia trials have included a systematic or objective assessment of daytime somnolence associated with drug treatment.Reference Harvey, Hassman and Mao 5 – Reference Fleischhacker, Heikkinen and Olié 8 The objective of this analysis was to evaluate the effects of 2 atypical antipsychotic agents, lurasidone and quetiapine XR, on daytime alertness, and the mediating effects of daytime sleepiness on clinical, cognitive, and functional outcomes in patients with an acute exacerbation of schizophrenia.
Materials and Methods
Design
This was a randomized, double-blind, placebo- and active-controlled study in which subjects with a primary diagnosis of schizophrenia who were recently hospitalized for an acute exacerbation of psychotic symptoms were randomly assigned to 6 weeks of double-blind treatment with once-daily, evening doses of lurasidone (80 mg or 160 mg), quetiapine XR (600 mg, included to establish assay sensitivity), or placebo. Study methods have been described in detail elsewhereReference Loebel, Cucchiaro and Sarma 9 and are summarized here. This multinational study was conducted between October 21, 2008, and June 2, 2010, and randomized a total of 488 subjects at 24 centers in the United States (n = 151 subjects) and 39 centers in the following countries: Russia (n = 87), India (n = 98), Ukraine (n = 75), Romania (n = 49), and Colombia (n = 26).Reference Loebel, Cucchiaro and Sarma 9 The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practices guidelines and with the ethical principles of the Declaration of Helsinki. An independent data and safety monitoring board reviewed safety data at regular intervals during the study.
Subjects
Hospitalized male and female subjects between the ages of 18 and 75 years who met Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR) criteria for a primary diagnosis of schizophrenia, which was confirmed by the Mini International Neuropsychiatric Interview-Plus (MINI-Plus),Reference Sheehan, Lecrubier and Sheehan 10 were enrolled. Subjects were required to have an illness duration greater than 1 year; the current acute exacerbation of psychotic symptoms was to be no longer than 2 months. At the screening and baseline visits, subjects were required to have a Clinical Global Impression-Severity (CGI-S) score ≥4 (moderately severe or greater) and a Positive and Negative Syndrome Scale (PANSS) total score ≥80, including a score ≥4 (moderately severe or greater) on 2 or more of the following PANSS items: delusions, conceptual disorganization, hallucinations, unusual thought content, and suspiciousness.
Study medication
All study medication was identically over-encapsulated to preserve the double-blind nature of the study. Subjects were evaluated for eligibility during a screening period of up to 14 days, during which all psychotropic medications were tapered off in a manner consistent with labeling recommendations and conventional medical practice. Subjects then completed a 3- to 7-day placebo washout period. At baseline (Day 0), subjects who continued to meetall study entry criteria were randomly assigned via an interactive voice response system (in a 1:1:1:1 ratio) to 1 of 4 treatment arms: lurasidone 80 mg/d (N = 125), lurasidone 160 mg/d (N = 121), quetiapine XR 600 mg/d (N = 119), or placebo (N = 121). Study medication was administered once daily in the evening with a meal or within 30 minutes after eating. Subjects assigned to lurasidone 80 mg/d started treatment at their target dose. Subjects assigned to lurasidone 160 mg/d started treatment at a dose of 120 mg/d for 2 days and were then increased to their target dose. Subjects assigned to quetiapine XR 600 mg/d were started at a dose of 300 mg/d for 2 days and were then increased to their target dose. The quetiapine XR dose of 600 mg/d was selected for use in this study because it is at the mid-point of the therapeutic dose range (400–800 mg/d) for this agent, and has been shown to be effective and generally well-tolerated in the treatment of patients with acute schizophrenia.Reference Baldwin and Scott 11 , Reference Zhornitsky, Potkin and Moteshafi 12 Subjects were eligible for hospital discharge after completing a minimum of 21 days of double-blind treatment if specific clinical stability criteria were met.
Assessments
Daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS) at the baseline, week 3, and week 6 visits. The ESS is a patient-reported questionnaire, which consists of 8 items that describe various routine daily life situations.Reference Johns 6 , Reference Johns 7 Subjects rate the likelihood of dozing or falling asleep (distinguishing dozing behavior from feelings of tiredness) in each of these situations, on a 4-point scale from 0 to 3; items are summed to produce a total score (range of 0–24). The ESS has been shown to be reliable and valid, in that it correlates reasonably well with laboratory measures of sleepiness [eg, the multiple sleep latency test (MSLT)]Reference Chervin 13 and has been shown to be sensitive to treatment-related improvement in sleep disorder populations. It has been validated in both case-control studies of normal subjects and several patient populations with different types of sleep disorders.Reference Johns 6 , Reference Johns 7 In addition, the ESS has been utilized for the assessment of daytime sleepiness in patients with schizophrenia.Reference Fleischhacker, Heikkinen and Olié 8 , Reference Lohr, Liu and Caligiuri 14 Scores higher than 10 are considered to indicate significant, i.e., markedly high, daytime sleepiness.Reference Johns 7 If one or more Epworth Sleepiness Scale items were missing at a visit, the total score would be set to missing. Primary efficacy outcomes were assessed using the PANSS total and subscale scores,Reference Kay, Fiszbein and Opler 15 and have been published in full elsewhere.Reference Loebel, Cucchiaro and Sarma 9 Agitation was assessed using the PANSS Excitement subscale (PANSS-EC).Reference Lindenmayer, Brown and Baker 16 Cognitive performance was assessed at baseline and week 6 with the CogState Computerized Schizophrenia Battery,Reference Pietrzak, Olver and Norman 17 using a composite Z score. Functional capacity was assessed using the University of California–San Diego (UCSD) Performance-Based Skills Assessment—Brief Version (UPSA-B) at baseline and week 6.Reference Mausbach, Harvey and Goldman 18 The UPSA-B total score ranges from 0 to 100 points, with higher scores reflecting better performance.
Statistical methods
A mediation regression approachReference Mackinnon, Fairchild and Matthew 19 , Reference Kraemer, Wilson and Fairburn 20 was applied to explore the potential effect of daytime sleepiness (mediator) and its association with study antipsychotic treatments (exogenous causal variable) on changes in the PANSS-EC, cognitive, and functional capacity. The parameters of the mediation regression models were estimated separately for each of these outcome measures. The effects of ESS change scores on mediating changes in the outcome measures (agitation, cognitive, and functional performance) were estimated by a series of analysis of covariance (ANCOVA) models, which included terms for baseline score, center, terms for main and interaction effects of ESS change scores, and treatment groups. The direct effect of treatment on change in ESS total scores (mediator) was estimated by ANCOVA, which included terms for baseline, center, and treatment. Because cognitive and UPSA-B assessments could be affected by cultural differences, analyses were performed for subjects in all sites as well as the subgroup recruited from U.S. sites.Reference Harvey and Velligan 21
Results
Baseline demographic and clinical characteristics were comparable among the 4 treatment groups (Table 1). Change in the ESS total score at week 6 (last observation carried forward; LOCF) was not significantly different for the lurasidone 80 mg/d (–1.1, SE 0.3) and 160 mg/d groups (–0.7, SE 0.3) compared with the placebo group (–0.9, SE 0.3). The quetiapine XR 600 mg/d group (+0.6, SE 0.3) was associated with a significant increase in the ESS total score compared to placebo (–0.9, SE 0.3, p = 0.001) and to lurasidone (p < 0.001 for lurasidone 80 mg; p < 0.01 for lurasidone 160 mg) (see Figure 1). The proportion of subjects who experienced markedly high sleepiness was significantly greater in the quetiapine XR group compared to the lurasidone 80 mg/d group [p = 0.021; number needed to treat/harm (NNTH) = 12], and was numerically higher compared to the lurasidone 160 mg/d group (p = 0.081; NNTH = 15).
Table 1 Baseline characteristics of patients with schizophrenia in a 6-week randomized, double-blind, placebo-, and quetiapine XR-controlled study of lurasidone (safety population)

‡Data for these parameters are based on the intent-to-treat population.
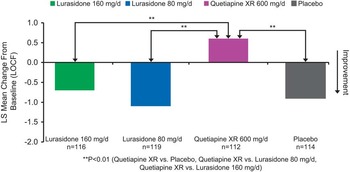
Figure 1 Change from baseline in Epworth Sleepiness Scale.
Change in each of the 8 ESS items at week 6 (LOCF) was not significantly different between either of the lurasidone groups and the placebo group (all p > 0.05). Change from baseline in 5 of the 8 ESS items was significantly greater (reflecting increased sedation) in the quetiapine XR group when compared with the placebo group (see Figure 2). The quetiapine XR group was also associated with a significant increase in sedation in 6 of the 8 ESS items compared with either the lurasidone 80 mg or 160 mg group (Figure 2).

Figure 2 Change from baseline in Epworth Sleepiness Scale: Item analysis.
Drug induced sedation: effect on agitation
Improvement in the PANSS-EC subscale score at week 6 was significantly greater for the lurasidone (both p < 0.001) and quetiapine XR (p < 0.001) groups compared to the placebo group (Figure 3). Increase in the ESS total score (increase in sedation) in the quetiapine XR group (versus placebo group) was associated with improvement in agitation, as assessed by the PANSS-EC subscale (p = 0.042), and interaction effect of change in ESS and quetiapine XR versus placebo, but not for either dose of lurasidone [p = 0.258, interaction effect of change in ESS and pooled lurasidone dose groups (versus placebo)]. Increase in the ESS total score was not significantly associated with improvement in PANSS total and subscales (positive and negative symptoms) scores in either lurasidone group nor the quetiapine XR group versus placebo (all p > 0.05).

Figure 3 Change in sleepiness as a mediator of change in PANSS excitement subscale: Mediation regression analysis.
Drug induced sedation: effect on cognitive and functional performance\
In patients whose neurocognitive testing results were evaluable (based on prespecified criteria for the validity of the cognitive tests, n = 267), the effect of lurasidone 160 mg/d on cognitive performance (LS mean change 0.5, SE 0.24) was superior to both placebo (–0.16, SE 0.23, p = 0.038) and quetiapine XR (–0.24, SE 0.23, p = 0.018), as assessed by the CogState composite Z-score.Reference Harvey, Siu and Hsu 22 We assessed the potential mediating relationship between each of the 8 ESS items and the effect on the CogState composite score, for all treatment groups. Notably, increase in the ESS item 6 score (“dozing when talking”) was associated with a worsening of overall cognitive performance in the quetiapine XR group, showing a significant difference in mediating relationship (slope) compared to the lurasidone 160 mg/d group (p = 0.006, U.S. sites; and p = 0.015, all subjects, but not with placebo or lurasidone 80 mg/d groups) (Figure 4). There were no consistently significant associations between changes in other ESS items and cognitive performance at week 6 for lurasidone or quetiapine XR (all subjects or U.S. sites).
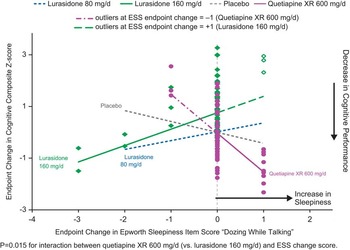
Figure 4 Change in sleepiness ESS item ‘dozing while talking’ and change composite Z score: Mediation regression analysis.
Among subjects recruited from U.S. sites, increase in the ESS total score (reflecting increased sedation) during quetiapine XR treatment was associated with a worsening in the UPSA-B total score (p = 0.003 for the difference in slopes between quetiapine and placebo, U.S. subjects only) (Figure 5); this relationship was not seen for the lurasidone treatment groups and was not observed in non-U.S., nor all subjects combined (p > 0.05).

Figure 5 Change in sleepiness and change in UPSA-B performance score: mediation regression analysis.
Adverse events
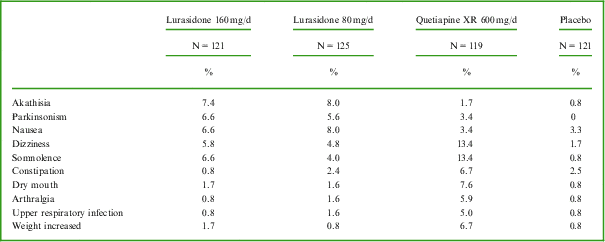
The most commonly reported adverse events in each active treatment group (incidence at least 5% and at least twice the placebo rate; see Table 2) were akathisia, parkinsonism, and nausea for the lurasidone 80 mg/d group; akathisia, parkinsonism, somnolence, nausea, and dizziness for the lurasidone 160 mg/d group; and dizziness, somnolence, dry mouth, constipation, weight increased, arthralgia, and upper respiratory tract infection for the quetiapine XR 600 mg/d group.
Table 2 Most commonly reported adverse events (incidence ≥5% and ≥2 placebo rate in each active treatment group) during 6 weeks of treatment with lurasidone, quetiapine XR, or placebo (safety population)
Discussion
This is the first placebo-controlled study in patients with acute schizophrenia to compare the effects of antipsychotic agents (lurasidone and quetiapine XR) on daytime sleepiness, using a validated self-report instrument, the Epworth Sleepiness Scale.Reference Johns 6 , Reference Johns 7 The proportion of patients experiencing somnolence or sedation in clinical trials is most commonly assessed based only on the spontaneously reported frequency of these adverse events.Reference Kane and Sharif 1 This approach may be limited by the ability and willingness of investigators to elicit and patients to report such adverse effects. In addition, we evaluated the effects of daytime sleepiness on key measures of treatment outcome, including severity of agitation, cognition, and functional capacity.
Change in the ESS total score was similar for lurasidone (at fixed daily doses of 80 mg and 160 mg) and placebo over 6 weeks in this study, with both groups showing a reduction in sleepiness from study baseline. Quetiapine XR (at a fixed daily dose of 600 mg) was associated with a significant mean increase in daytime sleepiness when compared to placebo or either dose of lurasidone. This effect persisted over the course of the study (6 weeks), which suggests that this adverse effect did not habituate over time. Markedly high daytime sleepiness (ESS total score >10) occurred significantly more often in the quetiapine XR group (19%) compared to placebo (13%) or either dose of lurasidone (10–12%). These findings indicate that high levels of daytime somnolence occur in association with lurasidone and placebo at comparable rates, but are observed more frequently in association with quetiapine XR treatment. These results are consistent with differences in the rates of treatment-emergent somnolence reported as an adverse event in this study (lurasidone, 5.3%; quetiapine XR, 13.4%; and placebo, 0.8%).Reference Sheehan, Lecrubier and Sheehan 10
We note that several prior studies have specifically examined sedating effects of quetiapine in patient populations. In a previous 1-day, crossover study of clinically stable patients with bipolar disorder, a single dose of quetiapine led to significant decrements in cognitive test performance and increased somnolence and daytime sleepiness compared to risperidone treatment.Reference Harvey, Hassman and Mao 5 Both quetiapine extended-release and quetiapine immediate-release have been shown to produce similar high levels of sedation intensity 4–14 hours postdose in a randomized, double-blind study involving inpatients with bipolar depression.Reference Riesenberg, Baldytcheva and Datto 23 The increased rates of sedation observed in quetiapine XR–treated patients in the current study are consistent with these observations.
Our findings that lurasidone is associated with lower levels of daytime sleepiness than quetiapine may be expected, based in part on the differential receptor pharmacology of these agents. Specifically, quetiapine is associated with high affinity at the H1 receptor where it acts as an antagonistReference Baldwin and Scott 11 ; this mechanism is associated with sedation in both animal models and human studies.Reference Witek, Canestrari and Miller 24 In contrast, lurasidone has no affinity at the H1 receptor subtype,Reference Ishibashi, Horisawa and Tokuda 25 and has not been associated with impairments in cognition or high levels of sedation in prior studies. The relative propensity to induce sedation among multiple antipsychotic agents has been examined by Leucht etalReference Leucht, Cipriani and Spineli 26 in a multiple-treatments meta-analysis. Lurasidone was ranked as 6 and quetiapine as 11 out of 15 antipsychotic agents (higher number indicates greater sedation).Reference Leucht, Cipriani and Spineli 26
In this 6-week study, both lurasidone 80 mg/d and 160 mg/d dose groups, as well as quetiapine XR 600 mg/d, were associated with comparable and significant reductions in agitation, as assessed using the PANSS-EC subscale. However, using mediator regression modeling, daytime sleepiness associated with quetiapine XR treatment was found to be a mediator of change in agitation for quetiapine XR, but not for either dose of lurasidone. These results suggest that reduction in agitation may be accounted for, in part, by induction of sleepiness in patients treated with quetiapine XR, but not lurasidone.
An analysis of evaluable neurocognitive testing scores in the current study (using the CogState computerized cognitive battery) found that lurasidone 160 mg/d was superior to both placebo and quetiapine XR in cognitive performance at week 6.Reference Harvey, Siu and Hsu 22 While the mechanistic basis for these observed improvements in cognition is not well understood, it is possible that the serotonin 5HT-7 receptor subtype (where lurasidone binds with high affinity and acts as an antagonist) plays a significant role in mediating these effects.Reference Horiguchi, Huang and Meltzer 27 The change in the ESS total score associated with both lurasidone 80 mg/d and 160 mg/d dose groups, and quetiapine XR 600 mg/d, was not a significant mediator of change in the cognitive composite performance score. However, analysis of the ESS item scores showed that an increase in the ESS item score 6 (“propensity to doze when talking to someone”) was found to be associated with a worsening of cognitive performance in the quetiapine XR group compared to the lurasidone 160 mg/d group. These results suggest that cognitive performance may be adversely affected by specific types of soporific effects, as characterized by selected ESS items. This finding is consistent with the psychometric basis for the ESS, where items were intended to assess the level of sleepiness in situations known to vary in their soporific qualities.Reference Johns 6 , Reference Johns 7 We note that the ESS composite score, which incorporates subjects’ impression of the likelihood to doze off or fall asleep while engaging in mostly passive activities, may not be a sufficiently sensitive correlate to cognitive performance. Among the 8 different situations, the ESS item “dozing while talking” is the only activity that involves the kind of attention, alertness, and active engagement required in social interactions. These results suggest that sleepiness experienced in specific situations, particularly sleepiness related to higher-level cognitive or social interactive tasks, may exert more influence on cognitive performance than other forms of somnolence.
Both the lurasidone 80 mg/d and 160 mg/d dose groups as well as the quetiapine XR 600 mg/d group were associated with comparable and significant improvement in functional capacity, as assessed using the UPSA-B. Daytime sleepiness associated with quetiapine XR treatment was found to be a mediator of change in functional capacity for quetiapine XR among subjects from U.S. sites, but not for either dose of lurasidone (Figure 4). Notably, this relationship was not observed in non-U.S. subjects (or in the full study sample). It is possible that cultural differences make the UPSA-B less sensitive to change in functional capacity in non-U.S. regions.Reference Harvey and Velligan 21 Our findings from the U.S. sites suggest that quetiapine XR–associated daytime sleepiness may lead to impaired functional performance; this relationship between daytime sedation and functional capacity change was not observed for lurasidone or placebo, perhaps due to the lack of soporific effects induced by these treatments.
Some limitations of these findings should be noted. The fixed-dose design of the placebo-controlled trial from which these data were derived did not permit investigators an opportunity to individualize treatment and therefore optimize tolerability for each agent, including with regard to the level of daytime sleepiness or sedation. Although several studies have reported that ESS total scores correlated significantly with laboratory measures of mean sleep latency, such as the MSLT, in patients with various sleep disorders,Reference Johns 6 , Reference Johns 7 others have reported lower correlations.Reference Guilleminault and Brooks 28 Since patients with schizophrenia may have reduced insight, this could have reduced the accuracy of patient report regarding perceived levels of daytime sleepiness; however the differences observed among the treatment groups in this study suggest that the ESS is sensitive to change in this patient population. The regression model used for analyzing the mediating effect of daytime sleepiness on various treatment outcomes involved a statistical interaction term for treatment and change in the ESS total score. It is possible that the absence of a mediating effect for some ESS items may have been due to a lack of statistical power and limited range of the variables. Further studies are warranted to verify these exploratory analysis findings utilizing other antipsychotic agents to determine the generalizability of these results. The effect of lurasidone and quetiapine XR on measures of daytime sleepiness in longer-term treatment will be reported elsewhere.
Conclusions
Our findings underscore the observation that second-generation, atypical antipsychotic agents have distinct efficacy and adverse effect profiles, and hence are not easily interchangeable.Reference Rosenheck 29 , Reference Leucht, Corves and Arbter 30 In this study, treatment with fixed-dose lurasidone 80 mg or 160 mg, administered for 6 weeks once daily in the evening, was associated with a reduction in daytime sleepiness, of similar magnitude to placebo, while treatment with quetiapine XR 600 mg was associated with a significant increase in self-reported daytime sleepiness compared to placebo. Our findings suggest that daytime somnolence may have a significant impact on cognitive and functional outcomes. In addition, these findings support the use of the ESS as a brief and practical tool for the assessment of daytime sleepiness in patients with schizophrenia and provide evidence for its sensitivity to change during treatment with antipsychotic agents.
Disclosures
This work was supported by Sunovion Pharmaceuticals Inc.
Dr. Siu has received payment for consulting from Pfizer Inc., Sunovion Pharmaceuticals Inc., and the Center for Addiction and Mental Health at the University of Toronto. Dr. Harvey serves as a consultant/advisory board member for Abbvie, Boeheringer Ingelheim, Bristol-Myers-Squibb, Forest Labs, Genentech, Roche, Shire, Sunovion, and Takeda. Drs. Cucchiaro, Loebel, and Pikalov are employees of Sunovion Pharmaceuticals Inc.









